Characterization of RAGE and CK2 Expressions in Human Fetal Membranes
Abstract
1. Introduction
2. Results
2.1. Ontogeny of RAGE and CK2 Subunit mRNA Expressions in Fetal Membranes during the Three Trimesters of Human Pregnancy
2.2. RAGE and CK2 Immunolocalizations in Fetal Membranes at Term in Case of Spontaneous Labor (TIL) and without Labor (TNL)
2.3. Molecular Characterization of RAGE and CK2 Subunits in Human Fetal Membranes at Term with Spontaneous Labor (TIL) and No Labor (TNL)
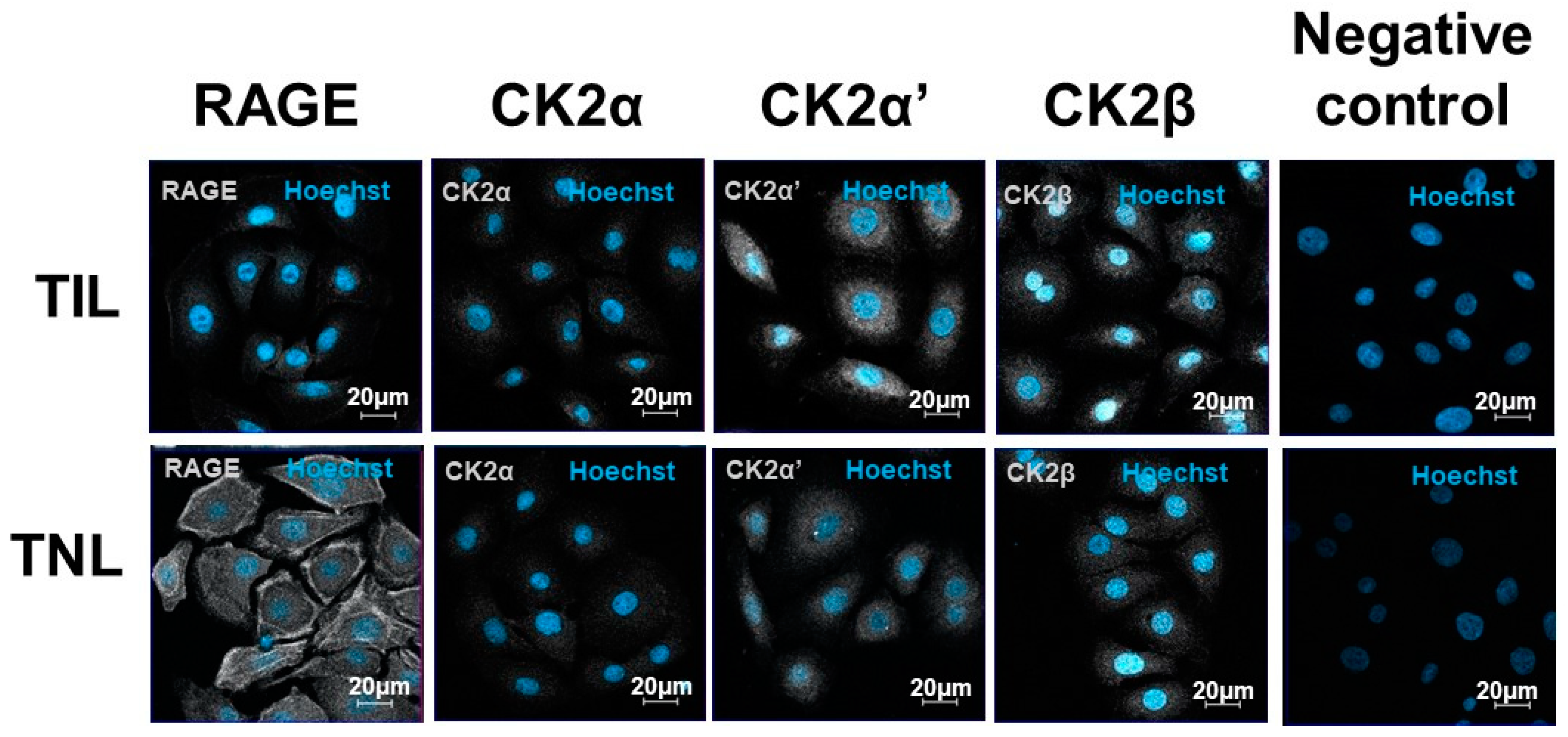
2.4. RAGE and CK2 Immunolocalizations in Primary Amniotic Epithelial Cells at Term in Case of Spontaneous Labor (TIL) and without Labor (TNL)
2.5. Molecular Characterization of RAGE and CK2 Subunits in Primary Amniocytes at Term in Case of Spontaneous Labor (TIL) and without Labor (TNL)
3. Discussion
4. Materials and Methods
4.1. Human Fetal Membrane Collection
4.2. Cell Cultures
4.3. CK2 and RAGE Immunofluorescence Staining
4.4. Quantitative RT-PCR
4.5. Western Blot Analysis
4.6. CK2 Activity Assay
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Méhats, C.; Schmitz, T.; Marcellin, L.; Breuiller-Fouché, M. Biochemistry of fetal membranes rupture. Gynecol. Obstet. Fertil. 2011, 39, 365–369. [Google Scholar] [CrossRef] [PubMed]
- El Khwad, M.; Stetzer, B.; Moore, R.M.; Kumar, D.; Mercer, B.; Arikat, S.; Redline, R.W.; Mansour, J.M.; Moore, J.J. Term Human Fetal Membranes Have a Weak Zone Overlying the Lower Uterine Pole and Cervix before Onset of Labor. Biol. Reprod. 2005, 72, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Richardson, L.S.; Lappas, M. Fetal Membrane Architecture, Aging and Inflammation in Pregnancy and Parturition. Placenta 2019, 79, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Moore, J.J. Fetal Membranes, Not a Mere Appendage of the Placenta, but a Critical Part of the Fetal-Maternal Interface Controlling Parturition. Obstet. Gynecol. Clin. N. Am. 2020, 47, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Padron, J.G.; Saito Reis, C.A.; Kendal-Wright, C.E. The Role of Danger Associated Molecular Patterns in Human Fetal Membrane Weakening. Front. Physiol. 2020, 11, 602. [Google Scholar] [CrossRef]
- Yan, H.; Zhu, L.; Zhang, Z.; Li, H.; Li, P.; Wang, Y.; Leng, M. HMGB1-RAGE Signaling Pathway in PPROM. Taiwan. J. Obstet. Gynecol. 2018, 57, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, D.; Giguère, Y.; Blanchon, L.; Bujold, E.; Pereira, B.; Bernard, N.; Gallot, D.; Sapin, V.; Forest, J.-C. Study of SRAGE, HMGB1, AGE, and S100A8/A9 Concentrations in Plasma and in Serum-Extracted Extracellular Vesicles of Pregnant Women With Preterm Premature Rupture of Membranes. Front. Physiol. 2020, 11, 609. [Google Scholar] [CrossRef]
- Richardson, L.S.; Taylor, R.N.; Menon, R. Reversible EMT and MET Mediate Amnion Remodeling during Pregnancy and Labor. Sci. Signal. 2020, 13, eaay1486. [Google Scholar] [CrossRef] [PubMed]
- de Castro Silva, M.; Richardson, L.S.; Kechichian, T.; Urrabaz-Garza, R.; da Silva, M.G.; Menon, R. Inflammation, but Not Infection, Induces EMT in Human Amnion Epithelial Cells. Reproduction 2020, 160, 627–638. [Google Scholar] [CrossRef]
- Janzen, C.; Sen, S.; Lei, M.Y.Y.; Gagliardi de Assumpcao, M.; Challis, J.; Chaudhuri, G. The Role of Epithelial to Mesenchymal Transition in Human Amniotic Membrane Rupture. J. Clin. Endocrinol. Metab. 2016, 102, 1261–1269. [Google Scholar] [CrossRef][Green Version]
- Lorthe, E. Épidémiologie, facteurs de risque et pronostic de l’enfant. RPC: Rupture prématurée des membranes avant terme CNGOF. Gynécologie Obs. Fertil. Sénologie 2018, 46, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Choltus, H.; Lavergne, M.; Belville, C.; Gallot, D.; Minet-Quinard, R.; Durif, J.; Blanchon, L.; Sapin, V. Occurrence of a RAGE-Mediated Inflammatory Response in Human Fetal Membranes. Front. Physiol. 2020, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Palanissami, G.; Paul, S.F.D. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer-a Review. Horm. Cancer 2018, 9, 295–325. [Google Scholar] [CrossRef] [PubMed]
- Egaña-Gorroño, L.; López-Díez, R.; Yepuri, G.; Ramirez, L.S.; Reverdatto, S.; Gugger, P.F.; Shekhtman, A.; Ramasamy, R.; Schmidt, A.M. Receptor for Advanced Glycation End Products (RAGE) and Mechanisms and Therapeutic Opportunities in Diabetes and Cardiovascular Disease: Insights From Human Subjects and Animal Models. Front. Cardiovasc. Med. 2020, 7, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, M.; Murata, H.; Yamamoto, K.; Ono, T.; Sakaguchi, Y.; Motoyama, A.; Hibino, T.; Kataoka, K.; Huh, N. TIRAP, an Adaptor Protein for TLR2/4, Transduces a Signal from RAGE Phosphorylated upon Ligand Binding. PLoS ONE 2011, 6, e23132. [Google Scholar] [CrossRef] [PubMed]
- Abi Nahed, R.; Reynaud, D.; Lemaitre, N.; Lartigue, S.; Roelants, C.; Vaiman, D.; Benharouga, M.; Cochet, C.; Filhol, O.; Alfaidy, N. Protein Kinase CK2 Contributes to Placental Development: Physiological and Pathological Implications. J. Mol. Med. 2020, 98, 123–133. [Google Scholar] [CrossRef]
- Bibby, A.C.; Litchfield, D.W. The Multiple Personalities of the Regulatory Subunit of Protein Kinase CK2: CK2 Dependent and CK2 Independent Roles Reveal a Secret Identity for CK2β. Int. J. Biol. Sci. 2005, 1, 67–79. [Google Scholar] [CrossRef]
- Borgo, C.; D’Amore, C.; Sarno, S.; Salvi, M.; Ruzzene, M. Protein Kinase CK2: A Potential Therapeutic Target for Diverse Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 183. [Google Scholar] [CrossRef]
- Deshiere, A.; Duchemin-Pelletier, E.; Spreux, E.; Ciais, D.; Combes, F.; Vandenbrouck, Y.; Couté, Y.; Mikaelian, I.; Giusiano, S.; Charpin, C.; et al. Unbalanced Expression of CK2 Kinase Subunits Is Sufficient to Drive Epithelial-to-Mesenchymal Transition by Snail1 Induction. Oncogene 2013, 32, 1373–1383. [Google Scholar] [CrossRef]
- Filhol, O.; Giacosa, S.; Wallez, Y.; Cochet, C. Protein Kinase CK2 in Breast Cancer: The CK2β Regulatory Subunit Takes Center Stage in Epithelial Plasticity. Cell. Mol. Life Sci. 2015, 72, 3305–3322. [Google Scholar] [CrossRef]
- Bredeson, S.; Papaconstantinou, J.; Deford, J.H.; Kechichian, T.; Syed, T.A.; Saade, G.R.; Menon, R. HMGB1 Promotes a P38MAPK Associated Non-Infectious Inflammatory Response Pathway in Human Fetal Membranes. PLoS ONE 2014, 9, e113799. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.M.; Bryant, A.H.; Rees, A.; Down, B.; Thornton, C.A. Production and Regulation of Interleukin-1 Family Cytokines at the Materno-Fetal Interface. Cytokine 2017, 99, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Liong, S.; Lappas, M. Endoplasmic Reticulum Stress Is Increased after Spontaneous Labor in Human Fetal Membranes and Myometrium Where It Regulates the Expression of Prolabor Mediators. Biol. Reprod. 2014, 91, 70. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in Inflammatory Diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef] [PubMed]
- Lestari, B.; Naito, S.; Endo, A.; Nishihara, H.; Kato, A.; Watanabe, E.; Denda, K.; Komada, M.; Fukushima, T. Placental Mammals Acquired Functional Sequences in NRK for Regulating the CK2–PTEN–AKT Pathway and Placental Cell Proliferation. Mol. Biol. Evol. 2022, 39, msab371. [Google Scholar] [CrossRef]
- Gupta, M.B.; Abu Shehab, M.; Nygard, K.; Biggar, K.; Singal, S.S.; Santoro, N.; Powell, T.L.; Jansson, T. IUGR Is Associated With Marked Hyperphosphorylation of Decidual and Maternal Plasma IGFBP-1. J. Clin. Endocrinol. Metab. 2019, 104, 408–422. [Google Scholar] [CrossRef]
- Li, X.; Gorodeski, G. Non-Muscle Myosin-II-B Filament Regulation of Paracellular Resistance in Cervical Epithelial Cells Is Associated with Modulation of the Cortical Acto-Myosin. J. Soc. Gynecol. Investig. 2006, 13, 579–591. [Google Scholar] [CrossRef][Green Version]
- Alvarado-Díaz, C.P.; Tapia, J.C.; Antonelli, M.; Moreno, R.D. Differential Localization of α’ and β Subunits of Protein Kinase CK2 during Rat Spermatogenesis. Cell Tissue Res. 2009, 338, 139. [Google Scholar] [CrossRef]
- Menon, R. Human Fetal Membranes at Term: Dead Tissue or Signalers of Parturition? Placenta 2016, 44, 1–5. [Google Scholar] [CrossRef]
- Filhol, O.; Cochet, C. Protein Kinase CK2 in Health and Disease: Cellular Functions of Protein Kinase CK2: A Dynamic Affair. Cell. Mol. Life Sci. 2009, 66, 1830–1839. [Google Scholar] [CrossRef]
- Axtell, R.C.; Xu, L.; Barnum, S.R.; Raman, C. CD5-CK2 Binding/Activation-Deficient Mice Are Resistant to Experimental Autoimmune Encephalomyelitis: Protection Is Associated with Diminished Populations of IL-17-Expressing T Cells in the Central Nervous System. J. Immunol. 2006, 177, 8542–8549. [Google Scholar] [CrossRef] [PubMed]
- Cozza, G.; Bortolato, A.; Moro, S. How Druggable Is Protein Kinase CK2? Med. Res. Rev. 2010, 30, 419–462. [Google Scholar] [CrossRef] [PubMed]
- Nakaniwa, T.; Kinoshita, T.; Sekiguchi, Y.; Tada, T.; Nakanishi, I.; Kitaura, K.; Suzuki, Y.; Ohno, H.; Hirasawa, A.; Tsujimoto, G. Structure of Human Protein Kinase CK2α2 with a Potent Indazole-Derivative Inhibitor. Acta Cryst. F 2009, 65, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, N.; Olsen, B.; Raaf, J.; Bretner, M.; Issinger, O.-G.; Niefind, K. Structure of the Human Protein Kinase CK2 Catalytic Subunit CK2α’ and Interaction Thermodynamics with the Regulatory Subunit CK2β. J. Mol. Biol. 2011, 407, 1–12. [Google Scholar] [CrossRef]
- Poletto, G.; Vilardell, J.; Marin, O.; Pagano, M.A.; Cozza, G.; Sarno, S.; Falqués, A.; Itarte, E.; Pinna, L.A.; Meggio, F. The Regulatory Beta Subunit of Protein Kinase CK2 Contributes to the Recognition of the Substrate Consensus Sequence. A Study with an EIF2 Beta-Derived Peptide. Biochemistry 2008, 47, 8317–8325. [Google Scholar] [CrossRef]
- Filhol, O.; Nueda, A.; Martel, V.; Gerber-Scokaert, D.; Benitez, M.J.; Souchier, C.; Saoudi, Y.; Cochet, C. Live-Cell Fluorescence Imaging Reveals the Dynamics of Protein Kinase CK2 Individual Subunits. Mol. Cell. Biol. 2003, 23, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Belville, C.; Ponelle-Chachuat, F.; Rouzaire, M.; Gross, C.; Pereira, B.; Gallot, D.; Sapin, V.; Blanchon, L. Physiological TLR4 Regulation in Human Fetal Membranes as an Explicative Mechanism of a Pathological Preterm Case. eLife 2022, 11, e71521. [Google Scholar] [CrossRef] [PubMed]
- Filhol, O.; Hesse, A.-M.; Bouin, A.-P.; Albigès-Rizo, C.; Jeanneret, F.; Battail, C.; Pflieger, D.; Cochet, C. CK2β Is a Gatekeeper of Focal Adhesions Regulating Cell Spreading. Front. Mol. Biosci. 2022, 9, 900947. [Google Scholar] [CrossRef]
- Rojas, A.; Schneider, I.; Lindner, C.; Gonzalez, I.; Morales, M.A. The RAGE/Multiligand Axis: A New Actor in Tumor Biology. Biosci. Rep. 2022, 42, BSR20220395. [Google Scholar] [CrossRef]
- Buckley, S.T.; Medina, C.; Kasper, M.; Ehrhardt, C. Interplay between RAGE, CD44, and Focal Adhesion Molecules in Epithelial-Mesenchymal Transition of Alveolar Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L548–L559. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Kinoshita, R.; Putranto, E.W.; Ruma, I.M.W.; Sumardika, I.W.; Youyi, C.; Tomonobu, N.; Yamamoto, K.-I.; Murata, H. Signal Diversity of Receptor for Advanced Glycation End Products. Acta Med. Okayama 2017, 71, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Montenarh, M.; Götz, C. Ecto-Protein Kinase CK2, the Neglected Form of CK2. Biomed. Rep. 2018, 8, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Menon, R. Fetal Inflammatory Response at the Fetomaternal Interface: A Requirement for Labor at Term and Preterm. Immunol. Rev. 2022, 308, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Radnaa, E.; Urrabaz-Garza, R.; Elrod, N.D.; de Castro Silva, M.; Pyles, R.; Han, A.; Menon, R. Generation and Characterization of Human Fetal Membrane and Decidual Cell Lines for Reproductive Biology Experiments†. Biol. Reprod. 2022, 106, 568–582. [Google Scholar] [CrossRef] [PubMed]
- Prat, C.; Bouvier, D.; Comptour, A.; Marceau, G.; Belville, C.; Clairefond, G.; Blanc, P.; Gallot, D.; Blanchon, L.; Sapin, V. All-Trans-Retinoic Acid Regulates Aquaporin-3 Expression and Related Cellular Membrane Permeability in the Human Amniotic Environment. Placenta 2015, 36, 881–887. [Google Scholar] [CrossRef]
- Marceau, G.; Gallot, D.; Borel, V.; Lémery, D.; Dastugue, B.; Dechelotte, P.; Sapin, V. Molecular and Metabolic Retinoid Pathways in Human Amniotic Membranes. Biochem. Biophys. Res. Commun. 2006, 346, 1207–1216. [Google Scholar] [CrossRef]
- Lavergne, M.; Belville, C.; Choltus, H.; Gross, C.; Minet-Quinard, R.; Gallot, D.; Sapin, V.; Blanchon, L. Human Amnion Epithelial Cells (AECs) Respond to the FSL-1 Lipopeptide by Engaging the NLRP7 Inflammasome. Front. Immunol. 2020, 11, 1645. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Songyang, Z.; Lu, K.P.; Kwon, Y.T.; Tsai, L.H.; Filhol, O.; Cochet, C.; Brickey, D.A.; Soderling, T.R.; Bartleson, C.; Graves, D.J.; et al. A Structural Basis for Substrate Specificities of Protein Ser/Thr Kinases: Primary Sequence Preference of Casein Kinases I and II, NIMA, Phosphorylase Kinase, Calmodulin-Dependent Kinase II, CDK5, and Erk1. Mol. Cell. Biol. 1996, 16, 6486–6493. [Google Scholar] [CrossRef]






| Human Gene | Sequence (5′→3′) | Product Length (bp) | NCBI Reference |
|---|---|---|---|
| hCK2α-S | TGTCCGAGTTGCTTCCCGATACTT | 104 | NM_177559 |
| hCK2α-A | TTGCCAGCATACAACCCAAACTCC | ||
| hCK2α′-S | AGCCCACCACCGTATATCAAACCT | 92 | NM_001896 |
| hCK2α′-A | ATGCTTTCTGGGTCGGGAAGAAGT | ||
| hCK2β-S | TTGGACCTGGAGCCTGATGAAGAA | 101 | NM_001320 |
| hCK2β-A | TAGCGGGCGTGGATCAATCCATAA | ||
| hRAGE-S | TGTGCTGATCCTCCCTGAGA | 139 | NM_001136.5 |
| hRAGE-A | CGAGGAGGGGCCAACTGCA | ||
| hRPLP0-S | AGGCTTTAGGTATCACCACT | 219 | NM_053275 |
| hRPLP0-A | GCAGAGTTTCCTCTGTGATA | ||
| hRPS17-S | TGCGAGGAGATCGCCATTATC | 170 | NM_001021 |
| hRPS17-A | AAGGCTGAGACCTCAGGAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coste, K.; Bruet, S.; Chollat-Namy, C.; Filhol, O.; Cochet, C.; Gallot, D.; Marceau, G.; Blanchon, L.; Sapin, V.; Belville, C. Characterization of RAGE and CK2 Expressions in Human Fetal Membranes. Int. J. Mol. Sci. 2023, 24, 4074. https://doi.org/10.3390/ijms24044074
Coste K, Bruet S, Chollat-Namy C, Filhol O, Cochet C, Gallot D, Marceau G, Blanchon L, Sapin V, Belville C. Characterization of RAGE and CK2 Expressions in Human Fetal Membranes. International Journal of Molecular Sciences. 2023; 24(4):4074. https://doi.org/10.3390/ijms24044074
Chicago/Turabian StyleCoste, Karen, Shaam Bruet, Caroline Chollat-Namy, Odile Filhol, Claude Cochet, Denis Gallot, Geoffroy Marceau, Loïc Blanchon, Vincent Sapin, and Corinne Belville. 2023. "Characterization of RAGE and CK2 Expressions in Human Fetal Membranes" International Journal of Molecular Sciences 24, no. 4: 4074. https://doi.org/10.3390/ijms24044074
APA StyleCoste, K., Bruet, S., Chollat-Namy, C., Filhol, O., Cochet, C., Gallot, D., Marceau, G., Blanchon, L., Sapin, V., & Belville, C. (2023). Characterization of RAGE and CK2 Expressions in Human Fetal Membranes. International Journal of Molecular Sciences, 24(4), 4074. https://doi.org/10.3390/ijms24044074








