Rare Genetic Variants in Human APC Are Implicated in Mesiodens and Isolated Supernumerary Teeth
Abstract
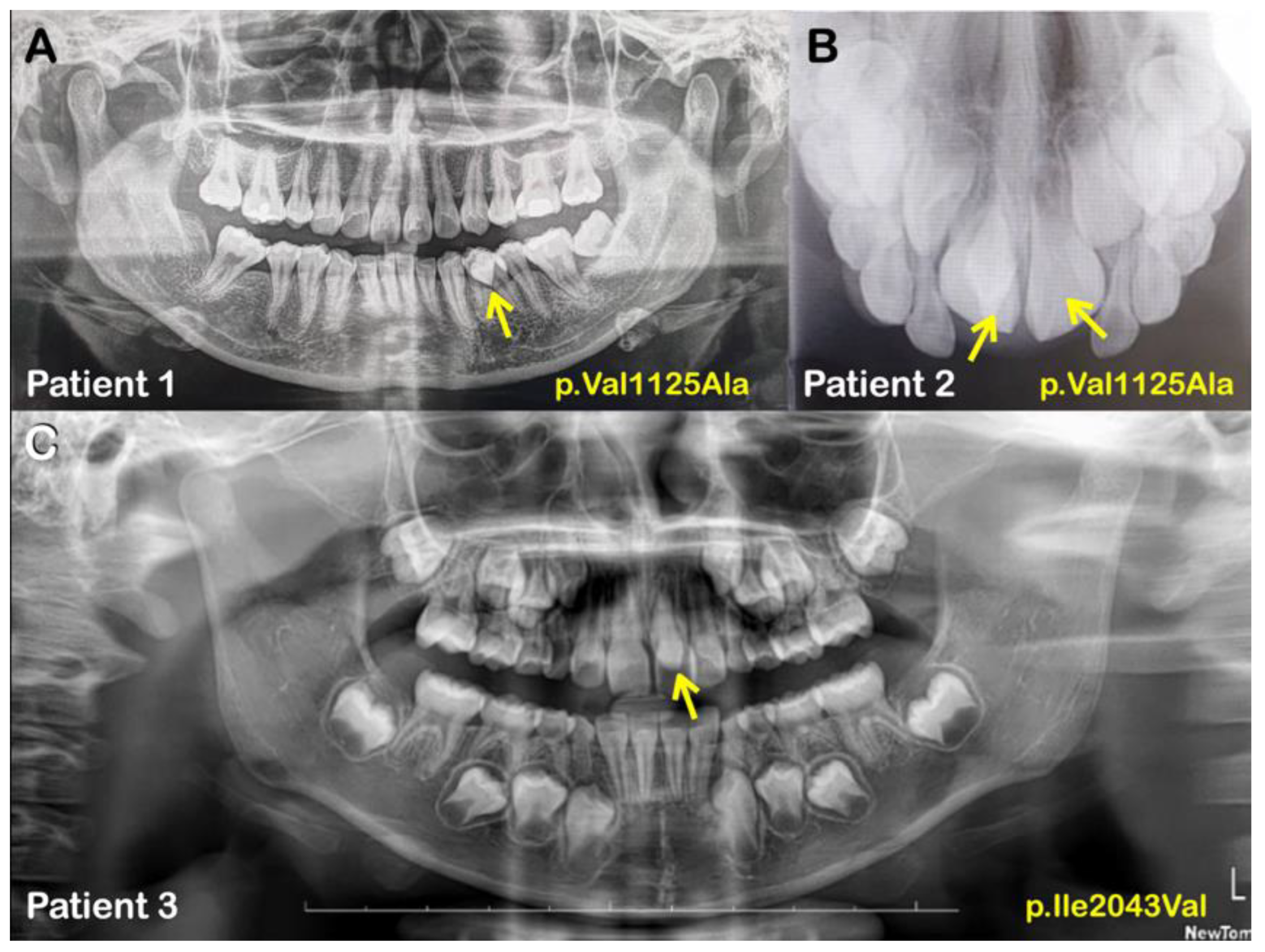
1. Introduction
2. Results
3. Discussion
3.1. APC Protein Structure and Possible Impact of Identified Variants on APC Function
3.2. APC Mutations, Ciliary Dysfunction, and Supernumerary Tooth Formation
4. Materials and Methods
4.1. Patient Recruitment
4.2. Whole Exome Sequencing, Mutation Analysis, and Bioinformatic Analyses
4.3. Structural Assessment of Variants
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajab, L.; Hamdan, M. Supernumerary teeth: Review of the literature and a survey of 152 cases. Int. J. Paediatr. Dent. 2002, 12, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Fan, J. Molecular genetics of supernumerary tooth formation. genesis 2011, 49, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Anthonappa, R.P.; King, N.M.; Rabie, A.B. Aetiology of supernumerary teeth: A literature review. Eur. Arch. Paediatr. Dent. 2013, 14, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, G.; Gokhale, N.; Mallineni, S.K.; Nuvvula, S. Prevalence of dental anomalies in deciduous dentition and its association with succedaneous dentition: A cross-sectional study of 4180 South Indian children. J. Indian. Soc. Pedod. Prev. Dent. 2017, 35, 56–62. [Google Scholar] [CrossRef]
- Davis, P.J. Hypodontia and hyperdontia of permanent teeth in Hong Kong schoolchildren. Community Dent. Oral Epidemiol. 1987, 15, 218–220. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Uehara, T.; Narita, T.; Tsutsumi, H.; Nakabayashi, S.; Araki, M. Prevalence and distribution of anomalies of permanent dentition in 9584 Japanese high school students. Odontology 2016, 104, 380–389. [Google Scholar] [CrossRef]
- Lu, X.; Yu, F.; Liu, J.; Cai, W.; Zhao, Y.; Zhao, S.; Liu, S. The epidemiology of supernumerary teeth and the associated molecular mechanism. Organogenesis 2017, 13, 71–82. [Google Scholar] [CrossRef]
- Kazanci, F.; Celikoglu, M.; Miloglu, O.; Yildirim, H.; Ceylan, I. The frequency and characteristics of mesiodens in a Turkish patient population. Eur. J. Dent. 2011, 5, 361–365. [Google Scholar] [CrossRef]
- Mukhopadhyay, S. Mesiodens: A clinical and radiographic study in children. J. Indian. Soc. Pedod. Prev. Dent. 2011, 29, 34. [Google Scholar] [CrossRef]
- Colak, H.; Uzgur, R.; Tan, E.; Hamidi, M.; Turkal, M.; Colak, T. Investigation of prevalence and characteristics of mesiodens in a non-syndromic 11256 dental outpatients. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2684–2689. [Google Scholar]
- Alarcón, J.; Guzmán, J.; Masuko, T.S.; Cáceres, P.N.; Fuentes, R. Non-Syndromic Familial Mesiodens: Presentation of Three Cases. Diagnostics 2022, 12, 1869. [Google Scholar] [CrossRef]
- Niswander, J.D.; Sujaku, C. Congenital anomalies of teeth in Japanese children. Am. J. Phys. Anthropol. 1963, 21, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.-Y.; Hsieh, C.-Y.; Tsai, T.-P. Clinical evaluation of the timing of mesiodens removal. J. Chin. Med. Assoc. 2016, 79, 345–350. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Doolan, B.; Onoufriadis, A.; Kantaputra, P.; McGrath, J. WNT10A, dermatology and dentistry. Br. J. Dermatol. 2021, 185, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; He, X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb. Perspect. Biol. 2012, 4, a007880. [Google Scholar] [CrossRef]
- Yu, F.; Cai, W.; Jiang, B.; Xu, L.; Liu, S.; Zhao, S. A novel mutation of adenomatous polyposis coli (APC) gene results in the formation of supernumerary teeth. J. Cell. Mol. Med. 2018, 22, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, F.; Cheng, X.; Wang, J.; Zhang, W.; Zhang, B.; Tang, Y.; Li, Q.; Zhou, C.; Tu, S. Wnt inhibition sensitizes PD-L1 blockade therapy by overcoming bone marrow-derived myofibroblasts-mediated immune resistance in tumors. Front. Immunol. 2021, 12, 619209. [Google Scholar] [CrossRef]
- Kantaputra, P.N.; Guven, Y.; Tripuwabhrut, K.; Adisornkanj, P.; Hatsadaloi, A.; Kaewgahya, M.; Olsen, B.; Ngamphiw, C.; Jatooratthawichot, P.; Tongsima, S. Mutations in LRP5 and BMP4 are associated with mesiodens, tooth agenesis, root malformation, and oral exostoses. Clin. Genet. 2022, 102, 333–338. [Google Scholar] [CrossRef]
- Kantaputra, P.; Jatooratthawichot, P.; Chintakanon, K.; Intachai, W.; Pradermdutsadeeporn, P.; Adisornkanj, P.; Tongsima, S.; Ngamphiw, C.; Olsen, B.; Tucker, A.S. Mutations in LRP6 highlight the role of WNT signaling in oral exostoses and dental anomalies. Arch. Oral. Biol. 2022, 142, 105514. [Google Scholar] [CrossRef]
- Kantaputra, P.; Tripuwabhrut, K.; Jatooratthawichot, P.; Adisornkanj, P.; Hatsadaloi, A.; Porntrakoolsaree, N.; Kaewgaya, M.; Olsen, B.; Tongsima, S.; Ngamphiw, C. Mutations in the WLS are associated with dental anomalies, torus palatinus, and torus mandibularis. Eur. J. Orthod. 2022, cjac068. Available online: https://pubmed.ncbi.nlm.nih.gov/36374649/ (accessed on 14 November 2022).
- Kantaputra, P.; Jatooratthawichot, P.; Kottege, N.; Anthonappa, R.P.; Kaewgahya, M.; Tongsima, S.; Ngamphiw, C.; Ketudat Cairns, J.R.; Predes, D.; He, X. DKK1 is a strong candidate for mesiodens and taurodontism. Clin. Genet. 2023. Available online: https://pubmed.ncbi.nlm.nih.gov/36601665/ (accessed on 4 January 2023). [CrossRef]
- Kantaputra, P.N.; Jatooratthawichot, P.; Adisornkanj, P.; Kitsadayurach, P.; Kaewgahya, M.; Olsen, B.; Ohazama, A.; Ngamphiw, C.; Tongsima, S.; Cox, T.C.; et al. Rare Variants in LRP4 Are Associated with Mesiodens, Root Maldevelopment, and Oral Exostoses in Humans. Biology 2023, 12, 220. [Google Scholar] [CrossRef]
- Munne, P.M.; Tummers, M.; Järvinen, E.; Thesleff, I.; Jernvall, J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development 2009, 136, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Ohazama, A.; Johnson, E.B.; Ota, M.S.; Choi, H.Y.; Porntaveetus, T.; Oommen, S.; Itoh, N.; Eto, K.; Gritli-Linde, A.; Herz, J.; et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS ONE 2008, 3, e4092. [Google Scholar] [CrossRef]
- García, E.B.G.; Knoers, N.V. Gardner’s syndrome (familial adenomatous polyposis): A cilia-related disorder. Lancet. Oncol. 2009, 10, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Lubinsky, M.; Kantaputra, P.N. Syndromes with supernumerary teeth. Am. J. Med. Genet. Part A 2016, 170, 2611–2616. [Google Scholar] [CrossRef]
- Otto, F.; Kanegane, H.; Mundlos, S. Mutations in the RUNX2 gene in patients with cleidocranial dysplasia. Hum. Mutat. 2002, 19, 209–216. [Google Scholar] [CrossRef]
- Hordyjewska-Kowalczyk, E.; Sowińska-Seidler, A.; Olech, E.M.; Socha, M.; Glazar, R.; Kruczek, A.; Latos-Bieleńska, A.; Tylzanowski, P.; Jamsheer, A. Functional analysis of novel RUNX2 mutations identified in patients with cleidocranial dysplasia. Clin. Genet. 2019, 96, 429–438. [Google Scholar] [CrossRef]
- Kantaputra, P.; Miletich, I.; Lüdecke, H.-J.; Suzuki, E.; Praphanphoj, V.; Shivdasani, R.; Wuelling, M.; Vortkamp, A.; Napierala, D.; Sharpe, P. Tricho-rhino-phalangeal syndrome with supernumerary teeth. J. Dent. Res. 2008, 87, 1027–1031. [Google Scholar] [CrossRef]
- Kunotai, W.; Ananpornruedee, P.; Lubinsky, M.; Pruksametanan, A.; Kantaputra, P.N. Making extra teeth: Lessons from a TRPS1 mutation. Am. J. Med. Genet. Part A 2017, 173, 99–107. [Google Scholar] [CrossRef]
- Kantaputra, P.N.; Coury, S.A.; Tan, W.-H. Impaired dentin mineralization, supernumerary teeth, hypoplastic mandibular condyles with long condylar necks, and a TRPS1 mutation. Arch. Oral. Biol. 2020, 116, 104735. [Google Scholar] [CrossRef]
- Nik Kantaputra, P.; Jotikasthira, D.; Carlson, B.; Wongmaneerung, T.; Quarto, N.; Khankasikum, T.; Powcharoen, W.; Intachai, W.; Tripuwabhrut, K. TRPS1 mutation associated with trichorhinophalangeal syndrome type 1 with 15 supernumerary teeth, hypoplastic mandibular condyles with slender condylar necks and unique hair morphology. J. Dermatol. 2020, 47, 774–778. [Google Scholar] [CrossRef]
- Rusan, N.M.; Peifer, M. Original CIN: Reviewing roles for APC in chromosome instability. J. Cell Biol. 2008, 181, 719–726. [Google Scholar] [CrossRef]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Gardner, E.J. A genetic and clinical study of intestinal polyposis, a predisposing factor for carcinoma of the colon and rectum. Am. J. Hum. Genet 1951, 3, 167. [Google Scholar]
- Järvinen, E.; Birchmeier, W.; Taketo, M.M.; Jernvall, J.; Thesleff, I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 18627–18632. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chu, E.Y.; Watt, B.; Zhang, Y.; Gallant, N.M.; Andl, T.; Yang, S.H.; Lu, M.-M.; Piccolo, S.; Schmidt-Ullrich, R. Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 2008, 313, 210–224. [Google Scholar] [CrossRef]
- Wang, X.-P.; O’Connell, D.J.; Lund, J.J.; Saadi, I.; Kuraguchi, M.; Turbe-Doan, A.; Cavallesco, R.; Kim, H.; Park, P.J.; Harada, H. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 2009, 136, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Hamada, F.; Bienz, M. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear beta-catenin from TCF. Dev. Cell 2004, 7, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Spink, K.E.; Polakis, P.; Weis, W.I. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 2000, 19, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Taketo, M.M. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J. Cell. Sci. 2007, 120, 3327–3335. [Google Scholar] [CrossRef] [PubMed]
- Kantaputra, P.; Jatooratthawichot, P.; Tantachamroon, O.; Nanekrungsan, K.; Intachai, W.; Olsen, B.; Tongsima, S.; Ngamphiw, C.; Cairns, J.R.K. Novel Dental Anomaly–associated Mutations in WNT10A Protein Binding Sites. Int. Dent. J. 2022, 73, 79–86. Available online: https://www.sciencedirect.com/science/article/pii/S0020653922000764 (accessed on 7 May 2022). [CrossRef]
- Zeng, Y.; Baugh, E.; Akyalcin, S.; Letra, A. Functional effects of wnt10a rare variants associated with tooth agenesis. J Dent Res 2021, 100, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.L.-H.; Ahn, V.E.; Choi, H.-J.; Daniels, D.L.; Nusse, R.; Weis, W.I. Structural studies of Wnts and identification of an LRP6 binding site. Structure 2013, 21, 1235–1242. [Google Scholar] [CrossRef]
- Tamai, K.; Semenov, M.; Kato, Y.; Spokony, R.; Liu, C.; Katsuyama, Y.; Hess, F.; Saint-Jeannet, J.-P.; He, X. LDL-receptor-related proteins in Wnt signal transduction. Nature 2000, 407, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Jho, E.-h. Regulation of the low-density lipoprotein receptor-related protein LRP6 and its association with disease: Wnt/β-catenin signaling and beyond. Front. Cell Dev. Biol. 2021, 9, 714330. [Google Scholar] [CrossRef] [PubMed]
- Kuraguchi, M.; Wang, X.-P.; Bronson, R.T.; Rothenberg, R.; Ohene-Baah, N.Y.; Lund, J.J.; Kucherlapati, M.; Maas, R.L.; Kucherlapati, R. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2006, 2, e146. [Google Scholar] [CrossRef]
- Fearnhead, N.S.; Britton, M.P.; Bodmer, W.F. The abc of apc. Hum. Mol. Genet. 2001, 10, 721–733. [Google Scholar] [CrossRef]
- Polakis, P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Acta 1997, 1332, F127–F147. [Google Scholar] [CrossRef]
- Juanes, M.A.; Fees, C.P.; Hoeprich, G.J.; Jaiswal, R.; Goode, B.L. EB1 Directly Regulates APC-Mediated Actin Nucleation. Curr Biol 2020, 30, 4763–4772.e4768. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, S.; Cheng, D.; Jing, W.; Helms, J. Primary cilia integrate hedgehog and Wnt signaling during tooth development. J. Dent. Res. 2014, 93, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hampl, M.; Cela, P.; Szabo-Rogers, H.L.; Kunova Bosakova, M.; Dosedelova, H.; Krejci, P.; Buchtova, M. Role of Primary Cilia in Odontogenesis. J. Dent. Res. 2017, 96, 965–974. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Sun, Y. Primary cilia in hard tissue development and diseases. Front. Med. 2021, 15, 657–678. [Google Scholar] [CrossRef] [PubMed]
- Hermans, F.; Hemeryck, L.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Intertwined signaling pathways governing tooth development: A give-and-take between canonical Wnt and Shh. Front. Cell Dev. Biol. 2021, 9, 758203. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, G.; Feng, L.; Jiang, Z.; Yu, M.; Bao, J.; Tian, W. Disruption of kif3a results in defective osteoblastic differentiation in dental mesenchymal stem/precursor cells via the Wnt signaling pathway. Mol. Med. Rep. 2016, 14, 1891–1900. [Google Scholar] [CrossRef]





| Families | Patients | Phenotypes | APC Variants NM_000038.6; NP_000029.2 | Prediction/Ranking |
|---|---|---|---|---|
| 1 | 1 (Female) | Supernumerary erupted mandibular left premolar | c.3374T>C p.Val1125Ala chr5-112174665-T-C rs377278397 MAF: 0.0005993 | MutationTaster: Disease causing Prob = 0.944130296883564 Polyphen-2: Benign; score = 0.001 SIFT: Tolerated; score = 0.244 CADD-PHRED score = 21.7 DANN: Benign; score = 0.9507 |
| 2 | 2 (Male) | Mesiodens (Double with normal orientation) | ||
| 3 | 3 (Female) | Mesiodens (Single; erupted) | c.6127A>G p.Ile2043Val chr5-112177418-A-G rs876660233 MAF: 0.000007085 | MutationTaster: Disease causing Prob = 0.999923872840111 Polyphen-2: Probably damaging; score = 0.958 SIFT: Damaging; score = 0.011 CADD-PHRED score = 24.9 DANN score = 0.9983 |
| 4 | Normal mother | No variants | MutationTaster: Disease causing Prob = 0.999223891277048 Polyphen-2: Probably damaging; score = 0.936 SIFT: Tolerated; score = 0.068 CADD-PHRED score = 22.2 DANN score = 0.9909 | |
| 4 (Male) | Mesiodens (Double; unerupted & inverted) | c.8383G>A p.Ala2795Thr chr5-112179674-G-A rs369264968 MAF:0.00004400 | ||
| 5 (Male) | Mesiodens (Double; both were erupted) | |||
| Normal sister | No variants | |||
| 5 | 6 (Male) | Mesiodens (Double; unerupted) One is inverted, the other had normal orientation | Variant 1 c.2740T>G p.Cys914Gly chr5-112174031-T-G rs1554084426 Not reported in gnomAD Variant 2 c.5722A>T p.Asn1908Tyr chr5-112177013-A-T No rs number Not reported in gnomAD | Variant 1 MutationTaster: Disease causing Prob = 0.99929839701033 Polyphen-2: Benign score = 0.055 SIFT: Tolerated; score = 0.127 CADD-PHRED score = 21.5 DANN score = 0.8956 Variant 2 MutationTaster: Polymorphism Prob = 0.999999988244843 Polyphen-2: Benign score = 0.214 SIFT: Damaging; score = 0.046 CADD-PHRED score = 17.19 DANN score = 0.9609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyarat, C.; Nakornchai, S.; Chintakanon, K.; Leelaadisorn, N.; Intachai, W.; Olsen, B.; Tongsima, S.; Adisornkanj, P.; Ngamphiw, C.; Cox, T.C.; et al. Rare Genetic Variants in Human APC Are Implicated in Mesiodens and Isolated Supernumerary Teeth. Int. J. Mol. Sci. 2023, 24, 4255. https://doi.org/10.3390/ijms24054255
Panyarat C, Nakornchai S, Chintakanon K, Leelaadisorn N, Intachai W, Olsen B, Tongsima S, Adisornkanj P, Ngamphiw C, Cox TC, et al. Rare Genetic Variants in Human APC Are Implicated in Mesiodens and Isolated Supernumerary Teeth. International Journal of Molecular Sciences. 2023; 24(5):4255. https://doi.org/10.3390/ijms24054255
Chicago/Turabian StylePanyarat, Chomchanok, Siriruk Nakornchai, Kanoknart Chintakanon, Niramol Leelaadisorn, Worrachet Intachai, Bjorn Olsen, Sissades Tongsima, Ploy Adisornkanj, Chumpol Ngamphiw, Timothy C. Cox, and et al. 2023. "Rare Genetic Variants in Human APC Are Implicated in Mesiodens and Isolated Supernumerary Teeth" International Journal of Molecular Sciences 24, no. 5: 4255. https://doi.org/10.3390/ijms24054255
APA StylePanyarat, C., Nakornchai, S., Chintakanon, K., Leelaadisorn, N., Intachai, W., Olsen, B., Tongsima, S., Adisornkanj, P., Ngamphiw, C., Cox, T. C., & Kantaputra, P. (2023). Rare Genetic Variants in Human APC Are Implicated in Mesiodens and Isolated Supernumerary Teeth. International Journal of Molecular Sciences, 24(5), 4255. https://doi.org/10.3390/ijms24054255








