Biochemical Characteristics of iPSC-Derived Dopaminergic Neurons from N370S GBA Variant Carriers with and without Parkinson’s Disease
Abstract
:1. Introduction
2. Results
2.1. Directed Differentiation of iPSCs to DA Neurons and Characteristics of the Obtained Neurons
2.2. Analysis of the GBA Gene Expression in DA Neurons before and after Treatment with Ambroxol
2.3. Lysosomal Enzymes Activity in DA Neurons
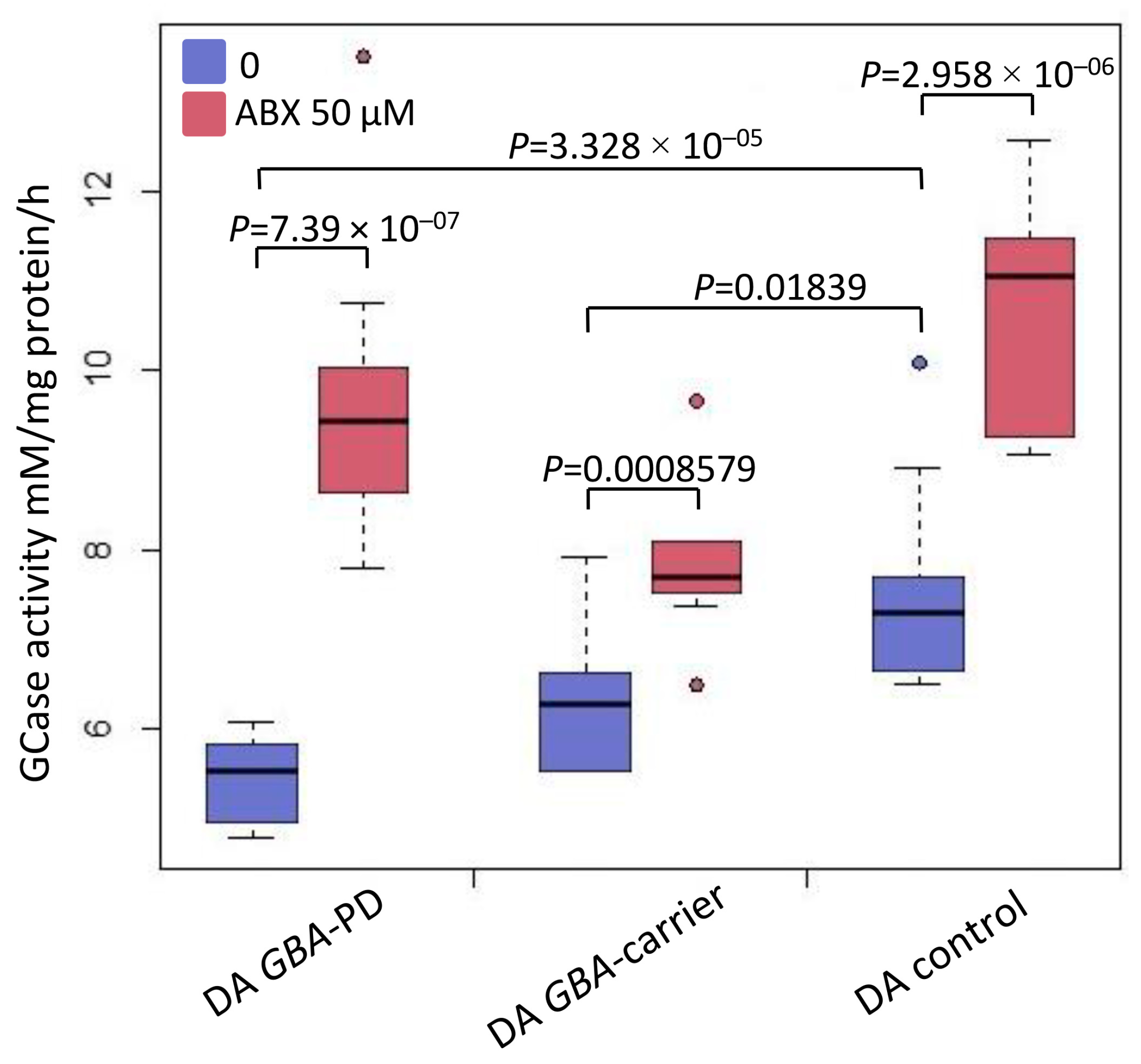
2.4. Analysis of GCase Activity in DA Neurons before and after Treatment with Ambroxol
2.5. Western Blot Analysis before and after Treatment with Ambroxol
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Whole-Exome Sequencing and Genotyping
4.3. DNA Isolation
4.4. Cultivation of iPSCs
4.5. Differentiation of Patient-Specific iPSCs into DA Neurons
4.6. Immunofluorescent Analysis
4.7. Flow Cytometry Analysis
4.8. Quantitative RT-PCR
4.9. Ambroxol Treatment of DA Neurons
4.10. Western Blot Analysis
4.11. Quantification of Lysosomal Enzymes Activity in DA-Neurons
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Schapira, A.H.V. GBA Variants and Parkinson Disease: Mechanisms and Treatments. Cells 2022, 11, 1261. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.K.; Usenko, T.S.; Tesson, C.; Senkevich, K.A.; Nikolaev, M.A.; Miliukhina, I.V.; Kopytova, A.E.; Timofeeva, A.A.; Yakimovsky, A.F.; Lesage, S.; et al. Mutation analysis of Parkinson’s disease genes in a Russian data set. Neurobiol. Aging 2018, 71, 267.e7–267.e10. [Google Scholar] [CrossRef] [PubMed]
- Sidransky, E.; Nalls, M.A.; Aasly, J.O.; Aharon-Peretz, J.; Annesi, G.; Barbosa, E.R.; Bar-Shira, A.; Berg, D.; Bras, J.; Brice, A.; et al. Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease. N. Engl. J. Med. 2009, 361, 1651–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, G.; Arias, S.; Cárdenas, L.; Zoghbi, D.; Paradisi, I. GBA mutations in Gaucher type I Venezuelan patients: Ethnic origins and frequencies. J. Genet. 2017, 96, 583–589. [Google Scholar] [CrossRef]
- Yilmazer, B.; Yagci, Z.B.; Bakar, E.; Ozden, B.; Ulgen, K.; Ozkirimli, E. Investigation of novel pharmacological chaperones for Gaucher Disease. J. Mol. Graph. Model. 2017, 76, 364–378. [Google Scholar] [CrossRef]
- Yang, S.Y.; Taanman, J.-W.; Gegg, M.; Schapira, A.H.V. Ambroxol reverses tau and α-synuclein accumulation in a cholinergic N370S GBA1 mutation model. Hum. Mol. Genet. 2022, 31, 2396–2405. [Google Scholar] [CrossRef]
- Yang, S.-y.; Gegg, M.; Chau, D.; Schapira, A. Glucocerebrosidase activity, cathepsin D and monomeric α-synuclein interactions in a stem cell derived neuronal model of a PD associated GBA1 mutation. Neurobiol. Dis. 2020, 134, 104620. [Google Scholar] [CrossRef]
- Kopytova, A.E.; Rychkov, G.N.; Nikolaev, M.A.; Baydakova, G.V.; Cheblokov, A.A.; Senkevich, K.A.; Bogdanova, D.A.; Bolshakova, O.I.; Miliukhina, I.V.; Bezrukikh, V.A.; et al. Ambroxol increases glucocerebrosidase (GCase) activity and restores GCase translocation in primary patient-derived macrophages in Gaucher disease and Parkinsonism. Parkinsonism Relat. Disord. 2021, 84, 112–121. [Google Scholar] [CrossRef]
- McNeill, A.; Magalhaes, J.; Shen, C.; Chau, K.Y.; Hughes, D.; Mehta, A.; Foltynie, T.; Cooper, J.M.; Abramov, A.Y.; Gegg, M.; et al. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain 2014, 137, 1481–1495. [Google Scholar] [CrossRef] [Green Version]
- Silveira, C.R.A.; MacKinley, J.; Coleman, K.; Li, Z.; Finger, E.; Bartha, R.; Morrow, S.A.; Wells, J.; Borrie, M.; Tirona, R.G.; et al. Ambroxol as a novel disease-modifying treatment for Parkinson’s disease dementia: Protocol for a single-centre, randomized, double-blind, placebo-controlled trial. BMC Neurol. 2019, 19, 20. [Google Scholar] [CrossRef] [Green Version]
- Mullin, S.; Smith, L.; Lee, K.; D’Souza, G.; Woodgate, P.; Elflein, J.; Hällqvist, J.; Toffoli, M.; Streeter, A.; Hosking, J.; et al. Ambroxol for the Treatment of Patients With Parkinson Disease with and without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial. JAMA Neurol. 2020, 77, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Alcalay, R.N.; Levy, O.A.; Waters, C.C.; Fahn, S.; Ford, B.; Kuo, S.H.; Mazzoni, P.; Pauciulo, M.W.; Nichols, W.C.; Gan-Or, Z.; et al. Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain 2015, 138, 2648–2658. [Google Scholar] [CrossRef] [Green Version]
- Bae, E.J.; Yang, N.Y.; Lee, C.; Lee, H.J.; Kim, S.; Sardi, S.P.; Lee, S.J. Loss of glucocerebrosidase 1 activity causes lysosomal dysfunction and α-synuclein aggregation. Exp. Mol. Med. 2015, 47, e153. [Google Scholar] [CrossRef] [Green Version]
- Pchelina, S.; Emelyanov, A.; Baydakova, G.; Andoskin, P.; Senkevich, K.; Nikolaev, M.; Miliukhina, I.; Yakimovskii, A.; Timofeeva, A.; Fedotova, E.; et al. Oligomeric α-synuclein and glucocerebrosidase activity levels in GBA-associated Parkinson’s disease. Neurosci. Lett. 2017, 636, 70–76. [Google Scholar] [CrossRef]
- Kopytova, A.E.; Usenko, T.S.; Baydakova, G.V.; Nikolaev, M.A.; Senkevich, K.A.; Izyumchenko, A.D.; Tyurin, A.A.; Miliukhina, I.V.; Emelyanov, A.K.; Zakharova, E.Y.; et al. Could Blood Hexosylsphingosine Be a Marker for Parkinson’s Disease Linked with GBA1 Mutations? Mov. Disord. 2022, 37, 1779–1781. [Google Scholar] [CrossRef]
- Aflaki, E.; Borger, D.K.; Moaven, N.; Stubblefield, B.K.; Rogers, S.A.; Patnaik, S.; Schoenen, F.J.; Westbroek, W.; Zheng, W.; Sullivan, P.; et al. A new glucocerebrosidase chaperone reduces α-synuclein and glycolipid levels in iPSC-derived dopaminergic neurons from patients with Gaucher disease and parkinsonism. J. Neurosci. 2016, 36, 7441–7452. [Google Scholar] [CrossRef]
- Fernandes, H.J.R.; Hartfield, E.M.; Christian, H.C.; Emmanoulidou, E.; Zheng, Y.; Booth, H.; Bogetofte, H.; Lang, C.; Ryan, B.J.; Sardi, S.P.; et al. ER Stress and Autophagic Perturbations Lead to Elevated Extracellular α-Synuclein in GBA-N370S Parkinson’s iPSC-Derived Dopamine Neurons. Stem Cell Rep. 2016, 6, 342–356. [Google Scholar] [CrossRef] [Green Version]
- Schöndorf, D.C.; Aureli, M.; McAllister, F.E.; Hindley, C.J.; Mayer, F.; Schmid, B.; Sardi, S.P.; Valsecchi, M.; Hoffmann, S.; Schwarz, L.K.; et al. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 2014, 5, 4028. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.Y.; Beavan, M.; Chau, K.Y.; Taanman, J.W.; Schapira, A.H.V. A Human Neural Crest Stem Cell-Derived Dopaminergic Neuronal Model Recapitulates Biochemical Abnormalities in GBA1 Mutation Carriers. Stem Cell Rep. 2017, 8, 728–742. [Google Scholar] [CrossRef]
- Woodard, C.M.; Campos, B.A.; Kuo, S.-H.; Nirenberg, M.J.; Nestor, M.W.; Zimmer, M.; Mosharov, E.V.; Sulzer, D.; Zhou, H.; Paull, D.; et al. iPSC-Derived Dopamine Neurons Reveal Differences between Monozygotic Twins Discordant for Parkinson’s Disease. Cell Rep. 2014, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigor’eva, E.V.; Drozdova, E.S.; Sorogina, D.A.; Malakhova, A.A.; Pavlova, S.V.; Vyatkin, Y.V.; Khabarova, E.A.; Rzaev, J.A.; Medvedev, S.P.; Zakian, S.M. Generation of induced pluripotent stem cell line, ICGi034-A, by reprogramming peripheral blood mononuclear cells from a patient with Parkinson’s disease associated with GBA mutation. Stem Cell Res. 2022, 59, 102651. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, A.A.; Grigor’eva, E.V.; Pavlova, S.V.; Malankhanova, T.B.; Valetdinova, K.R.; Vyatkin, Y.V.; Khabarova, E.A.; Rzaev, J.A.; Zakian, S.M.; Medvedev, S.P. Generation of induced pluripotent stem cell lines ICGi021-A and ICGi022-A from peripheral blood mononuclear cells of two healthy individuals from Siberian population. Stem Cell Res. 2020, 48, 101952. [Google Scholar] [CrossRef] [PubMed]
- Jonathan Niclis, C.; Gantner, C.W.; Alsanie, W.F.; McDougall, S.J.; Bye, C.R.; Elefanty, A.G.; Stanley, E.G.; Haynes, J.M.; Pouton, C.W.; Thompson, L.H.; et al. Efficiently Specified Ventral Midbrain Dopamine Neurons from Human Pluripotent Stem Cells Under Xeno-Free Conditions Restore Motor Deficits in Parkinsonian Rodents. Stem Cells Transl. Med. 2017, 6, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [Green Version]
- De Rus Jacquet, A. Preparation and Co-Culture of iPSC-Derived Dopaminergic Neurons and Astrocytes. Curr. Protoc. Cell Biol. 2019, 85, e98. [Google Scholar] [CrossRef] [Green Version]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Roussa, E.; Von Bohlen Und Halbach, O.; Krieglstein, K. TGF-β in dopamine neuron development, maintenance and neuroprotection. Adv. Exp. Med. Biol. 2009, 651, 81–90. [Google Scholar] [CrossRef]
- El-Akabawy, G.; Medina, L.M.; Jeffries, A.; Price, J.; Modo, M. Purmorphamine increases DARPP-32 differentiation in human striatal neural stem cells through the Hedgehog pathway. Stem Cells Dev. 2011, 20, 1873–1887. [Google Scholar] [CrossRef]
- Ma, L.; Hu, B.; Liu, Y.; Vermilyea, S.C.; Liu, H.; Gao, L.; Sun, Y.; Zhang, X.; Zhang, S.C. Human embryonic stem cell-derived GABA neurons correct locomotion deficits in quinolinic acid-lesioned mice. Cell Stem Cell 2012, 10, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Samata, B.; Doi, D.; Nishimura, K.; Kikuchi, T.; Watanabe, A.; Sakamoto, Y.; Kakuta, J.; Ono, Y.; Takahashi, J. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nat. Commun. 2016, 7, 13097. [Google Scholar] [CrossRef] [Green Version]
- Atashrazm, F.; Hammond, D.; Perera, G.; Dobson-Stone, C.; Mueller, N.; Pickford, R.; Kim, W.S.; Kwok, J.B.; Lewis, S.J.G.; Halliday, G.M.; et al. Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci. Rep. 2018, 8, 15446. [Google Scholar] [CrossRef] [Green Version]
- Gegg, M.E.; Burke, D.; Heales, S.J.R.; Cooper, J.M.; Hardy, J.; Wood, N.W.; Schapira, A.H.V. Glucocerebrosidase Deficiency in Substantia Nigra of Parkinson Disease Brains. Ann. Neurol. 2012, 72, 455. [Google Scholar] [CrossRef] [Green Version]
- Paciotti, S.; Gatticchi, L.; Beccari, T.; Parnetti, L. Lysosomal enzyme activities as possible CSF biomarkers of synucleinopathies. Clin. Chim. Acta 2019, 495, 13–24. [Google Scholar] [CrossRef]
- Ortega, R.A.; Torres, P.A.; Swan, M.; Nichols, W.; Boschung, S.; Raymond, D.; Barrett, M.J.; Johannes, B.A.; Severt, L.; Shanker, V.; et al. Glucocerebrosidase enzyme activity in GBA mutation Parkinson’s disease. J. Clin. Neurosci. 2016, 28, 185–186. [Google Scholar] [CrossRef] [Green Version]
- Omer, N.; Giladi, N.; Gurevich, T.; Bar-Shira, A.; Gana-Weisz, M.; Glinka, T.; Goldstein, O.; Kestenbaum, M.; Cedarbaum, J.M.; Mabrouk, O.S.; et al. Glucocerebrosidase Activity is not Associated with Parkinson’s Disease Risk or Severity. Mov. Disord. 2022, 37, 190–195. [Google Scholar] [CrossRef]
- Polo, G.; Burlina, A.P.; Ranieri, E.; Colucci, F.; Rubert, L.; Pascarella, A.; Duro, G.; Tummolo, A.; Padoan, A.; Plebani, M.; et al. Plasma and dried blood spot lysosphingolipids for the diagnosis of different sphingolipidoses: A comparative study. Clin. Chem. Lab. Med. 2019, 57, 1863–1874. [Google Scholar] [CrossRef]
- Surface, M.; Balwani, M.; Waters, C.; Haimovich, A.; Gan-Or, Z.; Marder, K.S.; Hsieh, T.; Song, L.; Padmanabhan, S.; Hsieh, F.; et al. Plasma Glucosylsphingosine in GBA1 Mutation Carriers with and without Parkinson’s Disease. Mov. Disord. 2022, 37, 416–421. [Google Scholar] [CrossRef]
- Robak, L.A.; Jansen, I.E.; van Rooij, J.; Uitterlinden, A.G.; Kraaij, R.; Jankovic, J.; Heutink, P.; Shulman, J.M.; Nalls, M.A.; Plagnol, V.; et al. Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 2017, 140, 3191–3203. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Huang, J.; Feng, X.; Zhang, A.; Li, J.; Pang, S.; Gu, K.; Dong, H.; Zhang, J.; Gao, H.; et al. Decreased expression of lysosomal alpha-Galactosiase a gene in sporadic parkinson’s disease. Neurochem. Res. 2011, 36, 1939–1944. [Google Scholar] [CrossRef]
- Alcalay, R.N.; Wolf, P.; Levy, O.A.; Kang, U.J.; Waters, C.; Fahn, S.; Ford, B.; Kuo, S.H.; Vanegas, N.; Shah, H.; et al. Alpha galactosidase A activity in Parkinson’s disease. Neurobiol. Dis. 2018, 112, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yan, B.; Wang, X.; Feng, X.; Zhang, A.; Xu, X.; Dong, H. Decreased activities of lysosomal acid alpha-D-galactosidase A in the leukocytes of sporadic Parkinson’s disease. J. Neurol. Sci. 2008, 271, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Usenko, T.S.; Senkevich, K.A.; Bezrukova, A.I.; Baydakova, G.V.; Basharova, K.S.; Zhuravlev, A.S.; Gracheva, E.V.; Kudrevatykh, A.V.; Miliukhina, I.V.; Krasakov, I.V.; et al. Impaired Sphingolipid Hydrolase Activities in Dementia with Lewy Bodies and Multiple System Atrophy. Mol. Neurobiol. 2022, 59, 2277–2287. [Google Scholar] [CrossRef] [PubMed]
- Alcalay, R.N.; Mallett, V.; Vanderperre, B.; Tavassoly, O.; Dauvilliers, Y.; Wu, R.Y.J.; Ruskey, J.A.; Leblond, C.S.; Ambalavanan, A.; Laurent, S.B.; et al. SMPD1 mutations, activity, and α-synuclein accumulation in Parkinson’s disease. Mov. Disord. 2019, 34, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Génisson, Y.; Ballereau, S.; Dehoux, C. Second-Generation Pharmacological Chaperones: Beyond Inhibitors. Molecules 2020, 25, 3145. [Google Scholar] [CrossRef]
- Maegawa, G.H.B.; Tropak, M.B.; Buttner, J.D.; Rigat, B.A.; Fuller, M.; Pandit, D.; Tang, L.; Kornhaber, G.J.; Hamuro, Y.; Clarke, J.T.R.; et al. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J. Biol. Chem. 2009, 284, 23502–23516. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martinez, A.; Beavan, M.; Gegg, M.E.; Chau, K.Y.; Whitworth, A.J.; Schapira, A.H.V. Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci. Rep. 2016, 6, 31380. [Google Scholar] [CrossRef] [Green Version]
- Migdalska-Richards, A.; Daly, L.; Bezard, E.; Schapira, A.H.V. Ambroxol effects in glucocerebrosidase and α-synuclein transgenic mice. Ann. Neurol. 2016, 80, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Migdalska-Richards, A.; Wegrzynowicz, M.; Rusconi, R.; Deangeli, G.; Di Monte, D.A.; Spillantini, M.G.; Schapira, A.H.V. The L444P Gba1 mutation enhances alpha-synuclein induced loss of nigral dopaminergic neurons in mice. Brain 2017, 140, 2706–2721. [Google Scholar] [CrossRef] [Green Version]
- Grigor’eva, E.V.; Malankhanova, T.B.; Surumbayeva, A.; Pavlova, S.V.; Minina, J.M.; Kizilova, E.A.; Suldina, L.A.; Morozova, K.N.; Kiseleva, E.; Sorokoumov, E.D.; et al. Generation of GABAergic striatal neurons by a novel iPSC differentiation protocol enabling scalability and cryopreservation of progenitor cells. Cytotechnology 2020, 72, 649–663. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2008, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Straniero, L.; Rimoldi, V.; Samarani, M.; Goldwurm, S.; Di Fonzo, A.; Krüger, R.; Deleidi, M.; Aureli, M.; Soldà, G.; Duga, S.; et al. The GBAP1 pseudogene acts as a ceRNA for the glucocerebrosidase gene GBA by sponging miR-22-3p. Sci. Rep. 2017, 7, 12702. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.K.; Elbin, C.S.; Chuang, W.L.; Cooper, S.K.; Marashio, C.A.; Beauregard, C.; Keutzer, J.M. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin. Chem. 2008, 54, 1725–1728. [Google Scholar] [CrossRef] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
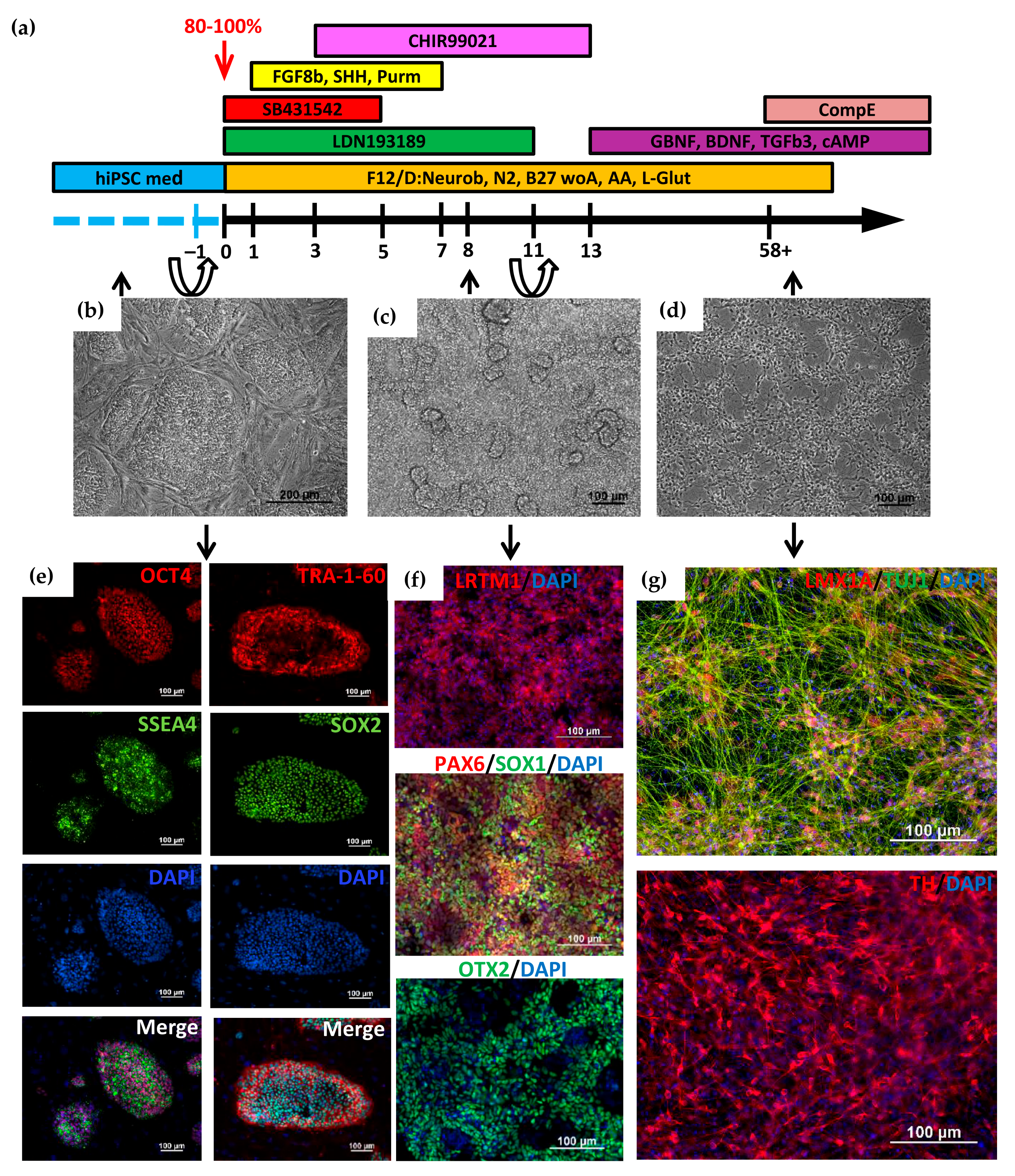


| Lysosomal Enzyme | GBA-PD (N370S/WT) | GBA-Carrier (N370S/WT) | Controls |
|---|---|---|---|
| GCase, nM/mg of protein/h | 5.8 (4.8–6.9) * p < 0.0001 # p = 0.025 | 6.6 (5.5–7.9) * p = 0.018 | 7.6 (6.5–10.1) |
| GALC, nM/mg of protein/h | 8.5 (7.3–10.6) # p = 0.045 | 10.6 (6.4–16.3) | 8.4 (6.6–13.1) |
| GAA, nM/mg of protein/h | 17.2 (12.4–20.3) | 17.6 (14.8–19.3) | 16.5 (8.8–18.6) |
| GLA, nM/mg of protein/h | 12.2 (8.9–14.6) * p = 0.008 # p = 0.002 | 9.6 (7.6–10.5) | 9.8 (8.8–12.1) |
| ASM, nM/mg of protein/h | 9.5 (6.9–11.1) | 9.9 (8.8–11.9) | 8.9 (7.8–11.8) |
| IDUA, nM/mg of protein/h | 20.9 (18.2–23.1) * p < 0.0001 # p < 0.0001 | 12.1 (8.4–16.9) | 10.9 (6.9–20.9) |
| Substance | Company | Concentration | Days |
|---|---|---|---|
| LDN193189 | Sigma-Aldrich, Darmstadt, Germany | 100 nM | 0–11 |
| SB431542 | Abcam, Cambridge, UK | 10 µM | 0–5 |
| Purmorphamine | Tocris, Ellisville, MO, USA | 2 µM | 1–7 |
| SHH C25II | PeproTech, Cranbury, NJ, USA | 100 ng/mL | 1–7 |
| FGF8b | PeproTech, Cranbury, NJ, USA | 100 ng/mL | 1–7 |
| CHIR99021 | Sigma-Aldrich, Darmstadt, Germany | 3 µM | 3–13 |
| BDNF | PeproTech, Cranbury, NJ, USA | 20 ng/mL | 13–to … |
| GDNF | PeproTech, Cranbury, NJ, USA | 20 ng/mL | 13–to … |
| TGFb3 | PeproTech, Cranbury, NJ, USA | 1 ng/mL | 13–to … |
| dbcAMP | PeproTech, Cranbury, NJ, USA | 0.5 mM | 13–to … |
| Compound E | Millipore, Burlington, VT, USA | 0.1 µM | terminal stage |
| Antibodies | Company | Cat. Ref. | Raised/Type | Dilution |
|---|---|---|---|---|
| Primary antibodies | ||||
| Anti-OCT4 | Abcam, Cambridge, UK | ab18976 | IgG rabbit polyclonal | 1:200 |
| Anti-SOX2 | Cell Signaling, Danvers, MA, USA | 3579 | IgG rabbit polyclonal | 1:500 |
| Anti-SSEA4 | Abcam, Cambridge, UK | ab16287 | IgG3 mouse monoclonal | 1:200 |
| Anti-TRA-1-60 | Abcam, Cambridge, UK | ab16288 | IgM mouse monoclonal | 1:200 |
| Anti-LRTM1 | EpiGentek, Farmingdale, NY, USA | A67852 | IgG rabbit polyclonal | 1:100 |
| Anti-PAX6 | Santa Cruz Biotechnology, Dallas, TX, USA | sc-81649 | IgM mouse monoclonal | 1:50 |
| Anti-SOX1 | R&D systems, Minneapolis, MN, USA | AF3369 | IgG goat polyclonal | 1:200 |
| Anti-OTX2 | R&D systems, Minneapolis, MN, USA | AF1979 | IgG goat polyclonal | 1:400 |
| Anti-TH | Millipore, Burlington, VT, USA | AB152 | IgG rabbit polyclonal | 1:400 |
| Anti-LMX1A | Abcam, Cambridge, UK | ab139726 | IgG rabbit polyclonal | 1:100 |
| Anti-TUJ1 | Covance, Princeton, NJ, USA | MMS-435P | IgG2a mouse monoclonal | 1:1000 |
| Secondary antibodies | ||||
| Alexa Fluor 488 goat anti-rabbit IgG (H+L) | Thermo Fisher Scientific, Waltham, MA, USA | A11008 | Made in goat | 1:400 |
| Alexa Fluor 568 goat anti-rabbit IgG (H+L) | Thermo Fisher Scientific, Waltham, MA, USA | A11011 | Made in goat | 1:400 |
| Alexa Fluor 488 goat anti-mouse IgG (H+L) | Thermo Fisher Scientific, Waltham, MA, USA | A11029 | Made in goat | 1:400 |
| Alexa Fluor 568 goat anti-mouse IgG (H+L) | Thermo Fisher Scientific, Waltham, MA, USA | A11031 | Made in goat | 1:400 |
| Alexa Fluor 488 goat anti-mouse IgG2a | Thermo Fisher Scientific, Waltham, MA, USA | A21131 | Made in goat | 1:400 |
| Alexa Fluor 488 donkey anti-goat IgG (H+L) | Thermo Fisher Scientific, Waltham, MA, USA | A11055 | Made in donkey | 1:400 |
| Alexa Fluor 594 rabbit anti-mouse IgG (H+L) | Thermo Fisher Scientific, Waltham, MA, USA | A11062 | Made in rabbit | 1:400 |
| Target | Forward/Reverse Primer (5′-3′) | |
|---|---|---|
| House-keeping gene (RT-qPCR) | beta-2-microglobulin | TAGCTGTGCTCGCGCTACT/ TCTCTGCTGGATGACGTGAG |
| TFRC | AGCAGTTGGCTGTTGTACCTCTC/ GTCGCTGGTCAGTTCGTGATT | |
| ACTB | GTGCGTGACATTAAGGAGAAG/ GAAGGAAGGCTGGAAGAGTG | |
| Gene expression analysis (RT-qPCR) [52] | GBA | TCCAGGTCGTTCTTCTGACT/ ATTGGGTGCGTAACTTTGTC |
| DA-specific gene expression (RT-qPCR) | TH | AAAGTGTCAGAGCTGGACAAG/ GAAGGCGATCTCAGCAATCA |
| LMX1A | CTTGCATTCTTGCTCTCTTTGG/ CAGGAGTCTGGGCTTTACATT | |
| NURR1 | CAGAGCTACAGTTACCACTCTTC/ TGGTGAGGTCCATGCTAAAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigor’eva, E.V.; Kopytova, A.E.; Yarkova, E.S.; Pavlova, S.V.; Sorogina, D.A.; Malakhova, A.A.; Malankhanova, T.B.; Baydakova, G.V.; Zakharova, E.Y.; Medvedev, S.P.; et al. Biochemical Characteristics of iPSC-Derived Dopaminergic Neurons from N370S GBA Variant Carriers with and without Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 4437. https://doi.org/10.3390/ijms24054437
Grigor’eva EV, Kopytova AE, Yarkova ES, Pavlova SV, Sorogina DA, Malakhova AA, Malankhanova TB, Baydakova GV, Zakharova EY, Medvedev SP, et al. Biochemical Characteristics of iPSC-Derived Dopaminergic Neurons from N370S GBA Variant Carriers with and without Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(5):4437. https://doi.org/10.3390/ijms24054437
Chicago/Turabian StyleGrigor’eva, Elena V., Alena E. Kopytova, Elena S. Yarkova, Sophia V. Pavlova, Diana A. Sorogina, Anastasia A. Malakhova, Tuyana B. Malankhanova, Galina V. Baydakova, Ekaterina Y. Zakharova, Sergey P. Medvedev, and et al. 2023. "Biochemical Characteristics of iPSC-Derived Dopaminergic Neurons from N370S GBA Variant Carriers with and without Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 5: 4437. https://doi.org/10.3390/ijms24054437
APA StyleGrigor’eva, E. V., Kopytova, A. E., Yarkova, E. S., Pavlova, S. V., Sorogina, D. A., Malakhova, A. A., Malankhanova, T. B., Baydakova, G. V., Zakharova, E. Y., Medvedev, S. P., Pchelina, S. N., & Zakian, S. M. (2023). Biochemical Characteristics of iPSC-Derived Dopaminergic Neurons from N370S GBA Variant Carriers with and without Parkinson’s Disease. International Journal of Molecular Sciences, 24(5), 4437. https://doi.org/10.3390/ijms24054437






