Small-Molecule-Mediated Suppression of BMP Signaling by Selective Inhibition of BMP1-Dependent Chordin Cleavage
Abstract
:1. Introduction
2. Results
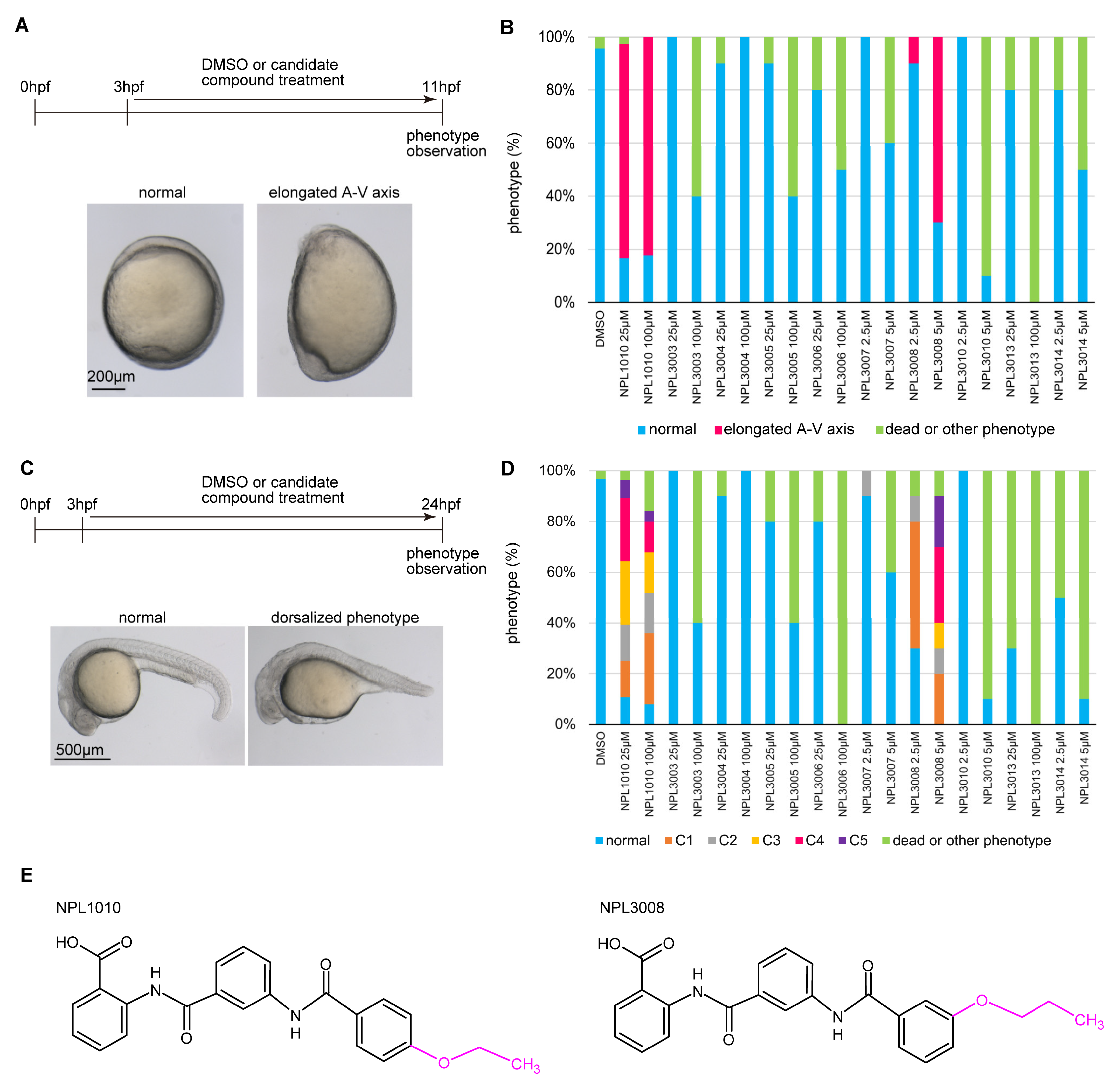
2.1. NPL1010 and NPL3008 Induced a Distinct Phenotype
2.2. NPL1010 and NPL3008 Induce a Dorsalized Phenotype
2.3. NPL1010 and NPL3008 Reduce the Expression of Ventral Genes
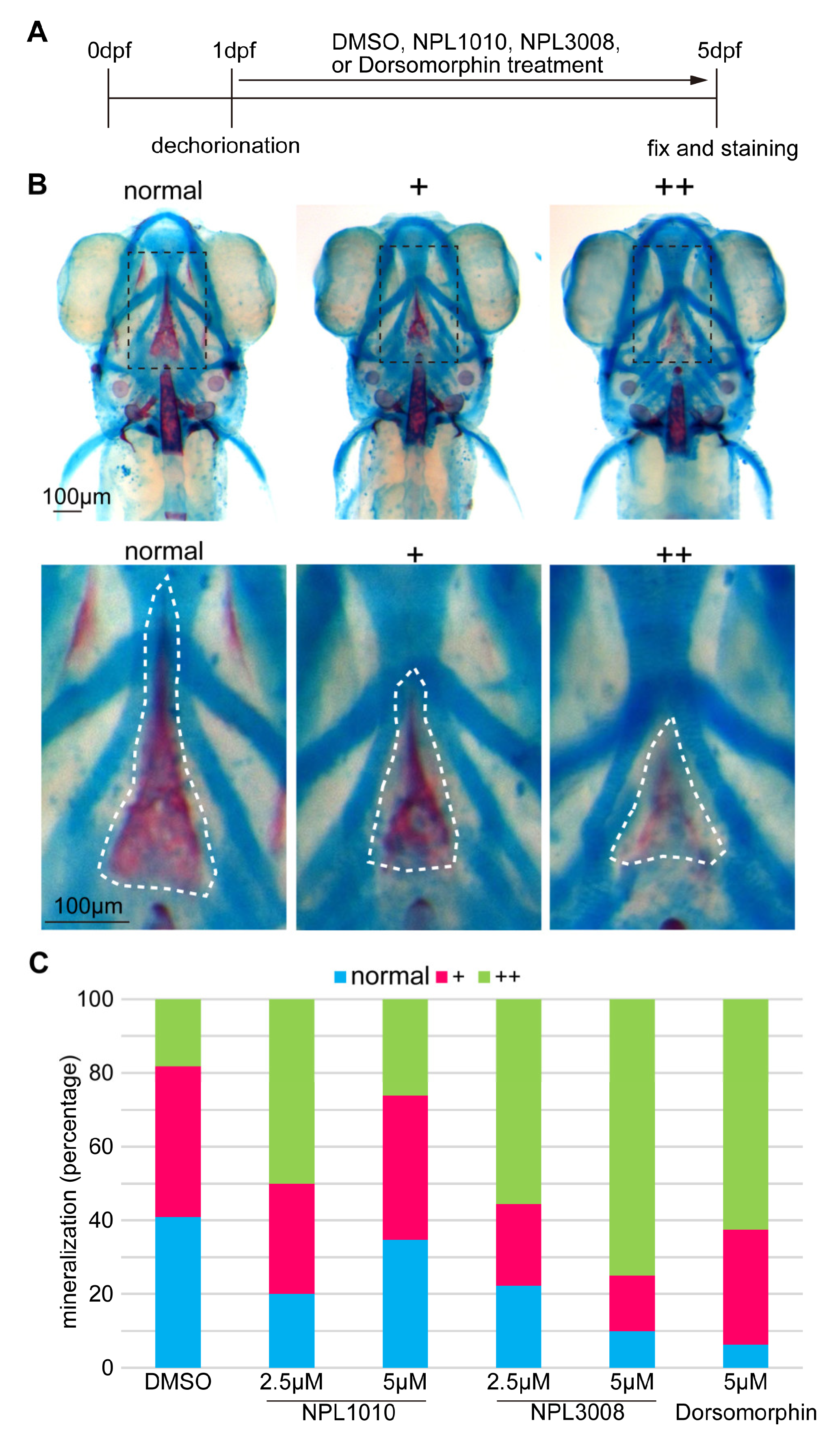
2.4. NPL1010 and NPL3008 Inhibit Bone Formation In Vivo
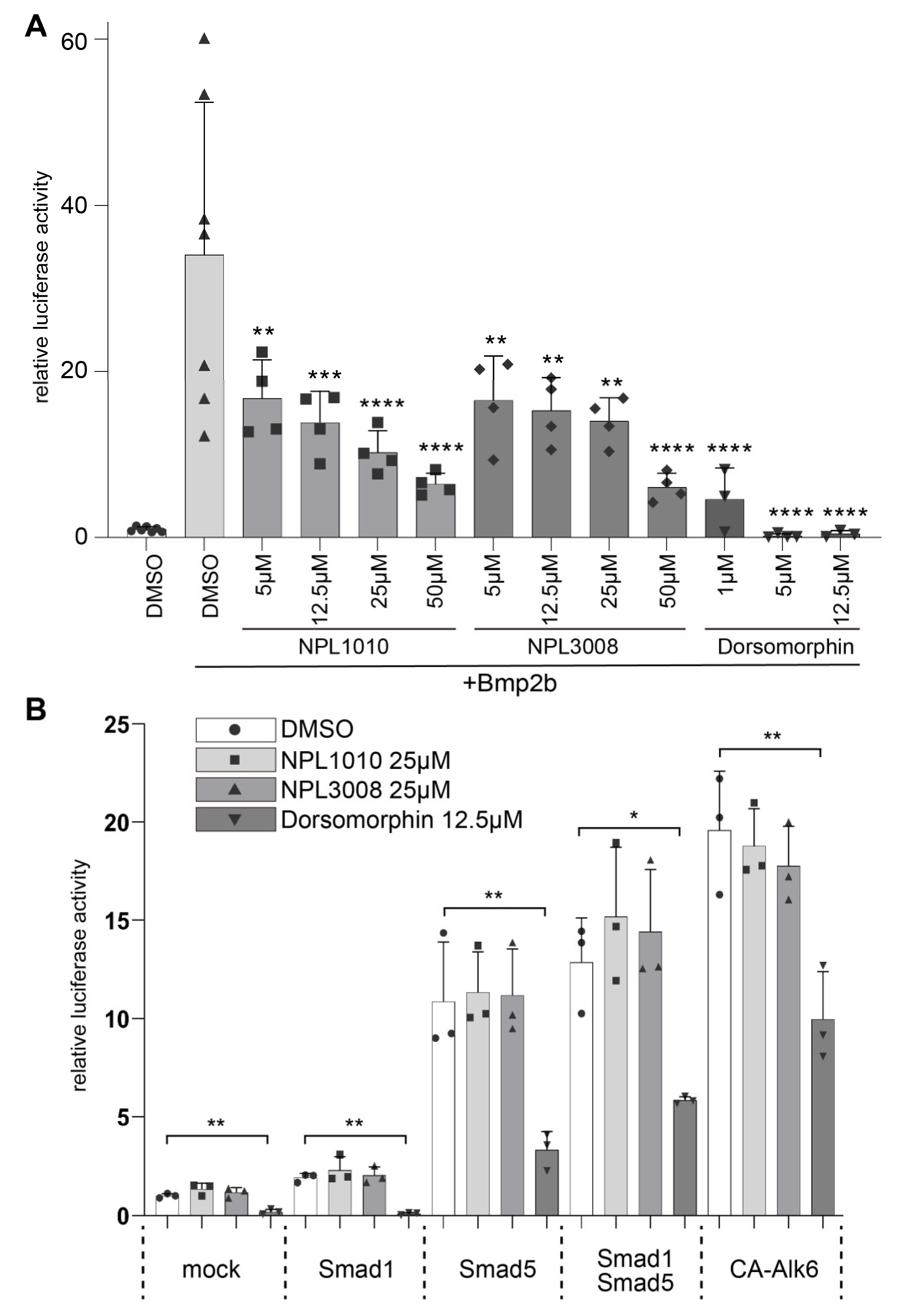
2.5. NPL1010 and NPL3008 Decrease Activity of the BMP Signaling Reporter
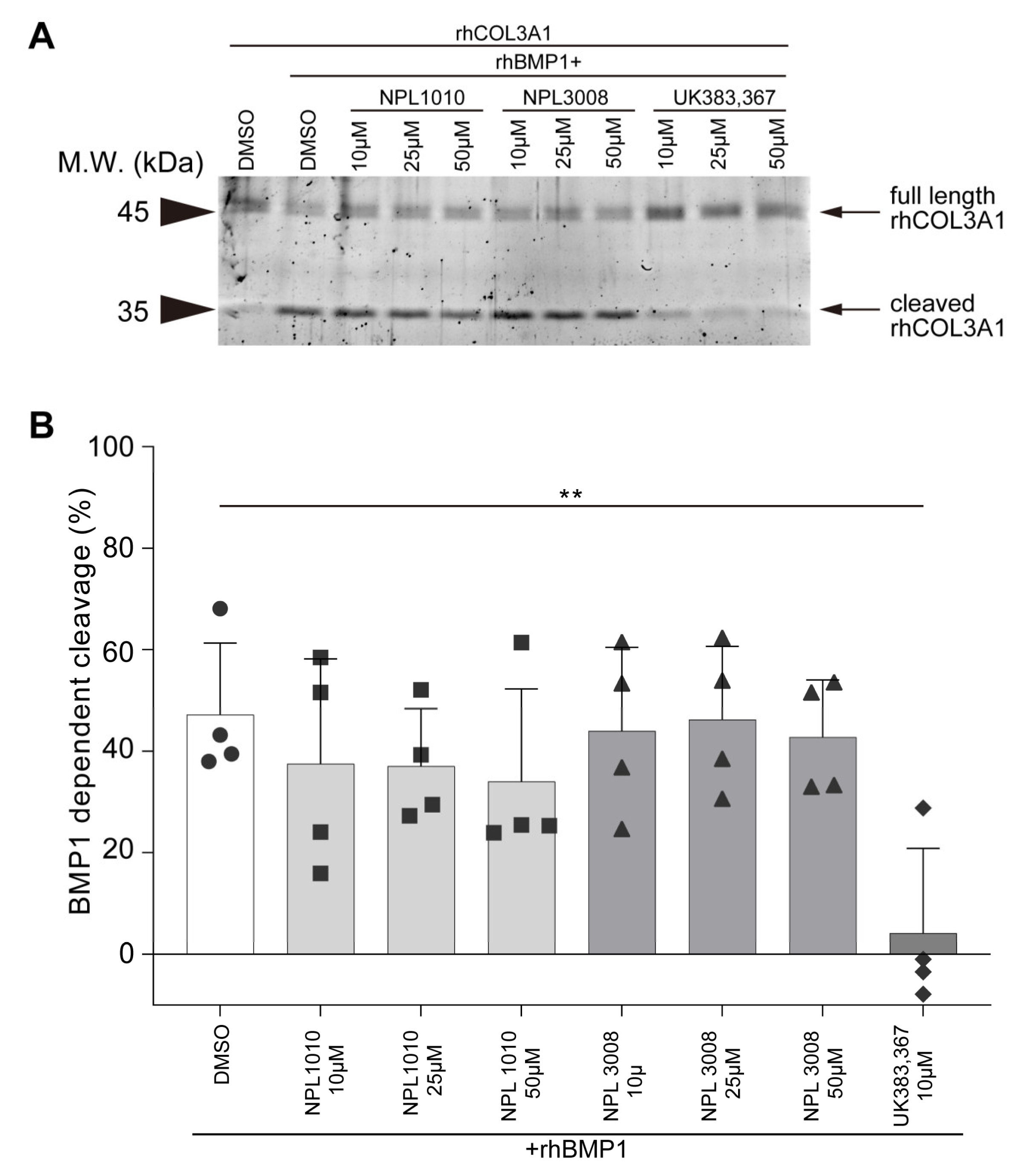
2.6. NPL1010 and NPL3008 Suppress BMP1a-Dependent Chrd Cleavage
2.7. NPL1010 and NPL3008 Potentially Bind to the Cleft in the BMP1 Catalytic Domain
3. Discussion
4. Materials and Methods
4.1. Screening of N-Substituted-2-Amino-Benzoic Acid Analogs
4.2. Preparation of Stock Solutions of Compounds and Protein
4.3. Dual-Luciferase Reporter Assay
4.4. Preparation of Chrd-Containing Conditioned Medium
4.5. Chrd Cleavage Assay
4.6. Western Blotting
4.7. COL3A1 Cleavage Assay
4.8. Fish Maintenance
4.9. Phenotypic Screening
4.10. RNA Probe
4.11. Whole-Mount In Situ Hybridization
4.12. Alizarin Red and Alcian Blue Staining
4.13. mRNA Synthesis and Phenotypic Rescue Experiment
4.14. Molecular Docking
4.15. Interaction Simulation
4.16. Statistical Analysis and Graphing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Robertis, E.M.; Moriyama, Y. The Chordin Morphogenetic Pathway. In Current Topics in Developmental Biology; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 116, pp. 231–245. ISBN 9780128029565. [Google Scholar]
- Kamiya, N.; Kobayashi, T.; Mochida, Y.; Yu, P.B.; Yamauchi, M.; Kronenberg, H.M.; Mishina, Y. Wnt Inhibitors Dkk1 and Sost Are Downstream Targets of BMP Signaling through the Type IA Receptor (BMPRIA) in Osteoblasts. J. Bone Miner. Res. 2010, 25, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cao, X. BMP Signaling and Skeletogenesis. In Proceedings of the Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2006; Volume 1068, pp. 26–40. [Google Scholar]
- Brooker, S.M.; Gobeske, K.T.; Chen, J.; Peng, C.Y.; Kessler, J.A. Hippocampal Bone Morphogenetic Protein Signaling Mediates Behavioral Effects of Antidepressant Treatment. Mol. Psychiatry 2017, 22, 910–919. [Google Scholar] [CrossRef] [Green Version]
- Meyers, E.A.; Gobeske, K.T.; Bond, A.M.; Jarrett, J.C.; Peng, C.Y.; Kessler, J.A. Increased Bone Morphogenetic Protein Signaling Contributes to Age-Related Declines in Neurogenesis and Cognition. Neurobiol. Aging 2016, 38, 164–175. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.N.; Gong, S.N.; Guan, W.; Wang, J.L.; Gao, T.T.; Wang, Y.; Sun, F.; Jiang, B. Hippocampal Overexpression of Chordin Protects against the Chronic Social Defeat Stress-Induced Depressive-like Effects in Mice. Brain Res. Bull. 2020, 158, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Yadav, P.S.; Prashar, P. BMP Signaling in Development and Diseases: A Pharmacological Perspective. Biochem. Pharmacol. 2013, 85, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP Signalling in Skeletal Development, Disease and Repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) Signaling in Development and Human Diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagita, M. BMP Antagonists: Their Roles in Development and Involvement in Pathophysiology. Cytokine Growth Factor Rev. 2005, 16, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, O.; Shimizu, T.; Yabe, T.; Nojima, H.; Bae, Y.K.; Hashimoto, H.; Hibi, M. Sizzled Controls Dorso-Ventral Polarity by Repressing Cleavage of the Chordin Protein. Nat. Cell Biol. 2006, 8, 329–338. [Google Scholar] [CrossRef]
- Vadon-Le Goff, S.; Hulmes, D.J.S.; Moali, C. BMP-1/Tolloid-like Proteinases Synchronize Matrix Assembly with Growth Factor Activation to Promote Morphogenesis and Tissue Remodeling. Matrix Biol. 2015, 44–46, 14–23. [Google Scholar]
- Yu, P.B.; Hong, C.C.; Sachidanandan, C.; Babitt, J.L.; Deng, D.Y.; Hoyng, S.A.; Lin, H.Y.; Bloch, K.D.; Peterson, R.T. Dorsomorphin Inhibits BMP Signals Required for Embryogenesis and Iron Metabolism. Nat. Chem. Biol. 2008, 4, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, J.; Daleo, M.A.; Murphy, C.K.; Yu, P.B.; Ho, J.N.; Hu, J.; Peterson, R.T.; Hatzopoulos, A.K.; Hong, C.C. Dorsomorphin, a Selective Small Molecule Inhibitor of BMP Signaling, Promotes Cardiomyogenesis in Embryonic Stem Cells. PLoS ONE 2008, 3, e2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Chhipa, R.R.; Nakano, I.; Dasgupta, B. The AMPK Inhibitor Compound C Is a Potent AMPK-Independent Antiglioma Agent. Mol. Cancer Ther. 2014, 13, 596–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vucicevic, L.; Misirkic, M.; Janjetovic, K.; Vilimanovich, U.; Sudar, E.; Isenovic, E.; Prica, M.; Harhaji-Trajkovic, L.; Kravic-Stevovic, T.; Bumbasirevic, V.; et al. Compound C Induces Protective Autophagy in Cancer Cells through AMPK Inhibition-Independent Blockade of Akt/MTOR Pathway. Autophagy 2011, 7, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Fish, P.V.; Allan, G.A.; Bailey, S.; Blagg, J.; Butt, R.; Collis, M.G.; Greiling, D.; James, K.; Kendall, J.; McElroy, A.; et al. Potent and Selective Nonpeptidic Inhibitors of Procollagen C-Proteinase. J. Med. Chem. 2007, 50, 3442–3456. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Ajioka, K.; Goda, N.; Shindo, A.; Takagishi, M.; Tenno, T.; Hiroaki, H. Discovery of Potent Disheveled/Dvl Inhibitors Using Virtual Screening Optimized With NMR-Based Docking Performance Index. Front. Pharmacol. 2018, 9, 983. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, Y.; Lee, K.H.; Zon, L.; Hammerschmidt, M.; Schulte-Merker, S. The Molecular Nature of Zebrafish Swirl: BMP2 Function Is Essential during Early Dorsoventral Patterning. Development 1997, 124, 4457–4466. [Google Scholar] [CrossRef]
- Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; Brand, M.; van Eeden, F.J.; Furutani-Seiki, M.; Granato, M.; Haffter, P.; Heisenberg, C.P.; et al. Genes Establishing Dorsoventral Pattern Formation in the Zebrafish Embryo: The Ventral Specifying Genes. Development 1996, 123, 81–93. [Google Scholar] [CrossRef]
- Little, S.C.; Mullins, M.C. Twisted Gastrulation Promotes BMP Signaling in Zebrafish Dorsal-Ventral Axial Patterning. Development 2004, 131, 5825–5835. [Google Scholar] [CrossRef] [Green Version]
- Tucker, J.A.; Mintzer, K.A.; Mullins, M.C. The BMP Signaling Gradient Patterns Dorsoventral Tissues in a Temporally Progressive Manner along the Anteroposterior Axis. Dev. Cell 2008, 14, 108–119. [Google Scholar] [CrossRef] [Green Version]
- Tuazon, F.B.; Mullins, M.C. Temporally Coordinated Signals Progressively Pattern the Anteroposterior and Dorsoventral Body Axes. Semin. Cell Dev. Biol. 2015, 42, 118–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schier, A.F.; Talbot, W.S. Molecular Genetics of Axis Formation in Zebrafish. Annu. Rev. Genet. 2005, 39, 561–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, L.; Jiang, H.; Wu, P.; Marlow, F.L. Negative Feedback Regulation of Wnt Signaling via N-Linked Fucosylation in Zebrafish. Dev. Biol. 2014, 395, 268–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller-Bertoglio, V.E.; Fisher, S.; Sánchez, A.; Mullins, M.C.; Halpern, M.E. Differential Regulation of Chordin Expression Domains in Mutant Zebrafish. Dev. Biol. 1997, 192, 537–550. [Google Scholar] [CrossRef] [Green Version]
- Yabe, T.; Shimizu, T.; Muraoka, O.; Bae, Y.K.; Hirata, T.; Nojima, H.; Kawakami, A.; Hirano, T.; Hibi, M. Ogon/Secreted Frizzled Functions as a Negative Feedback Regulator of Bmp Signaling. Development 2003, 130, 2705–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martyn, U.; Schulte-Merker, S. The Ventralized Ogon Mutant Phenotype Is Caused by a Mutation in the Zebrafish Homologue of Sizzled, a Secreted Frizzled-Related Protein. Dev. Biol. 2003, 260, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Yamanaka, Y.; Nojima, H.; Yabe, T.; Hibi, M.; Hirano, T. A Novel Repressor-Type Homeobox Gene, Ved, Is Involved in Dharma/Bozozok-Mediated Dorsal Organizer Formation in Zebrafish. Mech. Dev. 2002, 118, 125–138. [Google Scholar] [CrossRef]
- Ye, D.; Wang, X.; Wei, C.; He, M.; Wang, H.; Wang, Y.; Zhu, Z.; Sun, Y. Marcksb Plays a Key Role in the Secretory Pathway of Zebrafish Bmp2b. PLoS Genet. 2019, 15, e1008306. [Google Scholar] [CrossRef] [Green Version]
- Ramel, M.-C.; Buckles, G.R.; Baker, K.D.; Lekven, A.C. WNT8 and BMP2B Co-Regulate Non-Axial Mesoderm Patterning during Zebrafish Gastrulation. Dev. Biol. 2005, 287, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Windhausen, T.; Squifflet, S.; Renn, J.; Muller, M. BMP Signaling Regulates Bone Morphogenesis in Zebrafish through Promoting Osteoblast Function as Assessed by Their Nitric Oxide Production. Molecules 2015, 20, 7586–7601. [Google Scholar] [CrossRef] [Green Version]
- Korchynskyi, O.; Ten Dijke, P. Identification and Functional Characterization of Distinct Critically Important Bone Morphogenetic Protein-Specific Response Elements in the Id1 Promoter. J. Biol. Chem. 2002, 277, 4883–4891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morikawa, M.; Koinuma, D.; Tsutsumi, S.; Vasilaki, E.; Kanki, Y.; Heldin, C.H.; Aburatani, H.; Miyazono, K. ChIP-Seq Reveals Cell Type-Specific Binding Patterns of BMP-Specific Smads and a Novel Binding Motif. Nucleic Acids Res. 2011, 39, 8712–8727. [Google Scholar] [CrossRef] [PubMed]
- Kawai, S.; Faucheu, C.; Gallea, S.; Spinella-Jaegle, S.; Atfi, A.; Baron, R.; Roman, S.R. Mouse Smad8 Phosphorylation Downstream of BMP Receptors ALK-2, ALK-3, and ALK-6 Induces Its Association with Smad4 and Transcriptional Activity. Biochem. Biophys. Res. Commun. 2000, 271, 682–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The Harmonizome: A Collection of Processed Datasets Gathered to Serve and Mine Knowledge about Genes and Proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Sasai, Y.; Lu, B.; De Robertis, E.M. Dorsoventral Patterning in Xenopus: Inhibition of Ventral Signals by Direct Binding of Chordin to BMP-4. Cell 1996, 86, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Sasai, Y.; Lu, B.; Steinbeisser, H.; Geissert, D.; Gont, L.K.; De Robertis, E.M. Xenopus Chordin: A Novel Dorsalizing Factor Activated by Organizer-Specific Homeobox Genes. Cell 1994, 79, 779–790. [Google Scholar] [CrossRef]
- Asharani, P.V.; Keupp, K.; Semler, O.; Wang, W.; Li, Y.; Thiele, H.; Yigit, G.; Pohl, E.; Becker, J.; Frommolt, P.; et al. Attenuated BMP1 Function Compromises Osteogenesis, Leading to Bone Fragility in Humans and Zebrafish. Am. J. Hum. Genet. 2012, 90, 661–674. [Google Scholar] [CrossRef] [Green Version]
- Jasuja, R.; Voss, N.; Ge, G.; Hoffman, G.G.; Lyman-Gingerich, J.; Pelegri, F.; Greenspan, D.S. Bmp1 and Mini Fin Are Functionally Redundant in Regulating Formation of the Zebrafish Dorsoventral Axis. Mech. Dev. 2006, 123, 548–558. [Google Scholar] [CrossRef]
- Kallander, L.S.; Washburn, D.; Hilfiker, M.A.; Eidam, H.S.; Lawhorn, B.G.; Prendergast, J.; Fox, R.; Dowdell, S.; Manns, S.; Hoang, T.; et al. Reverse Hydroxamate Inhibitors of Bone Morphogenetic Protein 1. ACS Med. Chem. Lett. 2018, 9, 736–740. [Google Scholar] [CrossRef]
- Evans, R.; O’Neill, M.; Pritzel, A.; Antropova, N.; Senior, A.; Green, T.; Žídek, A.; Bates, R.; Blackwell, S.; Yim, J.; et al. Protein Complex Prediction with AlphaFold-Multimer. bioRxiv 2021. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. Hynes and Kenneth Yamada Additional Perspectives on Extracellular Matrix Biology Available at Cite This Article As. Cold Spring Harb. Perspect. Biol. 2011, 3, 4978–4979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petropoulou, V.; Garrigue-Antar, L.; Kadleri, K.E. Identification of the Minimal Domain Structure of Bone Morphogenetic Protein-1 (BMP-1) for Chordinase Activity: Chordinase Activity Is Not Enhanced by Procollagen C-Proteinase Enhancer-1 (PCPE-1). J. Biol. Chem. 2005, 280, 22616–22623. [Google Scholar] [CrossRef] [Green Version]
- Pulido, D.; Sharma, U.; Vadon-Le Goff, S.; Hussain, S.A.; Cordes, S.; Mariano, N.; Bettler, E.; Moali, C.; Aghajari, N.; Hohenester, E.; et al. Structural Basis for the Acceleration of Procollagen Processing by Procollagen C-Proteinase Enhancer-1. Structure 2018, 26, 1384–1392.e3. [Google Scholar] [CrossRef] [Green Version]
- Scott, I.C.; Blitz, I.L.; Pappano, W.N.; Imamura, Y.; Clark, T.G.; Steiglitz, B.M.; Thomas, C.L.; Maas, S.A.; Takahara, K.; Cho, K.W.Y.; et al. Mammalian BMP-1/Tolloid-Related Metalloproteinases, Including Novel Family Member Mammalian Tolloid-Like 2, Have Differential Enzymatic Activities and Distributions of Expression Relevant to Patterning and Skeletogenesis. Dev. Biol. 1999, 213, 283–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Fisher, S. Twisted Gastrulation Enhances BMP Signaling through Chordin Dependent and Independent Mechanisms. Development 2005, 132, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Oxtoby, E.; Jowett, T. Cloning of the Zebrafish Krox-20 Gene (Krx-20) and Its Expression during Hindbrain Development. Nucleic Acids Res. 1993, 21, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, E.S.; Allende, M.L.; Kelly, C.S.; Abdelhamid, A.; Murakami, T.; Andermann, P.; Doerre, O.G.; Grunwald, D.J.; Riggleman, B. Developmental Regulation of Zebrafish MyoD in Wild-Type, No Tail and Spadetail Embryos. Development 1996, 122, 271–280. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Blader, P.; Rastegar, S.; Fischer, N.; Strähle, U. Cleavage of the BMP-4 Antagonist Chordin by Zebrafish Tolloid. Science 1997, 278, 1937–1940. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, T.; Sugihara, Y.; Fukunishi, Y.; Nakamura, H. LigandBox: A Database for 3D Structures of Chemical Compounds. Biophysics 2013, 9, 113–121. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoguchi, T.; Mikami, S.; Yatou, M.; Kondo, Y.; Omaru, S.; Kuwabara, S.; Okura, W.; Noda, S.; Tenno, T.; Hiroaki, H.; et al. Small-Molecule-Mediated Suppression of BMP Signaling by Selective Inhibition of BMP1-Dependent Chordin Cleavage. Int. J. Mol. Sci. 2023, 24, 4313. https://doi.org/10.3390/ijms24054313
Mizoguchi T, Mikami S, Yatou M, Kondo Y, Omaru S, Kuwabara S, Okura W, Noda S, Tenno T, Hiroaki H, et al. Small-Molecule-Mediated Suppression of BMP Signaling by Selective Inhibition of BMP1-Dependent Chordin Cleavage. International Journal of Molecular Sciences. 2023; 24(5):4313. https://doi.org/10.3390/ijms24054313
Chicago/Turabian StyleMizoguchi, Takamasa, Shohei Mikami, Mari Yatou, Yui Kondo, Shuhei Omaru, Shuhei Kuwabara, Wataru Okura, Syouta Noda, Takeshi Tenno, Hidekazu Hiroaki, and et al. 2023. "Small-Molecule-Mediated Suppression of BMP Signaling by Selective Inhibition of BMP1-Dependent Chordin Cleavage" International Journal of Molecular Sciences 24, no. 5: 4313. https://doi.org/10.3390/ijms24054313




