Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols
Abstract
1. Introduction
2. Results and Discussion
2.1. GFY and Density
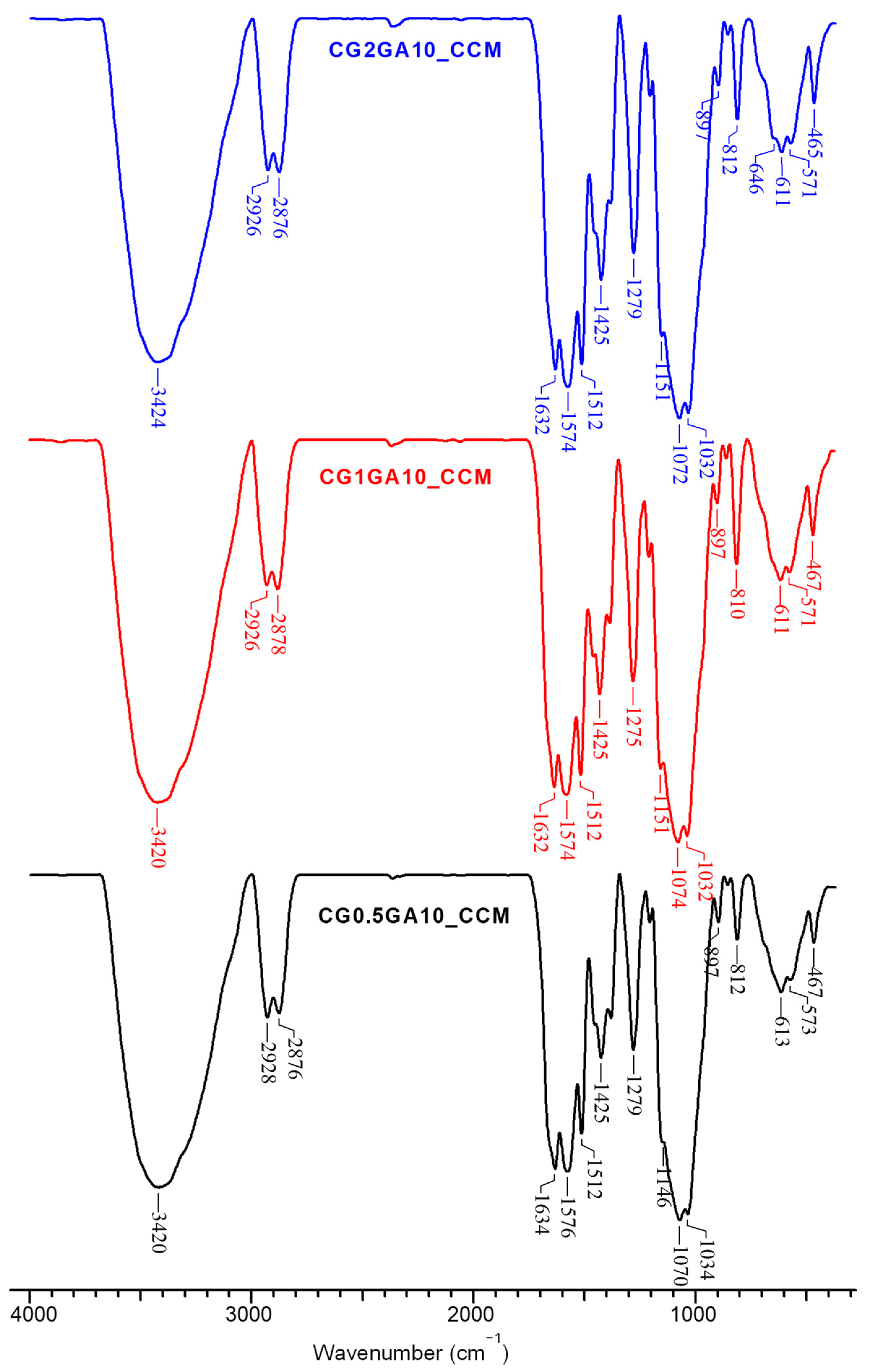
2.2. Structural Characterization
2.3. Morphology and Elemental Analysis
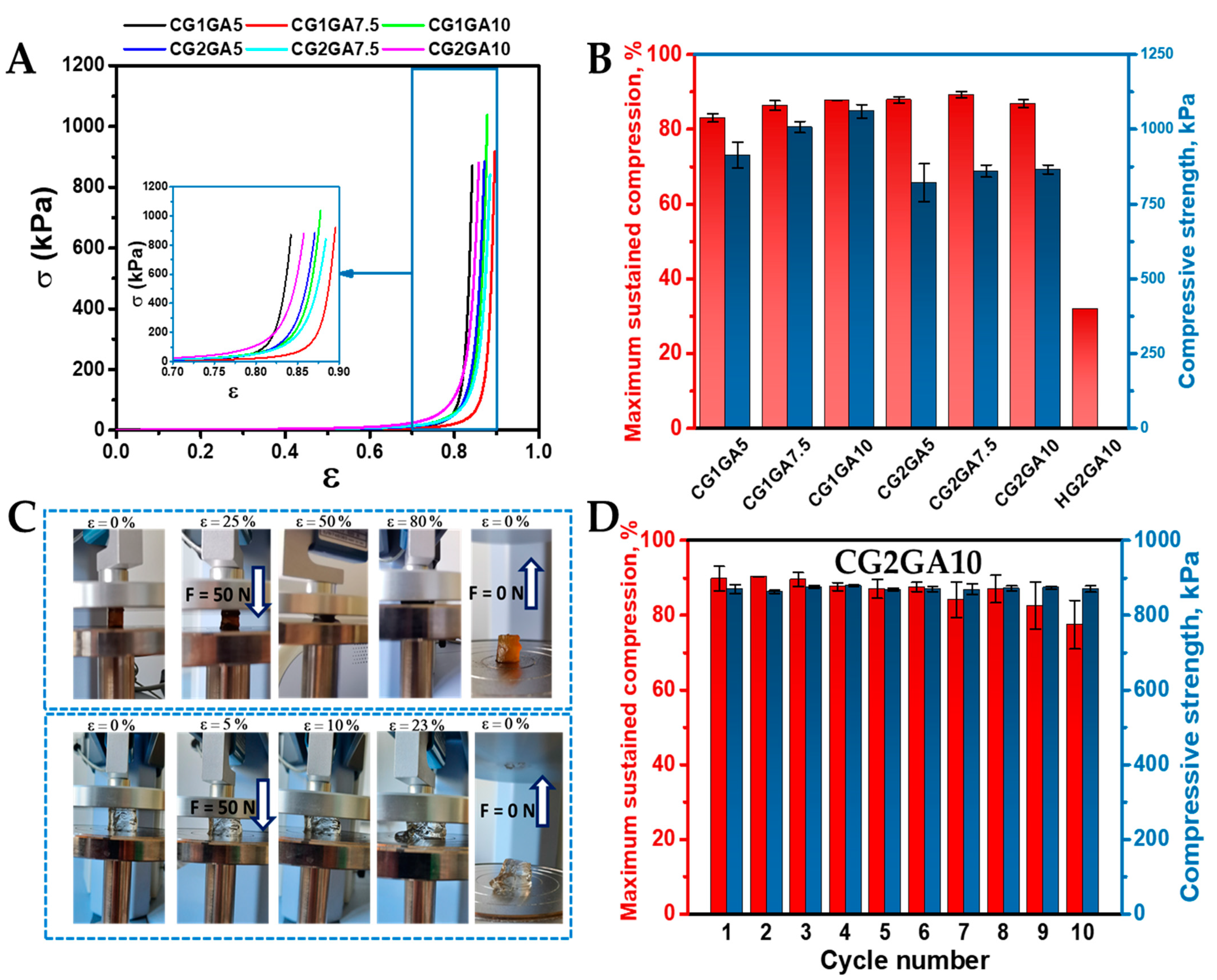
2.4. Mechanical Properties
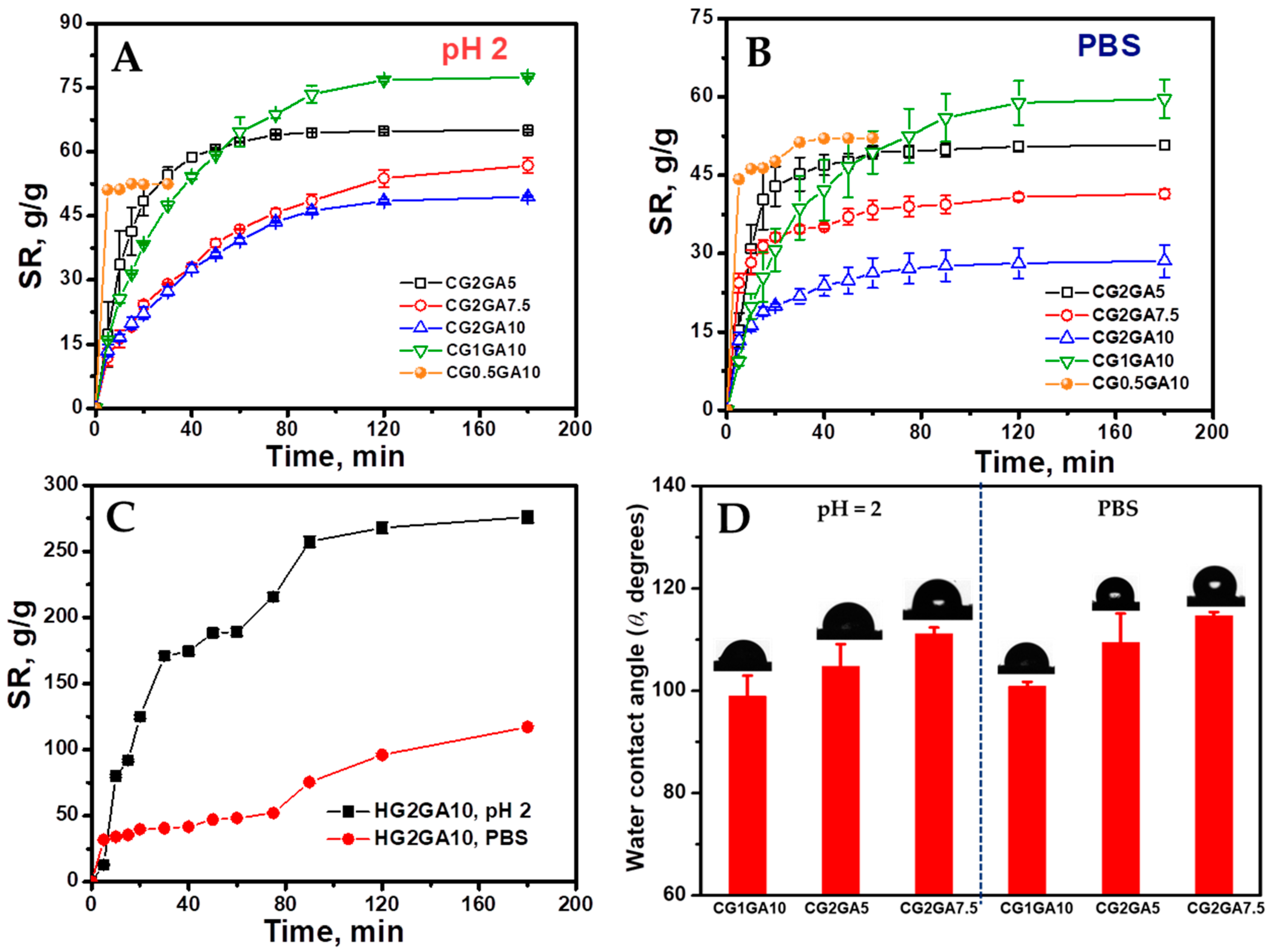
2.5. Swelling Behavior and Surface Wettability
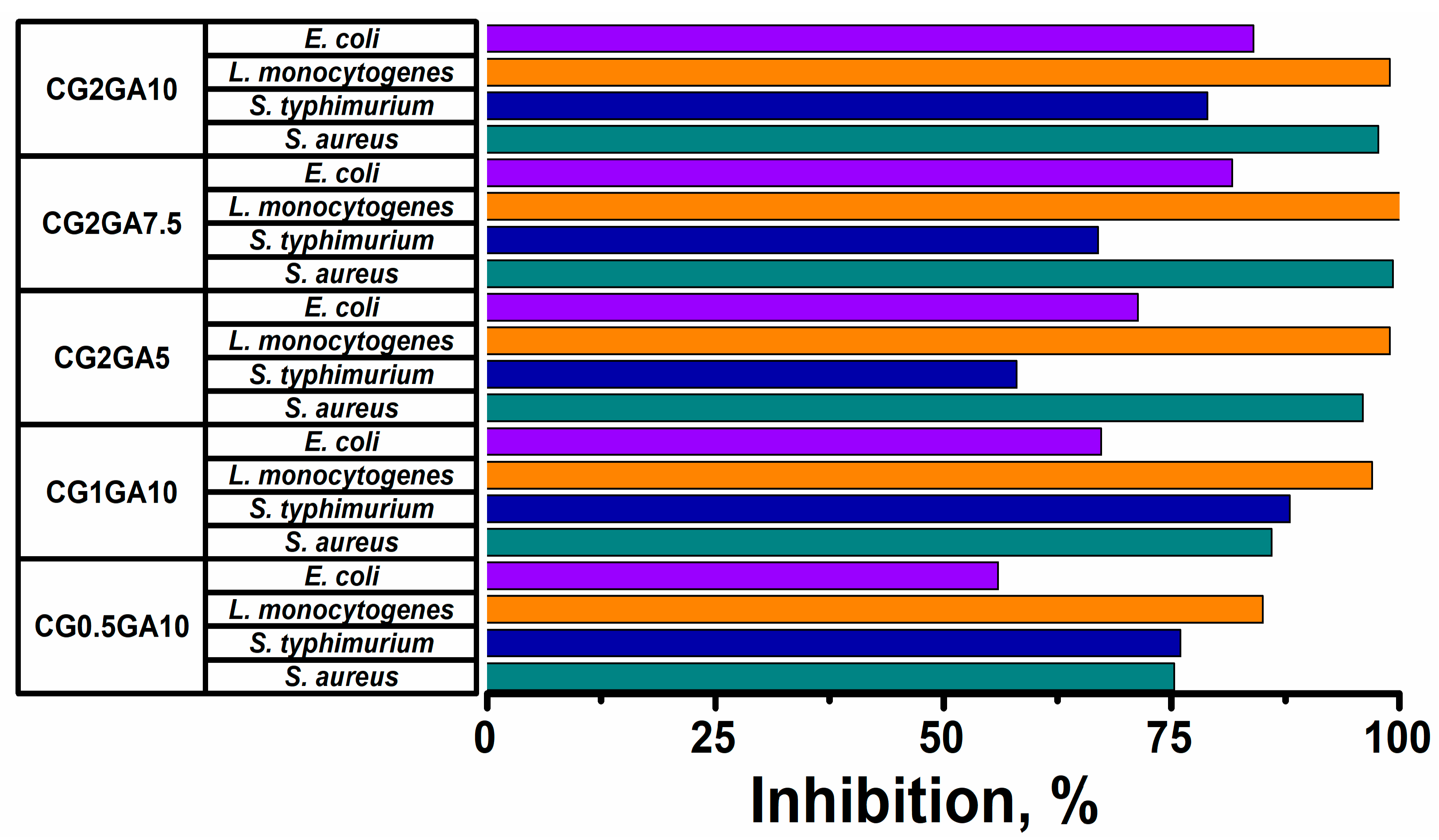
2.6. Antimicrobial Activity
2.7. CCM-Loaded CS Sponges
2.8. In Vitro Release of CCM
2.9. Antioxidant Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of CS Sponges
3.3. Characterization Methods
3.3.1. GFY
3.3.2. Density
3.3.3. FTIR Spectroscopy
3.3.4. SEM, EDX, and Pore Size Analysis
3.3.5. Mechanical Properties
3.3.6. Swelling Properties
3.3.7. Contact Angle Measurements
3.4. Loading and Release of CCM
3.5. Antimicrobial Activity
3.6. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical Polyphenols: New Analytical Challenges and Opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774–112786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, C.; Li, X.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Advances in Research on Interactions Between Polyphenols and Biology-Based Nano-Delivery Systems and Their Applications in Improving the Bioavailability of Polyphenols. Trends Food Sci. Technol. 2021, 116, 492–500. [Google Scholar] [CrossRef]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin Nanoformulations: A Review of Pharmaceutical Properties and Preclinical Studies and Clinical Data Related to Cancer Treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef] [PubMed]
- Malacrida, C.R.; Ferreira, S.; Zuanon, L.A.C.; Nicoletti Telis, V.R. Freeze-Drying for Microencapsulation of Turmeric Oleoresin using Modified Starch and Gelatin. J. Food Process. Preserv. 2015, 39, 1710–1719. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-Curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Ariyarathna, I.R.; Karunaratne, D.N. Microencapsulation Stabilizes Curcumin for Efficient Delivery in Food Applications. Food Packag. Shelf Life 2016, 10, 79–86. [Google Scholar] [CrossRef]
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.K.; Joshi, H.K.; Divakar, G. Enhanced Water Dispersibility of Curcumin Encapsulated in Alginate-polysorbate 80 Nano Particles and Bioavailability in Healthy Human Volunteers. Pharm. Nanotechnol. 2019, 7, 39–56. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Rafiee, Z.; Nejatian, M.; Daeihamed, M.; Jafari, S.M. Application of Curcumin-Loaded Nanocarriers for Food, Drug and Cosmetic Purposes. Trends Food Sci. Technol. 2019, 88, 445–458. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Ofoedu, C.E.; Hussain, S.Z.; Adeyanju, A.A.; Naseer, B.; Inyinbor, A.A.; Olaniran, A.F.; Kamal-Eldin, A. Application of biogels for bioactives delivery: Recent developments and future research insights. Appl. Food Res. 2022, 2, 100238. [Google Scholar] [CrossRef]
- Nayak, A.K.; Hasnain, M.S.; Aminabhavi, T.M. Drug delivery using interpenetrating polymeric networks of natural polymers: A recent update. J. Drug Deliv. Sci. Technol. 2021, 66, 102915. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.-k.; Mei, L.; Zeng, X. Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.; Akbari, T.; Hasani, M.; Sharifikolouei, E.; Raoufi, M.; Foroumadi, A.; Sharifzadeh, M.; Firoozpour, L.; Khoobi, M. Polysaccharide-based hydrogels containing herbal extracts for wound healing applications. Carbohydr. Polym. 2022, 294, 119808. [Google Scholar] [CrossRef]
- Kurczewska, J. Recent Reports on Polysaccharide-Based Materials for Drug Delivery. Polymers 2022, 14, 4189. [Google Scholar] [CrossRef] [PubMed]
- Richa, R.; Roy Choudhury, A. Exploration of Polysaccharide Based Nanoemulsions for Stabilization and Entrapment of Curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Goëlo, V.; Chaumun, M.; Gonçalves, A.; Estevinho, B.N.; Rocha, F. Polysaccharide-Based Delivery Systems for Curcumin and Turmeric Powder Encapsulation Using a Spray-Drying Process. Powder Technol. 2020, 370, 137–146. [Google Scholar] [CrossRef]
- Ma, Z.; Yao, J.; Wang, Y.; Jia, J.; Liu, F.; Liu, X. Polysaccharide-Based Delivery System for Curcumin: Fabrication and Characterization of Carboxymethylated Corn Fiber Gum/Chitosan Biopolymer Particles. Food Hydrocoll. 2022, 125, 107367. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent Advancements in Applications of Chitosan-Based Biomaterials for Skin Tissue Engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Pramanik, S.; Sali, V. Connecting the Dots in Drug Delivery: A Tour d’Horizon of Chitosan-Based Nanocarriers System. Int. J. Biol. Macrom. 2021, 169, 103–121. [Google Scholar] [CrossRef]
- Dinu, M.V.; Cocarta, A.I.; Dragan, E.S. Synthesis, Characterization and Drug Release Properties of 3D Chitosan/Clinoptilolite Biocomposite Cryogels. Carbohydr. Polym. 2016, 153, 203–211. [Google Scholar] [CrossRef]
- Mendes, A.C.; Gorzelanny, C.; Halter, N.; Schneider, S.W.; Chronakis, I.S. Hybrid Electrospun Chitosan-Phospholipids Nanofibers for Transdermal Drug Delivery. Int. J. Pharm. 2016, 510, 48–56. [Google Scholar] [CrossRef]
- Golchin, A.; Hosseinzadeh, S.; Staji, M.; Soleimani, M.; Ardeshirylajimi, A.; Khojasteh, A. Biological Behavior of the Curcumin Incorporated Chitosan/Poly(Vinyl Alcohol) Nanofibers for Biomedical Applications. J. Cell. Biochem. 2019, 120, 15410–15421. [Google Scholar] [CrossRef]
- Kamaraja, S.; Palanisamy, U.M.; Kadhar Mohamed, M.S.B.; Gangasalama, A.; Mariac, G.A.; Kandasamy, R. Curcumin Drug Delivery by Vanillin-Chitosan Coated with Calcium Ferrite Hybrid Nanoparticles as Carrier. Eur. J. Pharm. Sci. 2018, 116, 48–60. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T.; Ngawhirunpat, T.; Tonglairoum, P.; Opanasopit, P. Development of Chitosan-Based pH-Sensitive Polymeric Micelles Containing Curcumin for Colon-Targeted Drug Delivery. AAPS PharmSciTech 2018, 19, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, Y.; Jiang, X.; Wu, J.; Le, X. Molecular Interactions, Characterization and Antimicrobial Activity of Curcumin-Chitosan Blend Films. Food Hydrocoll. 2016, 52, 564–572. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Navarro, R. Metal Ion Biosorption on Chitosan for the Synthesis of Advanced Materials. J. Mater. Sci. 2014, 49, 5505–5518. [Google Scholar] [CrossRef]
- Rollini, M.; Mascheroni, E.; Capretti, G. Propolis and Chitosan as Antimicrobial and Polyphenols Retainer for the Development of Paper Based Active Packaging Materials. Food Packag. Shelf Life 2017, 14, 75–82. [Google Scholar] [CrossRef]
- Zemljic, L.F.; Tkavc, T.; Vesel, A. Chitosan Coatings onto Polyethylene Terephthalate for the Development of Potential Active Packaging Material. Appl. Surf. Sci. 2013, 265, 697–703. [Google Scholar] [CrossRef]
- Miteluț, A.C.; Tănase, E.E.; Popa, V.I. Sustainable Alternative for Food Packaging: Chitosan Biopolymer—A Review. AgroLife Sci. J. 2015, 4, 52–60. [Google Scholar]
- Mourya, V.K.; Inamdar, N.N. Chitosan-Modifications and Applications: Opportunities Galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Lungu, R.; Anisiei, A.; Rosca, I.; Sandu, A.-I.; Ailincai, D.; Marin, L. Double Functionalization of Chitosan Based Nanofibers Towards Biomaterials for Wound Healing. React. Funct. Polym. 2021, 167, 105028. [Google Scholar] [CrossRef]
- Iftime, M.-M.; Rosca, I.; Sandu, A.-I.; Marin, L. Chitosan Crosslinking with a Vanillin Isomer Toward Self-Healing Hydrogels with Antifungal Activity. Int. J. Biol. Macromol. 2022, 205, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Huang, R.-L.; Zha, X.-Q.; Li, Q.-M.; Pan, L.-H.; Luo, J.-P. Encapsulation and Sustained Release of Curcumin by a Composite Hydrogel of Lotus Root Amylopectin and Chitosan. Carbohydr. Polym. 2020, 232, 115810. [Google Scholar] [CrossRef]
- Nakagawa, K.; Sowasod, N.; Tanthapanichakoon, W.; Charinpanitkul, T. Hydrogel Based Oil Encapsulation for Controlled Release of Curcumin by Using a Ternary System of Chitosan, kappa-Carrageenan, and Carboxymethylcellulose Sodium Salt. LWT-Food Sci. Technol. 2013, 54, 600–605. [Google Scholar] [CrossRef]
- Zhang, C.; Wanga, X.; Xiao, M.; Ma, J.; Qu, Y.; Zou, L.; Zhang, J. Nano-in-Micro Alginate/Chitosan Hydrogel via Electrospray Technology for Orally Curcumin Delivery to Effectively Alleviate Alcerative Colitis. Mater. Des. 2022, 221, 110894. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in Porous Chitosan-Based Composite Hydrogels: Synthesis and Applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, H.; Guo, H.; Liu, K.; Mei, C.; Li, Y.; Duan, G.; He, S.; Han, J.; Zheng, J.; et al. Anisotropic Cellulose Nanofibril Composite Sponges for Electromagnetic Interference Shielding with Low Reflection Loss. Carbohydr. Polym. 2022, 276, 118799. [Google Scholar] [CrossRef]
- Peng, S.; Sun, Y.; Ma, C.; Duan, G.; Liu, Z.; Ma, C. Recent Advances in Dynamic Covalent Bond-Based Shape Memory Polymers. e-Polymers 2022, 22, 285–300. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryotropic Gelation of Poly(Vinyl Alcohol) Solutions. Russ. Chem. Rev. 1998, 67, 573–586. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 55. Retrospective view on the more than 40 years of studies performed in the A. N. Nesmeyanov Institute of Organoelement Compounds with Respect of the Cryostructuring Processes in Polymeric Systems. Gels 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Giannouli, P.; Morris, E.R. Cryogelation of Xanthan. Food Hydrocoll. 2003, 17, 495–501. [Google Scholar] [CrossRef]
- Nugent, M.J.D.; Higginbotham, C.L. Preparation of a Novel Freeze Thawed Poly(Vinyl Alcohol) Composite Hydrogel for Drug Delivery Application. Eur. J. Pharm. Biopharm. 2007, 67, 377–386. [Google Scholar] [CrossRef]
- Hedström, M.; Plieva, F.; Galaev, Y.I.; Mattiasson, B. Monolithic Macroporous Albumin/Chitosan Cryogel Structure: A New Matrix for Enzyme Immobilization. Anal. Bioanal. Chem. 2008, 390, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A.; Lazar, M.M.; Doroftei, F. Preparation and Characterization of Semi-IPN Cryogels Based on Polyacrylamide and Poly(N,N-dimethylaminoethyl methacrylate); Functionalization of Carrier with Monochlorotriazinyl-β-Cyclodextrin and Release Kinetics of Curcumin. Molecules 2021, 26, 6975. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-Based Polyelectrolyte Complex Cryogels with Elasticity, Toughness and Delivery of Curcumin Engineered by Polyions Pair and Cryostructuration Steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef]
- Dragan, E.S.; Ghiorghita, C.A.; Dinu, M.V.; Duceac, I.A.; Coseri, S. Fabrication of Self-Antibacterial Chitosan/Oxidized Starch Polyelectrolyte Complex Sponges for Controlled Delivery of Curcumin. Food Hydrocoll. 2023, 135, 108147. [Google Scholar] [CrossRef]
- Duceac, I.A.; Coseri, S. Chitosan Schiff-Base Hydrogels—A Critical Perspective Review. Gels 2022, 8, 779. [Google Scholar] [CrossRef]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Designing Smart Triple-Network Cationic Cryogels with Outstanding Efficiency and Selectivity for Deep Cleaning of Phosphate. Chem. Eng. J. 2021, 426, 131411. [Google Scholar] [CrossRef]
- Dinu, I.A.; Ghimici, L.; Raschip, I.E. Macroporous 3D Chitosan Cryogels for Fastac 10EC Pesticide Adsorption and Antibacterial Applications. Polymers 2022, 14, 3145. [Google Scholar] [CrossRef]
- Du Toit, J.P.; Pott, R.W.M. Transparent Polyvinyl-Alcohol Cryogel as Immobilisation Matrix for Continuous Biohydrogen Production by Phototrophic Bacteria. Biotechnol. Biofuels 2020, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice Templating Soft Matter: Fundamental Principles and Fabrication Approaches to Tailor Pore Structure and Morphology and Their Biomedical Applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Okay, O. Basic Principles of Cryotropic Gelation. Adv. Polym. Sci. 2014, 263, 49–101. [Google Scholar]
- Okay, O.; Lozinsky, V.I. Synthesis, structure-property relationships of cryogels. Adv. Polym. Sci. 2014, 263, 103–157. [Google Scholar]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, L.; Zhang, W.; Shi, J.; Cao, Y. Reinforced Low Density Alginate-Based Aerogels: Preparation, Hydrophobic Modification and Characterization. Carbohydr. Polym. 2012, 88, 1093–1099. [Google Scholar] [CrossRef]
- Tripathi, A.; Parsons, G.N.; Rojas, O.J.; Khan, S.A. Featherlight, Mechanically Robust Cellulose Ester Aerogels for Environmental Remediation. ACS Omega 2017, 2, 4297–4305. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically Cross-linked Chitosan/Dextrin Cryogels Entrapping Thymus Vulgaris Essential Oil with Enhanced Mechanical, Antioxidant and Antifungal Properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Humelnicu, D.; Lazar, M.M.; Ignat, M.; Dinu, I.A.; Dragan, E.S.; Dinu, M.V. Removal of Heavy Metal Ions from Multicomponent Aqueous Solutions by Eco-Friendly and Low-Cost Composite Sorbents with Anisotropic Pores. J. Hazard. Mater. 2020, 381, 120980. [Google Scholar] [CrossRef]
- Lazar, M.M.; Dinu, I.A.; Dinu, M.V. Synthesis of Ethylenediaminetetraacetic Acid-Functionalized Chitosan Cryogels as Potential Sorbents of Heavy Metal Ions. Mater. Plast. 2021, 58, 155–166. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Borchert, K.B.L.; Vasiliu, A.-L.; Zaharia, M.-M.; Schwarz, D.; Mihai, M. Porous Thiourea-Grafted-Chitosan Hydrogels: Synthesis and Sorption of Toxic Metal Ions from Contaminated Waters. Colloids Surf. A 2020, 607, 125504. [Google Scholar] [CrossRef]
- Dinu, M.V.; Ozmen, M.M.; Dragan, E.S.; Okay, O. Freezing as a Path to Build Macroporous Structures: Superfast Responsive Polyacrylamide Hydrogels. Polymer 2007, 48, 195–204. [Google Scholar] [CrossRef]
- Wang, S.; Yu, P.; Li, X.; Zhao, Z.; Dong, Y.; Li, X. Design and Fabrication of Functional Hydrogels with Specific Surface Wettability. Colloid Interface Sci. Commun. 2023, 52, 100697. [Google Scholar] [CrossRef]
- Yolcu, H. Analogies to Demonstrate the Effect of Roughness on Surface Wettability. Sci. Act. 2017, 54, 70–73. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact Angle Measurement on Porous Substrates: Effect of Liquid Absorption and Drop Size. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicribial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia Illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuang, S. Antibacterial Activity of Chitosan and Its Derivatives and Their Interaction Mechanism with Bacteria: Current State and Perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Ailincai, D.; Rosca, I.; Ursu, L.; Dascalu, A. Chitosan Oligomers—Synthesis, Characterization, and Properties. Cellul. Chem. Technol. 2022, 56, 767–776. [Google Scholar] [CrossRef]
- Economou, V.; Tsitsos, A.; Theodoridis, A.; Ambrosiadis, I.; Arsenos, G. Effects of Chitosan Coatings on Controlling Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus in Beef and Mutton Cuts. Appl.Sci. 2022, 12, 11345. [Google Scholar] [CrossRef]
- Liang, J.; Wang, R.; Chen, R. The impact of cross-linking mode on the physical and antimicrobial properties of a chitosan/bacterial cellulose composite. Polymers 2019, 11, 491. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Nikolić, L.; Urošević, M.; Nikolić, V.; Gajić, I.; Dinić, A.; Miljković, V.; Rakić, S.; Đokić, S.; Kesić, J.; Ilić-Stojanović, S.; et al. The Formulation of Curcumin:2-Hydroxypropyl-β-Cyclodextrin Complex with Smart Hydrogel for Prolonged Release of Curcumin. Pharmaceutics 2023, 15, 382. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Tan, S.; Tan, G.; Zhang, H.; Xia, N.; Jiang, L.; Ren, H.; Rayan, A.M. Intelligent Colorimetric Soy Protein Isolate-Based Films Incorporated with Curcumin Through an Organic Solvent-Free pH-Driven Method: Properties, Molecular Interactions, and Application. Food Hydrocoll. 2022, 133, 107904. [Google Scholar] [CrossRef]
- Wang, G.; Sukumar, S. Characteristics and Antitumor Activity of Polysorbate 80 Curcumin Micelles Preparation by Cloud Point Cooling. J. Drug Deliv. Sci. Technol. 2020, 59, 101871. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: A Review on the Most Used Mathematical Models for Drug Release. Processes 2022, 10, 1094. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.A.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From Molecule to Biological Function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Randey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Dragan, E.S.; Perju, M.M. Preparation and Swelling Behavior of Chitosan/Poly(N-2-Aminoethyl Acrylamide) Composite Hydrogels. Soft Mater. 2010, 8, 49–62. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Fu, Z.; Lu, L.; Cheng, J.; Fei, Y. A ‘Top Modification’ Strategy for Enhancing the Ability of a Chitosan Aerogel to Efficiently Capture Heavy Metal Ions. J. Colloid Interface Sci. 2021, 594, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Raschip, I.E.; Paduraru-Mocanu, O.M.; Nita, L.E.; Dinu, M.V. Antibacterial Porous Xanthan-Based Films Containing Flavoring Agents Evaluated by Near Infrared Chemical Imaging Technique. J. Appl. Polym. Sci. 2020, 137, e49111. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-Drug Delivery System Based on the Hydrogels of Alginate and Sodium Carboxymethyl Cellulose for Colorectal Cancer Treatment. Carbohydr. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Siepman, J.; Peppas, N.A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Raschip, I.E.; Fifere, N.; Dinu, M.V. A Comparative Analysis on the Effect of Variety of Grape Pomace Extracts on the Ice-Templated 3D Cryogel Features. Gels 2021, 7, 76. [Google Scholar] [CrossRef]
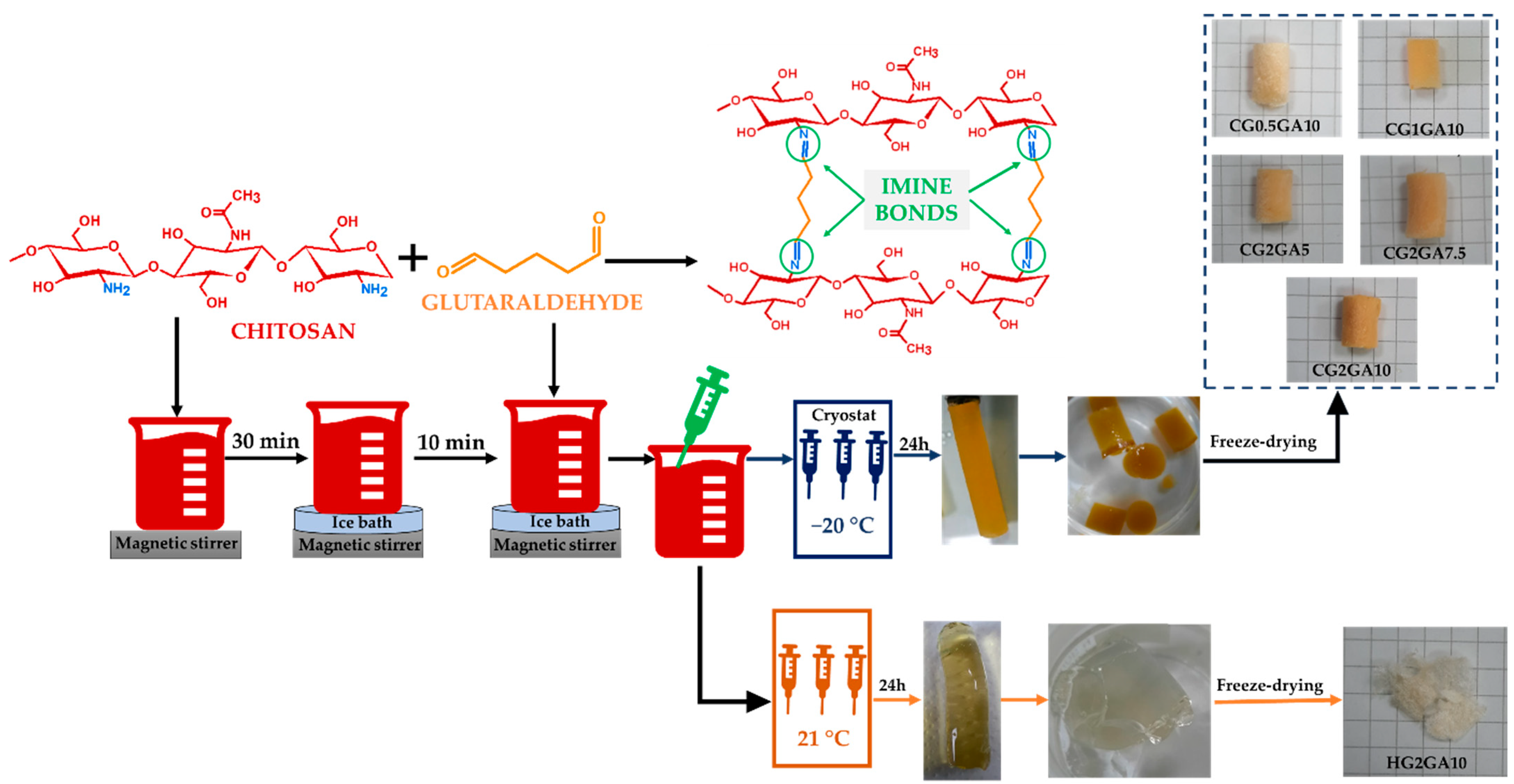


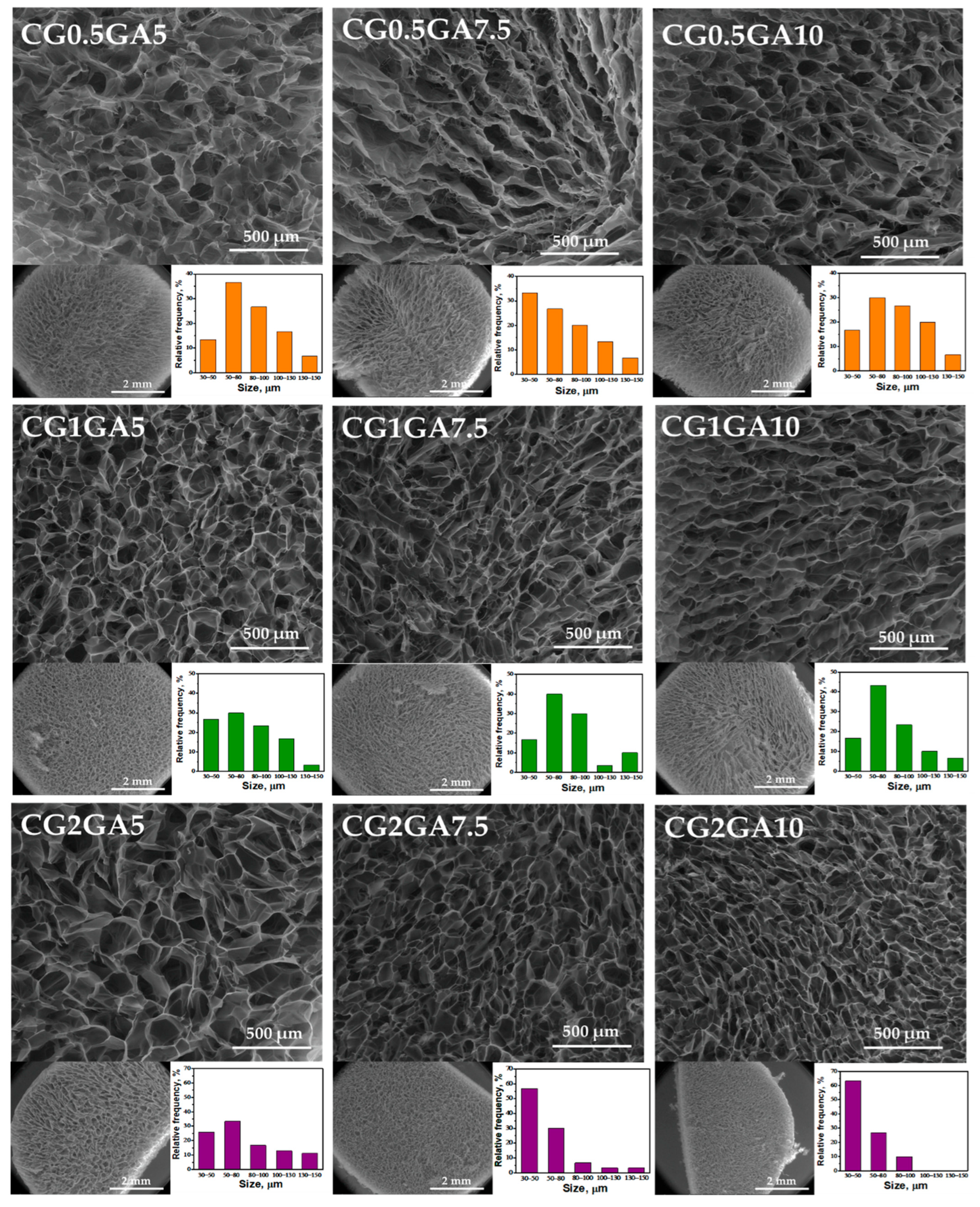




| a Sample Code | CS, wt. % | b GA, wt. % | c GA, μmol | d T, °C | e GFY, % |
|---|---|---|---|---|---|
| CG0.5GA5 | 0.5 | 5 | 0.14 | −20 ± 2 | 86.28 ± 0.65 |
| CG0.5GA7.5 | 0.5 | 7.5 | 0.22 | −20 ± 2 | 88.96 ± 0.83 |
| CG0.5GA10 | 0.5 | 10 | 0.29 | −20 ± 2 | 89.37 ± 0.48 |
| CG1GA5 | 1 | 5 | 0.14 | −20 ± 2 | 86.28 ± 0.80 |
| CG1GA7.5 | 1 | 7.5 | 0.22 | −20 ± 2 | 86.07 ± 1.11 |
| CG1GA10 | 1 | 10 | 0.29 | −20 ± 2 | 87.47 ± 0.46 |
| CG2GA5 | 2 | 5 | 0.14 | −20 ± 2 | 86.60 ± 0.42 |
| CG2GA7.5 | 2 | 7.5 | 0.22 | −20 ± 2 | 86.60 ± 0.01 |
| CG2GA10 | 2 | 10 | 0.29 | −20 ± 2 | 86.64 ± 0.52 |
| HG2GA10 | 2 | 10 | 0.29 | 21 ± 2 | 93.71 ± 2.4 |
| a CCS | 0.5 wt. % | 1 wt. % | 2 wt. % | |
|---|---|---|---|---|
| T °C | ||||
| −20 °C | Opaque; Light yellow | Opaque; Light yellow | Opaque; Dark brown | |
| 21 °C | No gel | No gel | Transparent; Light yellow | |
| Sample | wt. % T80 | Higuchi | Korsmeyer-Peppas | First Order | ||||
|---|---|---|---|---|---|---|---|---|
| kH | R2 | nr | kKP (min−nr) | R2 | k1 | R2 | ||
| CG0.5GA10 | 0.25 | 8.6678 | 0.8392 | 0.3607 | 6.3913 | 0.8807 | −0.0093 | 0.7682 |
| CG1GA10 | 0.25 | 8.3991 | 0.7875 | 0.3511 | 6.5265 | 0.8585 | −0.0089 | 0.7073 |
| CG2GA5 | 0.1 | 6.2567 | 0.9168 | 0.4236 | 5.7488 | 0.9386 | −0.0053 | 0.8225 |
| 0.25 | 9.2292 | 0.9264 | 0.3766 | 6.0836 | 0.9395 | −0.0101 | 0.8774 | |
| 0.5 | 30.0719 | 0.9629 | 0.3428 | 6.3910 | 0.9292 | −0.0412 | 0.9813 | |
| CG2GA7.5 | 0.25 | 6.7136 | 0.9236 | 0.3571 | 6.1903 | 0.9542 | −0.0061 | 0.8432 |
| CG2GA10 | 0.1 | 4.9026 | 0.8915 | 0.4841 | 5.4034 | 0.9201 | −0.0038 | 0.7731 |
| 0.25 | 5.9882 | 0.8522 | 0.3583 | 6.3565 | 0.9264 | −0.0051 | 0.7387 | |
| 0.5 | 7.4126 | 0.9498 | 0.3583 | 6.1163 | 0.9708 | −0.0071 | 0.8869 | |
| HG2GA10 | 0.25 | 4.9796 | 0.8904 | 0.2133 | 7.4776 | 0.9508 | −0.0047 | 0.7983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Platon, I.-V.; Ghiorghita, C.-A.; Lazar, M.M.; Raschip, I.E.; Dinu, M.V. Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols. Int. J. Mol. Sci. 2023, 24, 4452. https://doi.org/10.3390/ijms24054452
Platon I-V, Ghiorghita C-A, Lazar MM, Raschip IE, Dinu MV. Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols. International Journal of Molecular Sciences. 2023; 24(5):4452. https://doi.org/10.3390/ijms24054452
Chicago/Turabian StylePlaton, Ioana-Victoria, Claudiu-Augustin Ghiorghita, Maria Marinela Lazar, Irina Elena Raschip, and Maria Valentina Dinu. 2023. "Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols" International Journal of Molecular Sciences 24, no. 5: 4452. https://doi.org/10.3390/ijms24054452
APA StylePlaton, I.-V., Ghiorghita, C.-A., Lazar, M. M., Raschip, I. E., & Dinu, M. V. (2023). Chitosan Sponges with Instantaneous Shape Recovery and Multistrain Antibacterial Activity for Controlled Release of Plant-Derived Polyphenols. International Journal of Molecular Sciences, 24(5), 4452. https://doi.org/10.3390/ijms24054452









