Abstract
Among several opioid-associated endocrinopathies, opioid-associated adrenal insufficiency (OIAI) is both common and not well understood by most clinicians, particularly those outside of endocrine specialization. OIAI is secondary to long-term opioid use and differs from primary adrenal insufficiency. Beyond chronic opioid use, risk factors for OIAI are not well known. OIAI can be diagnosed by a variety of tests, such as the morning cortisol test, but cutoff values are not well established and it is estimated that only about 10% of patients with OIAI will ever be properly diagnosed. This may be dangerous, as OIAI can lead to a potentially life-threatening adrenal crisis. OIAI can be treated and for patients who must continue opioid therapy, it can be clinically managed. OIAI resolves with opioid cessation. Better guidance for diagnosis and treatment is urgently needed, particularly in light of the fact that 5% of the United States population has a prescription for chronic opioid therapy.
1. Introduction
Opioids have been and remain among the most prescribed analgesics in the developed world, despite concerns about tolerance, use disorder, diversion, and adverse effects [1]. Approximately 9% to 29% of chronic opioid therapy patients develop opioid-induced adrenal insufficiency (OIAI), which is challenging to diagnose and may be treatment limiting [2,3]. OIAI symptoms include nausea, vomiting, lethargy, weight loss, dizziness, and muscle aches and pain. Some of these diffuse symptoms may be difficult to assess as they can overlap with the patient’s prescribing indication [2]. OIAI can occur with any opioid and despite its prevalence, has not been the subject of great academic interest [2]. Note that OIAI occurs secondary to prolonged exposure to opioids and differs in many ways from primary adrenal insufficiency.
The hypothalamic-pituitary-adrenal (HPA) axis describes a complex network of interacting endocrine pathways in the brain, which allow for communications among the hypothalamus gland, the anterior pituitary gland, and the adrenal system [4]. The HPA axis regulates the body’s physiological responses to stress as well as regulating the appropriate release of hormones for sexual development. When it is functioning optimally, the HPA allows for physiological homeostasis, but disruptions and imbalances can trigger alterations in neuropeptide and neurotransmitter synthesis, some of which may have long-lasting consequences [4]. HPA dysfunction is common in those in long-term opioid therapy [5].
Despite the opioid epidemic, chronic opioid use in large subpopulations, and the widespread use of medication-assisted treatments for opioid rehabilitation which require prolonged exposure to opioids, opioid-induced endocrinopathies remain under-diagnosed and underappreciated [6]. The aim of this article is to provide a narrative overview of what is known about OIAI and its treatment.
2. Methods
This is a narrative review. The search term “opioid-induced adrenal insufficiency” was searched in December 2022 in PubMed with no delimiters and yielded 17 results. The keywords “opioid and adrenal insufficiency” with no delimiters yielded 86 results. The bibliographies of these articles were searched. The Cochrane Library database was searched for that same search time (0 results) and “adrenal insufficiency” (0 results). There were two results for “endocrinopathy” in the Cochrane database but neither related to opioids or adrenal insufficiency. Google Scholar was searched as well. There were some duplications among the results. This is a narrative review and there is limited published literature. There are no published clinical trials or randomized clinical trials on opioid-induced adrenal insufficiency in the literature. It should be noted that almost all of the peer-reviewed material published on this topic has appeared in the last five years.
3. Results
Located throughout the body, the main G-protein-coupled opioid receptors, mu-opioid receptors (MOR), delta opioid receptors (DOR), and kappa opioid receptors (KOR), respond to endogenous and exogenous opioids and suppress the HPA axis. Not unsurprisingly, there is scant evidence investigating HPA-axis suppression with only a few studies, which use different definitions, opioids, routes of administration, and doses, as well as different patient populations and markedly different study designs [2]. Different definitions make epidemiologic reference difficult. When HPA axis suppression is defined as a basal cortisol level of <5 µg/dL, then its incidence is 9.2% for those taking intrathecal morphine or hydromorphone, but if an insulin tolerance test is used to define HPA axis suppression, the rate of HPA axis suppression in the same population is 15%. Furthermore, if a 24 h cortisol-free urine assay below the reference range (20 to 90 µg/24 h) is used to define HPA axis suppression, then the rate is 20% [7].
The HPA axis may be thought of as a complex network of neuroendocrine pathways and feedback loops with the purpose of maintaining physiologic homeostasis [4]. The HPA axis is bounded by the hypothalamus, the anterior pituitary gland, and the adrenal system. The HPA axis develops in the fetus, but it is not until puberty that it develops fully under the influence of circulating gonadal hormones. Derangement in this system can alter neuropeptides, change neurotransmitter synthesis in the central nervous system, and disrupt glucocorticoid synthesis in the periphery [4]. HPA axis dysfunction can result not only in changes in the neuroendocrine system, but it can also cause changes in behavior, autonomic function, and metabolism [4]. Short-term exposure to opioids can suppress the HPA axis. Paradoxically, brief opioid exposure has the opposite effect in many animal studies and tends to increase corticotropin and glucocorticoids [2], making it challenging to use animal studies to investigate HPA axial suppression caused by brief opioid use. On the other hand, long-term exposure to opioids in both humans and animals has been associated with suppression of the HPA axis [2]. Studying HPA axial suppression is further confounded by marked intersubject variations. The mechanisms to explain why and how prolonged exposure to opioids can suppress the HPA axis include genetic polymorphisms of the opioid receptors, genetic variations in interleukin beta (ILβ), as well as differences that trace back to study design or methodology [2].
Peripheral (primary) and central (secondary and tertiary) forms of adrenal insufficiency occur because of direct adrenal gland disorders, such as rare genetic conditions or autoimmune diseases and secondary to ACTH deficiency, or by suppression of CRH by exogenous glucocorticoids or other medications, such as opioids, respectively [8,9]. In serum, cortisol has a 90-min half-life and the relative or absolute absence of endogenous cortisol, an important glucocorticoid that promotes homeostasis, has numerous consequences, including increased pro-inflammatory cytokines, altered populations of immune cells, and vascular consequences that can cause vasodilatation and profound, even life-threatening hypotension [10].
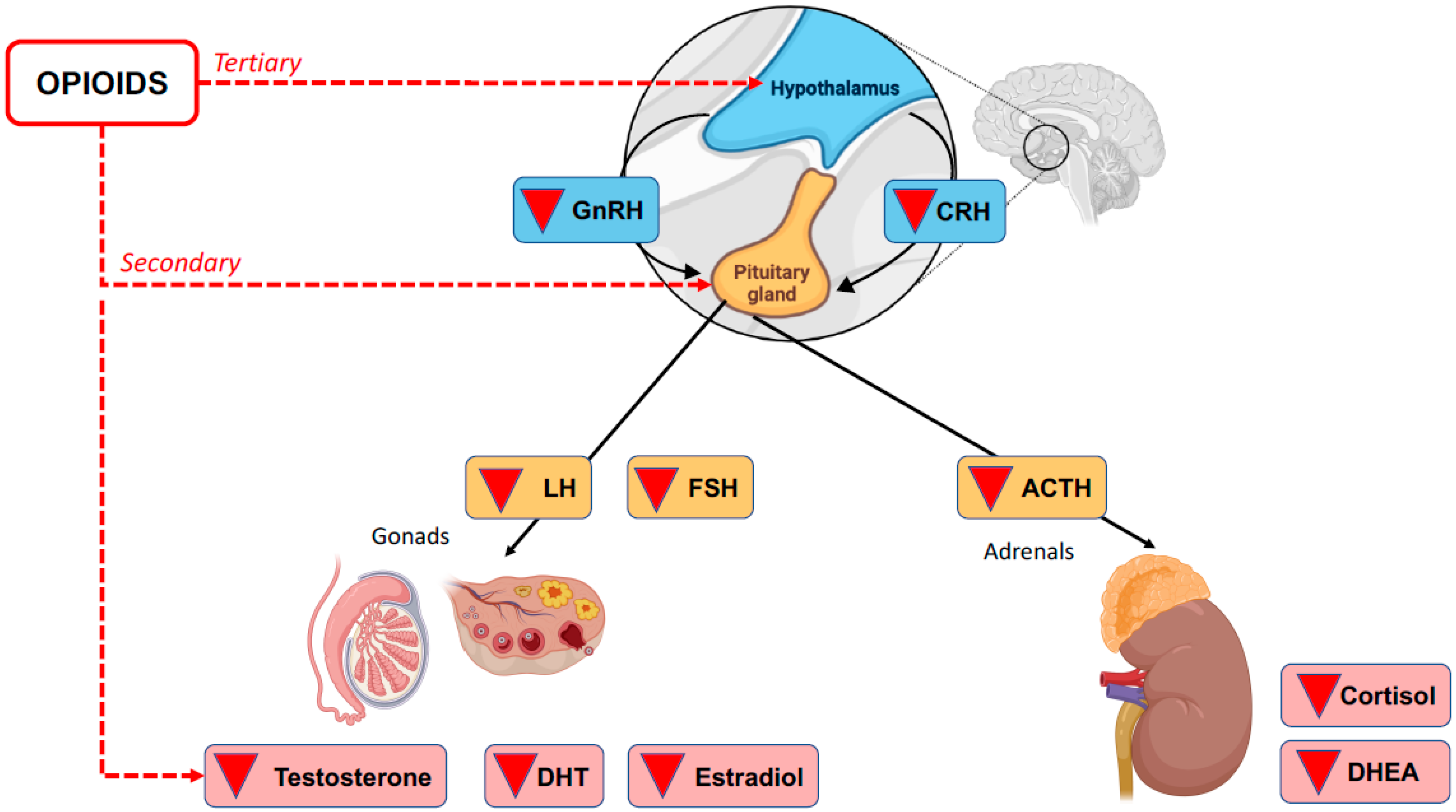
The deterioration of HPA axis communication can lead to any number of conditions, including OIAI. This is a secondary/tertiary form of adrenal insufficiency because it is a direct consequence of the inhibitory activity of opioids on the HPA axis rather than damage to the adrenal cortex itself [2]. See Figure 1.
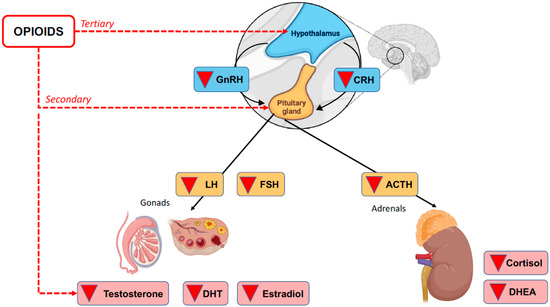
Figure 1.
Opioid-induced secondary and tertiary adrenal insufficiency.
4. Epidemiology of OIAI
Most opioids are prescribed to manage moderate-to-severe acute pain, but opioids are also prescribed long term to patients with cancer pain or chronic non-cancer pain [11]. In fact, over 5% of the United States population has a prescription for chronic opioid therapy [12]. Chronic opioid therapy has been associated with OIAI, but specific risk factors (agent, dose, duration, route of administration) have not been conclusively determined [13].
Since it was thought that the prevalence of OIAI was underestimated, a prospective study at a pain center was designed to evaluate adults taking opioids for ≥90 days [14]. Morning cortisol levels were evaluated at baseline. Based on endocrine testing, 5% of the patient population (n = 162) had OIAI and those patients with diagnosed OIAI were taking more morphine milligram equivalents (MME) per day than those without OIAI [14].
5. Risk Factors for OIAI
While suppression of the HPA axis is known to be a factor in the development of OIAI, the risk factors for HPA-axis suppression in chronic opioid therapy patients are not known [2]. Another opioid-associated endocrinopathy, opioid-induced androgen deficiency (OIAD), has been linked to high doses of long-acting opioids [15], but there is no conclusive evidence this is the case with OIAI [2]. It is not known if age, sex, or genetic polymorphisms contribute to OIAI and no study has yet evaluated whether OIAI is more or less associated with specific opioids or specific routes of administration [13]. It has been suggested that long-acting opioids may confer a greater risk of OIAI than other opioid formulations, but there is limited evidence to support this [16].
It may be that long-term exposure to opioids is the strongest risk factor for secondary adrenal insufficiency [17]. Opioid use disorder (OUD) may lead to OIAI, as a case report in the literature found, and the opioid epidemic may be at the root of many cases of OIAI [18]. A 28-year-old female presented at the emergency department with palpitations, malaise, hot flashes, and lightheadedness [19]. She had a history of hypertension, carpal tunnel syndrome, depression, and anxiety for which she was being treated. She had used marijuana regularly for the past 13 years and in the past five months had started using synthetic marijuana as well. She was prescribed 15 mg of oxycodone twice daily for her carpal tunnel syndrome, she was taking 60 mg/day by mouth and 60 mg intranasally per day for the past two years (120 mg/day). A random cortisol test and cosyntropin suppression test suggested poor adrenocorticotropic hormone (ACTH) response. She was diagnosed with OIAI. Magnetic resonance imaging showed a normal-sized pituitary gland without any focal lesions. She was administered systemic glucocorticoids and mineralocorticoids and prescribed a course of oral steroids. She was referred to substance use counseling [19].
6. Diagnosis
Since OIAI can result in significant morbidity, prompt recognition and treatment are optimal [20]. However, the presenting symptoms of OIAI tend to be vague and diffuse and might be readily attributed to the underlying condition rather than the opioid therapy [20]. Secondary adrenal insufficiency in general is challenging to diagnose [21,22]. Diagnostic delays are common, with an estimated six-month lag time between the onset of symptoms and clinical diagnosis [23]. In fact, 20% of OIAI patients wait over five years for an accurate diagnosis, and two-thirds (68%) are misdiagnosed before getting an accurate diagnosis [23]. It has been estimated that only about 10% of patients with OIAI will be diagnosed accurately [20]. Signs and symptoms of OIAI include musculoskeletal pain, weight loss, fatigue, gastrointestinal symptoms, and sexual dysfunction in patients with prolonged exposure to opioids [16,20].
There is no consensus definition for an OIAI diagnosis. Since OIAI is typically associated with a low level of cortisol, a low concentration of corticotropin, and a failure to launch an appropriate cortisol response when synthetic corticotropin is given, these tests are typically used. Confounding factors include the use of exogenous glucocorticosteroids or an abnormal pituitary gland. It is important to note that OIAI is caused by HPA-axis suppression rather than damaged or destroyed adrenal cortex regions.
Although OIAI is a known clinical entity, it is not easily diagnosed and thus may not be adequately treated [2]. In fact, the optimal diagnostic workup for a patient on chronic opioid therapy suspected of having OIAI remains a subject of controversy, in part, because opioids can acutely suppress cortisol and may disrupt the circadian rhythm of cortisol production, which can affect the diagnostic tests [24,25].
A variety of diagnostic tests have been described in the literature and are summarized in Table 1 [18,21,24,25].

Table 1.
Diagnostic testing strategies.
In healthy humans, serum glucocorticoid concentrations in the blood can change five-fold over the course of the day with the highest levels observed upon arising and the lowest during sleep; cortisol is also released in response to physical and emotional trauma apart from the circadian schedule [26]. When healthy subjects are administered intravenous morphine, their plasma cortisol levels will drop within 60 min. Cortisol production can also be thrown off by abnormal circadian rhythms, which are common among people with OUD [27,28]. For example, the morning cortisol test anticipates the subject will have peak daily cortisol at approximately 30 min to two hours after arising, but this only occurs in people with normal circadian rhythms, that is, it excludes most of the population with suspected OIAI [29].
The European Society of Endocrinology has recommended morning cortisol testing as a first-line diagnostic approach for adrenal insufficiency and recommends a cosyntropin stimulation test if the cortisol test is inconclusive [30]. However, this guidance relates to primary adrenal insufficiency and the guidelines do not specifically address how such recommendations apply to patients taking chronic opioid therapy. The cutoff values for cortisol levels can also vary. In many cases, 500 nmol/L is used. As might be suspected, when using a lower value, such as 405 nmol/L, fewer patients will be diagnosed with OIAI [25]. Measuring cortisol in the urine is not considered a helpful test, but it may be of value when considering the patient’s overall hormonal status [2].
A corticotropin (cosyntropin) stimulation test (CST) can be performed and is recommended if the cortisol test is inconclusive, but OIAI has been known to occur in patients with normal CST results [2]. From a clinical vantage point, it is presumed that people with OIAI will have below-normal levels of serum cortisol and a low corticotropin concentration; it is also thought that OIAI will cause the body to be unable to offer an appropriate cortisol response following the administration of corticotropin [2]. It should be noted that CST scores may be influenced by atrophy of the adrenal cortex which can be caused by primary adrenal insufficiency and/or an extended period of time without corticotropin [2]. Administration of synthetic corticotropin may stimulate the adrenal cortex to the degree it can provide a cortisol response of 18 to 20 µg/dL, but the exact cutoff values for OIAI diagnosis remain disputed and can vary, even across different hospitals and testing facilities [2].
Perhaps the gold standard diagnostic test for OIAI is the insulin tolerance test [13], but it is not always available at all locations. The cosyntropin stimulation test (CST) may be taken as a surrogate, because its results align with the insulin tolerance test [31]. Note that the CST has a sensitivity of 0.64 and specificity of 0.93, when the standard cutoff of 10 to 20 µg/dl is used [32]. The overnight test using metyrapone, a reversible 11β-mono-oxygenase inhibitor, may also be helpful [13]. Screening for basal cortisol levels should be followed by an insulin tolerance test, when available, if the cortisol level is low or inconclusive [33].
In a clinical study of 40 chronic opioid patients diagnosed with OIAI, patients took a mean of 105 MME/day (range 60 to 200 mg) for a median of 60 months upon inclusion into the study [20]. OIAI was diagnosed by low morning cortisol levels, baseline ACTH and/or DHEA-A, or an abnormal cosyntropin stimulation test [20].
Note that OIAI may present in unusual ways. The literature reports on the case of a 25-year-old man with OIAI presenting as hypercalcemia [34]. Following treatment for a critical illness that involved prolonged opioid exposure, he was diagnosed with hypercalcemia and treated with a three-day course of intravenous saline and glucocorticoid replacement. His hypercalcemia resolved, but his hypoadrenalism persisted until he discontinued opioids [34]. This case serves as an example of two concomitant and interacting endocrinopathies. Hypercalcemia can occur secondary to adrenal insufficiency and may be the presenting symptom [34].
7. The Clinical Course
The clinical course of OIAI has yet to be thoroughly elucidated [2]. A retrospective study of 40 OIAI patients conducted between 2006 and 2018 at a single center described the natural course of OIAI [20]. The population was 75% female with a median age of 49 years; the vast majority (95%) took opioids daily while 5% used opioids only as needed, with a median daily dose of 105 MME (range: 60 to 200 MME). The most frequently reported symptoms were fatigue (73%), musculoskeletal pain (53%), weight loss (43%), headache (30%), and nausea (20%). An adrenal crisis occurred in one patient during the study, but no patient presented with an adrenal crisis. A variety of laboratory values were used to diagnose OIAI and included low morning cortisol, baseline ACTH and/or DHEAS values, and CST testing. Median values of serum cortisol were 3 (range 1.4 to 5) µg/dL (normal > 7); ACTH was 9.7 (range 4.7 to 15) pg/mL (normal > 10); and DHEAS was 20 (range 15–32) µg/dL (normal > 50). Twenty-one percent were diagnosed with opioid-induced hypogonadism, which was more common in men. Most patients were prescribed hydrocortisone (95%) and of the subset of patients with follow-up data (n = 33), 42% showed symptomatic improvement [20].
8. Management of OIAI
The objective of OIAI treatment is the replication of the deficient cycles of physiologic cortisol, which should eliminate the OIAI symptoms. This seems reasonable in theory, but the currently marketed glucocorticoids are somewhat limited. The most commonly prescribed exogenous glucocorticoid, hydrocortisone, is typically administered in two or three daily divided doses for a total of 15 to 25 mg/dL [2]. Oral glucocorticoid medications can be used to regulate cortisol release, but the dose must be carefully determined to avoid excessive replacement [2]. Other glucocorticoid products, such as prednisone, prednisolone, and dexamethasone, offer the advantage of longer half-lives, but they are associated with increased glucocorticoid effects and may result in greater suppression of endogenous cortisol [2]. Dosing is based on symptomatic relief. Improved delivery systems and formulations for exogenous glucocorticosteroids are in development or new to market [2].
Complete discontinuation of opioids reverses OIAI, but the timeline for full recovery has not been conclusively established [2]. Note that long-term opioid therapy must be tapered rather that discontinued abruptly in order to mitigate or even avoid withdrawal symptoms [35]. It may be advisable for clinicians to monitor adrenal function during opioid tapering and after discontinuation [2].
Ideally, patients should be informed about the potential risks for adrenal insufficiency, hypogonadism, and other opioid-associated side effects before opioids are initiated [36]. Patients already taking chronic opioids should be periodically informed about these conditions and encouraged to report related symptoms which they may not necessarily associate with opioid use [36].
When opioid therapy must continue, OIAI can be managed if the patient can be educated and caregivers are willing to assist. When patients are under particular stress (major psychological stress, trauma, impending or recent surgical procedures), they should increase their intake of corticosteroids [2]. If the patient falls ill, the dose of corticosteroids should be doubled or even tripled for two or three days. If symptoms are too severe or oral intake is not possible, intramuscular corticosteroids may be used [2]. Patients with OIAI should wear a medical alert bracelet for emergency responders [2].
In a case report, a 46-year-old woman who had been treated for non-cancer pain for four years with a transdermal fentanyl patch (90 to 120 MME/day) developed fatigue, decreased energy, and loss of appetite in her second year of treatment [37]. Seeking treatment for worsening opioid-induced constipation and abdominal pain, a diagnosis of OIAI was reached based on her long-term opioid exposure, laboratory tests including the corticotropin-releasing hormone stimulation test, and the exclusion of other hypothalamic-pituitary disorders. Oral corticosteroid therapy was prescribed but did not relieve her constipation or abdominal pain; opioid tapering, rotation, or discontinuation was not possible because of her painful symptoms and anxiety [37].
9. Adrenal Insufficiency in the COVID Era
There is some controversy as to whether patients with primary or secondary adrenal insufficiency, including OIAI, have an elevated risk of contracting COVID-19 and face a greater risk of a complicated course, because the infection may trigger a possibly life-threatening adrenal crisis [38,39,40,41]. In cases of a patient with both COVID-19 and OIAI, an increased dose of glucocorticoid replacement therapy is recommended [38]. Other autoimmune endocrinopathies have been reported in case studies associated with COVID-19 infection and certainly adrenal dysfunction has occurred in other infectious diseases [42,43].
An interesting academic speculation is whether so-called “long COVID” is actually an adrenal problem that emerges after the acute COVID-19 symptoms resolve [43]. The symptoms of secondary adrenal insufficiency and long COVID overlap somewhat, leading to the hypothesis that long COVID may be COVID-induced adrenal insufficiency [43]. Viral tropism to adrenal glands is not unusual in viral infections, and adrenal insufficiency has been reported in patients with meningococcal sepsis and tuberculosis adrenalitis [44]. Autopsies of COVID-19 patients have reported both fibrin and microthrombi deposits in the adrenal capillaries [45]. Adrenal insufficiency in the aftermath of acute COVID infection would likely require several weeks to develop [43]. It goes beyond the scope of this review of OIAI to explore the connections between COVID-19 and adrenal insufficiency, but care should be taken with OIAI patients who contract COVID-19.
10. Adrenal Androgens
Adrenal androgens contribute far more to the overall androgen stores in females than in males [2]. The serum levels of the weak adrenal androgen, dehydroepiandrosterone sulfate (DHEA-S), are lower in long-term opioid users compared to those who do not use opioids [46]. The effect of DHEA-S supplementation in patients with OIAI has not been well studied and is not generally recommended, but remains an area for investigation [2]. For young women with OIAI and receiving optimal glucocorticoid replacement therapy, DHEA-S supplementation is sometimes considered a means to treat symptoms of diminished libido, depression, and low energy [2].
11. Adrenal Crisis
Adrenal crisis is a potentially lethal acute emergency that may occur in primary or secondary adrenal insufficiency, including OIAI. Adrenal crisis differs from adrenal insufficiency, although the term acute adrenal insufficiency is sometimes used interchangeably with adrenal crisis [10]. In some instances, an adrenal crisis is the initial presentation of patients with OIAI or other forms of adrenal insufficiency. There is no expert consensus definition of adrenal crisis, but the condition is characterized by hypotension that can be treated effectively with parenteral glucocorticoids [10]. Other symptoms may mirror those that occur with OIAI or adrenal insufficiency, such as confusion and abdominal pain [10]. There is no clear-cut evidence as to what can trigger an adrenal crisis or why crises occur in some patients but not others [10]. Infection, chemotherapy or immunotherapy, and nonadherence to glucocorticoid regimens in patients with adrenal insufficiency have been implicated in an adrenal crisis [10].
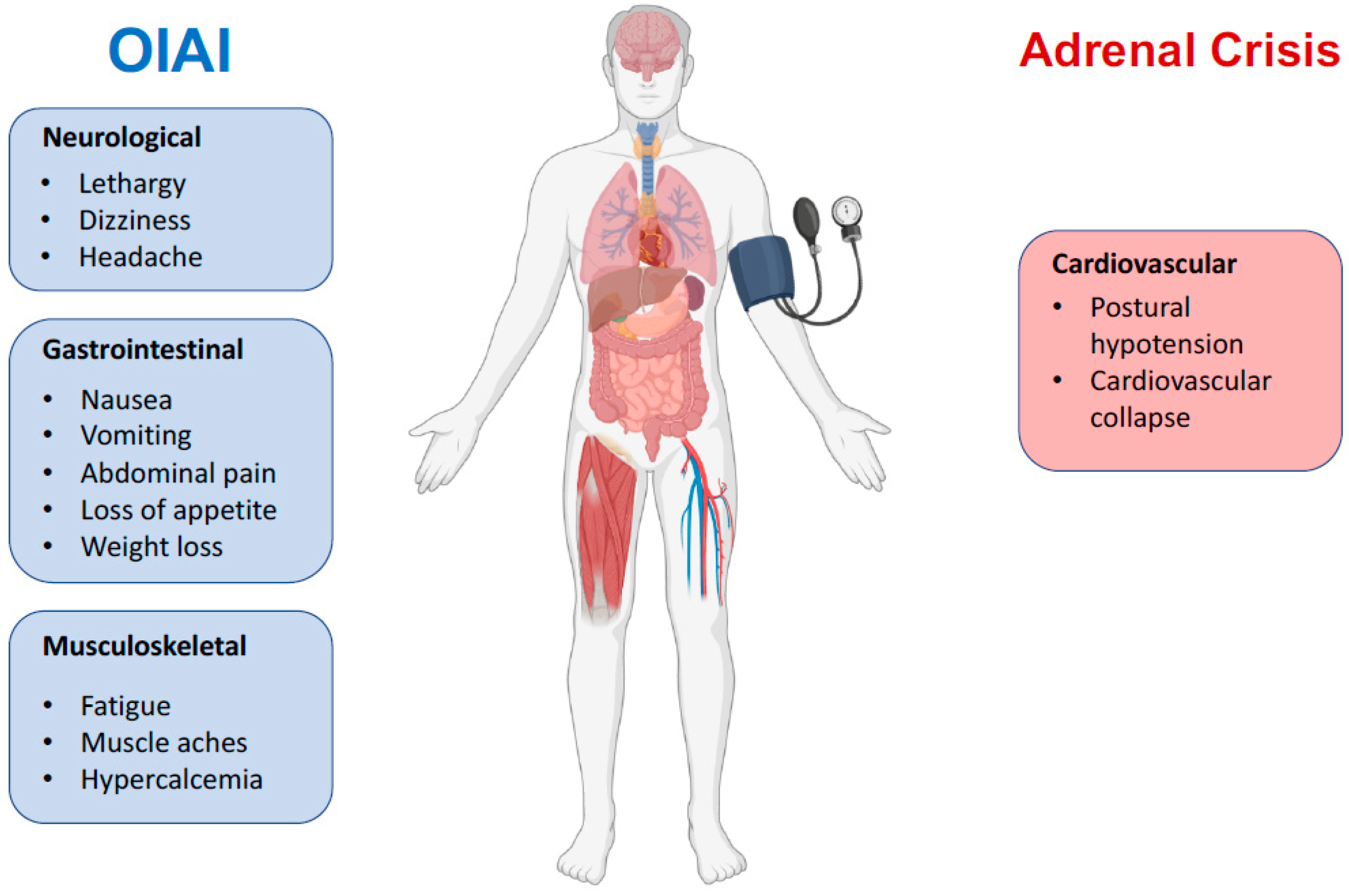
In addition to the symptoms of OIAI, patients in an adrenal crisis may suffer postural hypotension, nausea, vomiting, myalgia, and cardiovascular effects, even escalating to cardiovascular collapse [2]. Treatment should be hydration resuscitation (1 L of 0.9% saline over 1 h followed by infusion as needed), parenteral glucocorticosteroids (100 mg bolus dose of IV hydrocortisone, then 200 to 300 mg of hydrocortisone per day) [10,47]. Few studies of an adrenal crisis have assessed patients for OIAI [13].
12. Clinical Considerations for Daily Practice
All patients treated with long-term opioid therapy should be assessed for OIAI, particularly those who experience symptoms of weight loss, fatigue, lethargy, and nausea [13]. Secondary adrenal insufficiency is associated with diminished work capacity and reduced quality of life [8]. Unfortunately, fatigue, myalgia, and gastrointestinal symptoms are very common among chronic pain patients regardless of the presence of OIAI [48,49]. This is probably the main reason why this condition has long been unknown among physicians and is still underdiagnosed in current clinical practice. Moreover, glucocorticoids are often part of the analgesic plan, particularly among cancer patients with bone metastases [50]; therefore, OIAI is pharmacologically masked and reveals no clinical signs or symptoms. Postural symptoms suggestive of an adrenal disorder should also be assessed but they are not as frequent because OIAI does not affect aldosterone secretion [9]. Laboratory testing should be conducted and overall management should include glucocorticoid replacement and, ideally, the reduction or discontinuation of opioid therapy [13]. Since MME was found to be the sole predictor for the development of OIAI in one prospective study [14], it is recommended that patients taking ≥ 20 MME/day be monitored for potential OIAI [14].
Unlike other-induced endocrinopathies [51], clinical awareness of OIAI is limited. In a cross-sectional survey of internal medicine healthcare professionals who care for patients in chronic opioid therapy (n = 91 respondents out of 300), 19% stated they felt comfortable in their knowledge of opioid-associated side effects and 68% of non-endocrine specialists recognized OIAI as an opioid-induced endocrinopathy [33]. Significantly more endocrinologists (38%) correctly identified the symptoms of OIAI compared to other healthcare providers in the survey (9%), p < 0.01. The majority of respondents thought online resources, continuing education activities, and other training interventions could improve knowledge about how to diagnose and treat OIAI [33].
Despite limited awareness of OIAI, this form of central adrenal insufficiency is more prevalent than most clinicians appreciate. There is no consensus as to diagnostic testing, but frequently used are a combination of a morning serum cortisol test, ACTH test, and CST stimulation test. Note that cortisol tests at random times other than morning have less clinical utility for OIAI diagnosis [18]. Low ACTH suggests a central etiology and the CST stimulation test suggests a need for glucocorticoid replacement therapy. High doses of opioids can suppress the HPA axis and cause hypoadrenalism, but the adrenal glands may retain their ability to respond to corticotropin; sometimes normal or near-normal CST stimulation tests occur in patients with OIAI [18].
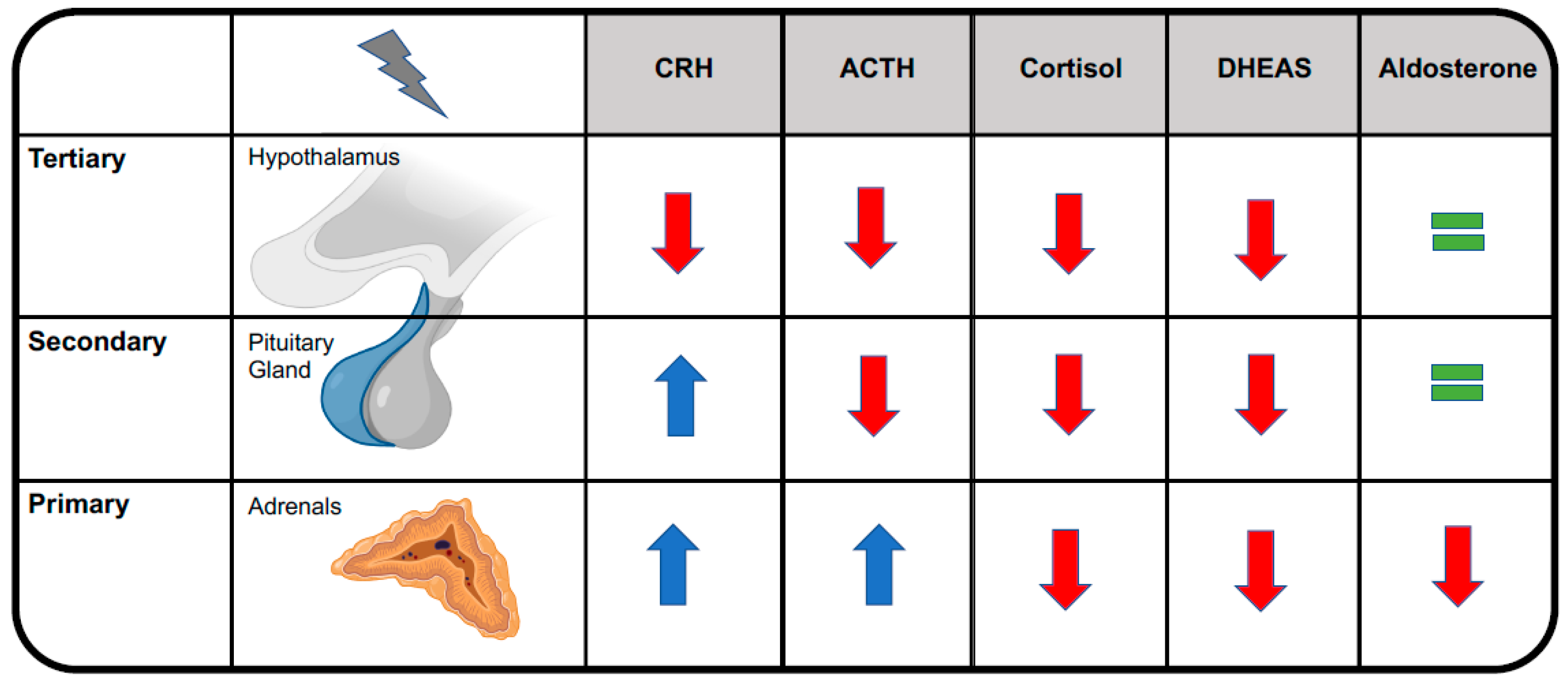
Central adrenal insufficiency usually does not reduce aldosterone levels, conversely to primary adrenal in-sufficiency, because aldosterone secretion by the adrenal glands is mainly regulated by the renin-angiotensin-aldosterone system [9]. See Figure 2. This is the reason why OIAI usually does not affect plasma levels of sodium and potassium and why it does not alter blood volume or blood pressure. Cardiovascular effects become clinically evident only during adrenal crisis, when they add to other OIAI symptoms, leading to a potentially life-threatening condition that may necessitate resuscitation [8]. See Figure 3.
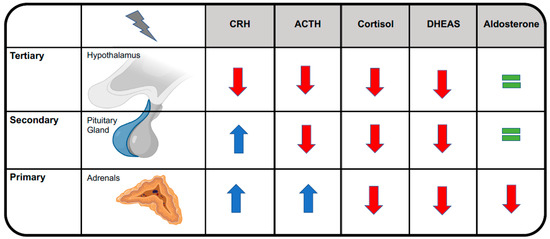
Figure 2.
Distinguishing among primary, secondary, and tertiary forms of adrenal insufficiency. ACTH, adrenocorticotropic hormone; CRH, corticotropin-releasing hormone; DHEAS, dehydroepiandrosterone sulfate.
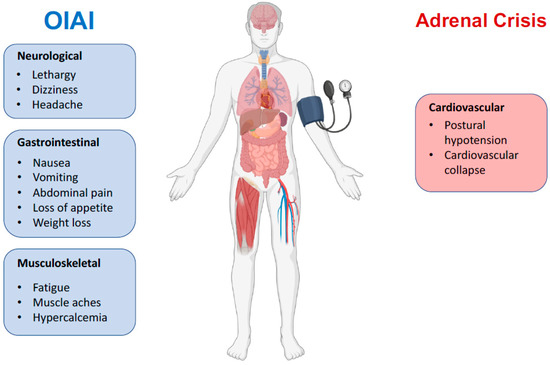
Figure 3.
Key clinical features of OIAI versus adrenal crisis. Opioids usually do not cause cardiovascular symptoms except in the context of adrenal crisis.
Central adrenal insufficiency may be caused by exogenous steroids, pituitary tumors, infiltrative diseases, head trauma, and apoplexy, so these must be ruled out in any diagnosis [18]. Cessation of opioid therapy resolves the condition [52], but this is not possible for all patients. When complete discontinuation cannot be achieved, the lowest effective dose of opioid should be used [53]. Opioid-tolerant patients should be tapered to avoid or reduce withdrawal [54].
While there are two case studies describing tramadol-induced adrenal insufficiency, differences between weak and strong opioids with respect to OIAI remain to be elucidated [52,55]. However, regarding other endocrine effects of opioids, such as OPIAD syndrome, opioids with reduced mu load, such as tapentadol, have been shown to have a lower impact on sex hormone concentrations compared with strong opioids, such as oxycodone and morphine [56]. Therefore, further studies are warranted to elucidate the clinical impact of different opioids on OIAI.
13. Experimental Evidence of the Peripheral Effects of Opioids
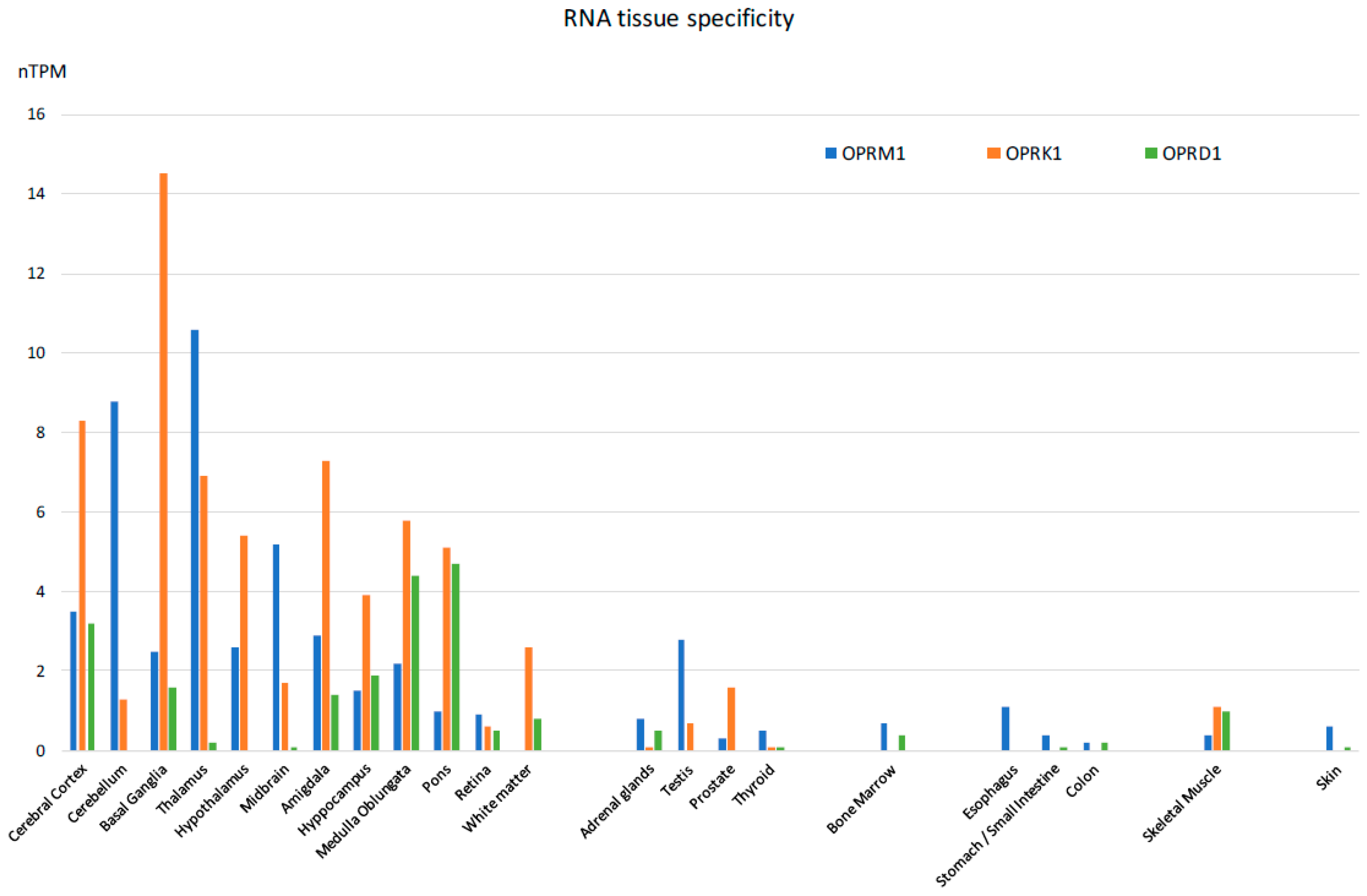
The majority of reported studies regarding the effects of opioids focus on the central nervous system. However, experimental evidence, mostly derived from studies of animal models, indicates that the expression of opioid receptors is not restricted to the central nervous system, [57] suggesting that opioid-induced effects can also be elicited by activation of these receptors outside of the CNS, in agreement with previous observations [58,59,60]. In fact, besides the classical localization in the central nervous system, they can also be detected both at the mRNA and protein levels in a wide variety of peripheral tissues of most species investigated so far, including humans. Specifically, they have been detected in the peripheral sensory neurons, where their activation exerts analgesic effects without inducing centrally mediated side effects [61]. They have been identified in testicular germ cells, where they are actively involved in spermatogenesis [62]. In the heart, they are thought to play a protective role in heart failure [63]. In the gastrointestinal system [64], they play a role in the regulation of gastrointestinal fluid secretion and gut motility [65]. In the immune system, they modulate immune response [66,67]. In the synovium of patients with joint trauma, osteoarthritis, and rheumatoid arthritis, it is believed they act locally to counteract pain and inflammation [68]. Last but not least, they are found in the mononuclear immune cells located inside and surrounding cancer tissues, where they appear to mediate a thermal hypoalgesic response [69,70]. An extensive list of mRNA distribution of the three opioid receptors (mu-1OPRM1, kappa 1-OPRK1, and delta 1 OPRD1) has been published on the Human Protein Atlas website and elsewhere [71,72]. The mRNA expressions of the three opioid receptor subtypes in human tissues appear in Figure 4.
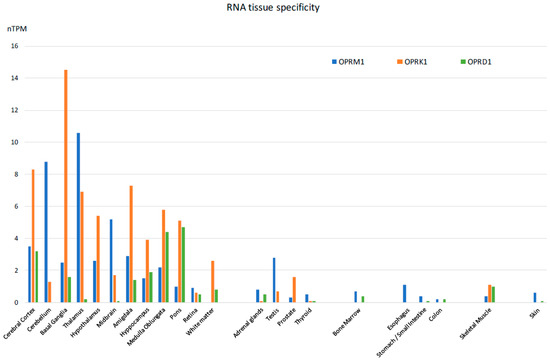
Figure 4.
Tissue-specific RNA expression of the three opioid receptors in humans [Adapted from [71]].
The expression of the genes coding for the three major subtypes of opioid receptors, namely the MOR, DOR, and KOR, was detected at high levels in the adrenal glands of rats [73], sheep [74], mice [75], pigs [76,77], bovines [78], guinea pigs [79], and humans [57].
The presence of these opioid receptors in the adrenal glands is consistent with a possible direct role of the opioids in modulating adrenal gland function. In addition, enkephalin and enkephalin-like peptides have been detected in the adrenal glands of different species with much higher quantities in bovine, canine, and human adrenals than in guinea pig or rat adrenals [80,81,82]. Relatively high levels of enkephalin have also been found in the adrenal medulla, contained within the same storage vesicles as catecholamines [83].
In this regard, experiments performed on an intact perfused rate adrenal preparation showed that endogenous opioid peptides may stimulate aldosterone and corticosterone secretion [84,85] and modulate adrenal vascular tone [86]. This experimental evidence was confirmed by other authors, who demonstrated that all of the opioid peptides tested, namely the β-endorphin, the Leu-enkephalin, the Met-enkephalin, and its long-acting analog D-ala2-met5-enkephalinamide (DALA) were capable of exerting a stimulatory effect on aldosterone secretion by cells of the zona glomerulosa, the most superficial layer of the adrenal cortex. With the exception of Leu-enkephalin, all of these were able to stimulate corticosterone secretion by the cells of the inner fasciculata/reticularis zones as well [87]. These stimulatory effects did not depend on or correlate to the stimulatory effects of ACTH. The effects of opioid peptides in peripheral tissues appear to be specifically mediated by the different opioid receptors, with the MOR able to mediate such effects on the cells of both the zona glomerulosa and inner zones, presumably through Ca2+ influx and activation of phospholipase C [88].
Evidence in support of the direct effects of opioids on the adrenal glands, independent from their effect on the HPA axis, has been reported in humans. Since such studies are complicated by the inevitable influence of the central effects on the central nervous system and, in particular, on the hypothalamus, a group of patients with hypothalamic-pituitary disconnection due to various pathological conditions (including craniopharyngioma, chordoma, suprasellar meningioma, or pituitary macroadenoma) was enrolled in a study [89]. In these patients, the administration of naloxone increased circulating cortisol but not ACTH levels when compared to control patients administered saline solution. This suggests that naloxone might exert a direct effect on cortisol secretion at the adrenal gland level. Another possible way to demonstrate the direct effect of opioids on adrenal glands is to isolate human adrenocortical cells [90]. In such a cell model, the β-endorphin stimulation exerted a steroidogenic effect, especially on the production of androstenedione and DHEA, greater than that observed after stimulation with ACTH.
The full physiological role of opioid peptides in the periphery remains to be elucidated. The presence of opioid receptors in the adrenal glands suggests a paracrine or autocrine role in adrenal function [57]. A more in-depth knowledge of the specific expression of opioid receptors and their ligands in the adrenal glands—both of which control stress, mood, pain, and inflammation—may allow for the design and development of peripherally restricted ligands that could offer analgesic benefits while avoiding the centrally mediated adverse effects of opioids.
14. Discussion
Opioids are used widely in the developed world, both by pain patients and recreational drug users, two distinct populations with some overlap. While many opioid-associated side effects, such as opioid-induced constipation, lethargy, nausea, vomiting, dizziness, and pruritus are well known, opioid-associated endocrinopathies are underappreciated [13]. In addition to those who take opioids medically or recreationally, OIAI may also occur in those on medication-assisted opioid therapy, that is, the population using methadone or buprenorphine in their recovery and rehabilitation. Although this represents a substantial and growing population of those who have prolonged exposure to opioids, there are almost no studies of the functional effects of such treatments [91]. The lack of a consensus definition of OIAI (or any opioid-associated endocrinopathy) has led to lack of awareness of the condition and no specific guidance as to how it should be diagnosed or treated.
While opioid mortality is frequently discussed in scientific literature as well as in the news media, opioid morbidity is less often discussed. Like OIAI, opioid morbidity remains a term that most clinicians understand but few can diagnose. Opioid morbidity would likely encompass conditions such as opioid-induced hyperalgesia [92], endocrinopathies [93], and disorders of bone metabolism associated with opioid use [94]. These are important topics that are not often the topic of investigation or inquiry. Mental disorders also occur concomitantly with opioid use disorder, but it is not clear if mental health conditions might precipitate opioid use or result from it. Since opioid clinical trials typically exclude patients with diagnosed mental health conditions, there is not a lot of evidence for clinicians to understand how opioids, particularly long-term use of recreational opioids, can affect such individuals [95]. Further study is urgently needed to better understand opioid-associated endocrinopathies and how to treat them.
15. Conclusions
OIAI is an under-recognized condition of adrenal insufficiency secondary to long-term exposure to opioids. OIAI can cause symptoms, and may result in potentially life-threatening adrenal crises, but can be managed. This management may be crucial for that subset of chronic opioid therapy patients who cannot or do not see the benefit of reducing or ceasing opioid treatment. Understanding how to diagnose and treat OIAI is crucial, particularly since opioids are widely used. Further controlled clinical studies are warranted to better analyze the central versus peripheral opioid effects on adrenal gland hormone production.
Author Contributions
F.C., M.R. and J.P. designed and supervised the review; F.C., J.A.K.L., M.S.S. and S.S. collected literature data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
No funds have been received for this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boudreau, D.; Von Korff, M.; Rutter, C.M.; Saunders, K.; Ray, G.T.; Sullivan, M.D.; Campbell, C.I.; Merrill, J.O.; Silverberg, M.J.; Banta-Green, C.; et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol. Drug Saf. 2009, 18, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Donegan, D.; Bancos, I. Opioid-Induced Adrenal Insufficiency. Mayo Clin. Proc. 2018, 93, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cunningham, J.L.; Gilliam, W.P.; Loukianova, L.; Donegan, D.M.; Bancos, I. Prevalence of Opioid-Induced Adrenal Insufficiency in Patients Taking Chronic Opioids. J. Clin. Endocrinol. Metab. 2020, 105, e3766–e3775. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Rhodin, A.; Stridsberg, M.; Gordh, T. Opioid Endocrinopathy: A Clinical Problem in Patients with Chronic Pain and Long-term Oral Opioid Treatment. Clin. J. Pain 2010, 26, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Fountas, A.; Van Uum, S.; Karavitaki, N. Opioid-induced endocrinopathies. Lancet Diabetes Endocrinol. 2020, 8, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Nenke, M.A.; Haylock, C.L.; Rankin, W.; Inder, W.J.; Gagliardi, L.; Eldridge, C.; Rolan, P.; Torpy, D.J. Low-dose hydrocortisone replacement improves wellbeing and pain tolerance in chronic pain patients with opioid-induced hypocortisolemic responses. A pilot randomized, placebo-controlled trial. Psychoneuroendocrinology 2015, 56, 157–167. [Google Scholar] [CrossRef]
- Husebye, E.S.; Pearce, S.H.; Krone, N.P.; Kämpe, O. Adrenal insufficiency. Lancet 2021, 397, 613–629. [Google Scholar] [CrossRef]
- Hahner, S.; Ross, R.J.; Arlt, W.; Bancos, I.; Burger-Stritt, S.; Torpy, D.J.; Husebye, E.S.; Quinkler, M. Adrenal insufficiency. Nat. Rev. Dis. Primers 2021, 7, 19. [Google Scholar] [CrossRef]
- Rushworth, R.L.; Torpy, D.J.; Falhammar, H. Adrenal Crisis. N. Engl. J. Med. 2019, 381, 852–861. [Google Scholar] [CrossRef]
- Price, S.M.; O’Donoghue, A.C.; Rizzo, L.; Sapru, S.; Aikin, K.J. Opioid Education and Prescribing Practices. J. Am. Board Fam. Med. 2021, 34, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Mojtabai, R. National trends in long-term use of prescription opioids. Pharmacoepidemiol. Drug Saf. 2018, 27, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Donegan, D. Opioid induced adrenal insufficiency: What is new? Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cunningham, J.; Gilliam, W.; Loukianova, L.; Donegan, D.; Bancos, I. SAT-383 Prevalence of Opioid Induced Adrenal Insuf-ficiency in Patients Taking Chronic Opioids. J. Endocr. Soc. 2019, 3, SAT-383. [Google Scholar] [CrossRef]
- Coluzzi, F.; Billeci, D.; Maggi, M.; Corona, G. Testosterone deficiency in non-cancer opioid-treated patients. J. Endocrinol. Investig. 2018, 41, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Sorbello, J.; Jang, C.; Torpy, D.J.; Inder, W.J. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur. J. Endocrinol. 2018, 179, 353–362. [Google Scholar] [CrossRef]
- Gibb, F.W.; Stewart, A.; Walker, B.R.; Strachan, M.W.J. Adrenal insufficiency in patients on long-term opioid analgesia. Clin. Endocrinol. 2016, 85, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Bessesen, D.; Cunningham, J. Secondary adrenal insufficiency: An insidious consequence of the opioid epidemic? Post-Grad. Med. J. 2021, 97, 432–433. [Google Scholar] [CrossRef]
- Gordin, Y.; Le, M.; Quarde, A.; Kinard, S.R.; Gordin, V. Recognizing Adrenal Insufficiency in Substance Use Disorder: A Case Study of Opioid-Related Suppression of Hypothalamo-Pituitary-Adrenal Axis. Pain Med. 2019, 20, 607. [Google Scholar]
- Li, T.; Donegan, D.; Hooten, W.; Bancos, I. Clinical Presentation and Outcomes of Opioid-Induced Adrenal Insufficiency. Endocr. Pract. 2020, 26, 1291–1297. [Google Scholar] [CrossRef]
- Gruber, L.M.; Bancos, I. Secondary Adrenal Insufficiency: Recent Updates and New Directions for Diagnosis and Management. Endocr. Pract. 2022, 28, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.R.; Batista, R.L.; Biscotto, I.; Carvalho, L.R. Central adrenal insufficiency: Who, when, and how? From the evidence to the controversies—An exploratory review. Arch. Endocrinol. Metab. 2022, 66, 541–550. [Google Scholar] [CrossRef]
- Bleicken, B.; Ventz, M.; Quinkler, M.; Hahner, S. Delayed Diagnosis of Adrenal Insufficiency Is Common: A Cross-Sectional Study in 216 Patients. Am. J. Med Sci. 2010, 339, 525–531. [Google Scholar] [CrossRef]
- Grossman, A.B. Clinical Review#: The diagnosis and management of central hypoadrenalism. J. Clin. Endocrinol. Metab. 2010, 95, 4855–4863. [Google Scholar]
- Colling, C.; Nachtigall, L.; Biller, B.M.K.; Miller, K.K. The biochemical diagnosis of adrenal insufficiency with modern cortisol assays: Reappraisal in the setting of opioid exposure and hospitalization. Clin. Endocrinol. 2022, 96, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, J.C. Glucocorticoids: Exemplars of multi-tasking. Br. J. Pharmacol. 2006, 147, S258–S268. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Volpe, A.; Farci, G.; Petraglia, F.; Porro, C.A.; Barbieri, G.; Cioni, A.; Balestrieri, A.; Genazzani, A.R. Hypothalamus-pituitary-adrenal axis of heroin addicts. Drug Alcohol Depend. 1985, 15, 361–366. [Google Scholar] [CrossRef]
- Facchinetti, F.; Grasso, A.; Petraglia, F.; Parrini, D.; Volpe, A.; Genazzani, A. Impaired circadian rhythmicity of beta-lipotropin be-ta-endorphin and ACTH in heroin addicts. Acta Endocrinol. 1984, 105, 149–155. [Google Scholar]
- Krieger, D.T.; Allen, W.; Rizzo, F.; Krieger, H.P. Characterization of the Normal Temporal Pattern of Plasma Corticosteroid Levels. J. Clin. Endocrinol. Metab. 1971, 32, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef]
- Ospina, N.S.; Al Nofal, A.; Bancos, I.; Javed, A.; Benkhadra, K.; Kapoor, E.; Lteif, A.N.; Natt, N.; Murad, M.H. ACTH Stimulation Tests for the Diagnosis of Adrenal Insufficiency: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2016, 101, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Baid, S.; Calis, K.; Raff, H.; Sinaii, N.; Nieman, L. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur. J. Endocrinol. 2010, 162, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Z.I.; Bancos, I.; Donegan, D. Current knowledge and practices of heath care professionals on opioid-induced adrenal insufficiency. Endocr. Pract. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Lee, A.S.; Twigg, S.M. Opioid-induced secondary adrenal insufficiency presenting as hypercalcaemia. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 150035. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Pocket Guide: Tapering Opioids for Chronic Pain. CDC. 2018. Available online: https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf (accessed on 15 April 2019).
- Gadelha, M.R.; Karavitaki, N.; Fudin, J.; Bettinger, J.J.; Raff, H.; Ben-Shlomo, A. Opioids and pituitary function: Expert opinion. Pituitary 2022, 25, 52–63. [Google Scholar] [CrossRef]
- Kondo, A.; Murakami, T.; Fujii, T.; Tatsumi, M.; Ueda-Sakane, Y.; Ueda, Y.; Inagaki, N. Opioid-induced adrenal insufficiency in transdermal fentanyl treatment: A revisited di-agnosis in clinical setting. Endocr. J. 2022, 69, 209–215. [Google Scholar] [CrossRef]
- Arlt, W.; Baldeweg, S.E.; Pearce, S.H.S.; Simpson, H.L. Endocrinology in the time of COVID-19: Management of adrenal insufficiency. Eur. J. Endocrinol. 2020, 183, G25–G32. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Nishigaki, T.; Kuramoto, N.; Oh, K.; Konishi, H. Secondary Adrenal Insufficiency After COVID-19 Diagnosed by Insulin Tolerance Test and Corticotropin-Releasing Hormone Test. Cureus 2022, 14, e23021. [Google Scholar] [CrossRef]
- Naik, D.; Suryadevara, V.; Merugu, C.; Perumal, N.; Sahoo, J.; Kamalanathan, S. Adrenal crisis in a patient with APS2 due to COVID-19: A case report. J. Fam. Med. Prim. Care 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Society for Endocrinology. Adrenal Crisis during COVID-19 Pandemic. Society for Endocrinology. 2022. Available online: https://www.endocrinology.org/clinical-practice/clinical-guidance/adrenal-crisis/covid-19-adrenal-crisis-information/ (accessed on 15 December 2022).
- Beshay, L.; Yang, Q. ODP580 A Case of New Onset Primary Adrenal Insufficiency in COVID Infection. J. Endocr. Soc. 2022, 6, A74. [Google Scholar] [CrossRef]
- Kanczkowski, W.; Beuschlein, F.; Bornstein, S.R. Is there a role for the adrenal glands in long COVID? Nat. Rev. Endocrinol. 2022, 18, 451–452. [Google Scholar] [CrossRef]
- Bornstein, S.R. Predisposing Factors for Adrenal Insufficiency. N. Engl. J. Med. 2009, 360, 2328–2339. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Ledderose, S.; Bartsch, H.; Sun, N.; Soliman, S.; Märkl, B.; Rudelius, M. Adrenal tropism of SARS-CoV-2 and adrenal findings in a post-mortem case series of patients with severe fatal COVID-19. Nat. Commun. 2022, 13, 1589. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.W. DHEAS deficiency during consumption of sustained-action prescribed opioids: Evidence for opioid-induced in-hibition of adrenal androgen production. J. Pain 2006, 7, 901–907. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Torpy, D.J. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef]
- Mercadante, S.; Coluzzi, F. Factors Influencing Pain Expression in Patients with Cancer: An Expert Opinion. Pain Ther. 2021, 10, 765–775. [Google Scholar] [CrossRef]
- Coluzzi, F.; Scerpa, M.S.; Pergolizzi, J. Naldemedine: A New Option for OIBD. J. Pain Res. 2020, 13, 1209–1222. [Google Scholar] [CrossRef]
- Coluzzi, F.; Di Bussolo, E.; Mandatori, I.; Mattia, C. Bone metastatic disease: Taking aim at new therapeutic targets. Curr. Med. Chem. 2011, 18, 3093–3115. [Google Scholar] [CrossRef]
- Coluzzi, F.; Mattia, C.; Raffa, R.R.; Pergolizzi, J. The unsolved case of “bone-impairing analgesics”: The endocrine effects of opioids on bone metabolism. Ther. Clin. Risk Manag. 2015, 11, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Chan, S.; Rolfe, C.; Jones, T.H. Tramadol-induced adrenal insufficiency. Eur. J. Clin. Pharmacol. 2011, 67, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Morlion, B.J.; Mueller-Lissner, S.A.; Vellucci, R.; Leppert, W.; Coffin, B.C.; Dickerson, S.L.; O’Brien, T. Oral Prolonged-Release Oxycodone/Naloxone for Managing Pain and Opi-oid-Induced Constipation: A Review of the Evidence. Pain Pract. 2018, 18, 647–665. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Bifulco, F.; Cuomo, A.; Dauri, M.; Leonardi, C.; Melotti, R.M.; Natoli, S.; Romualdi, P.; Savoia, G.; Corcione, A. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag. 2017, 13, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Gonzalvo Gómez, I.; Ortiz Bescós, V.; Luz Miguel, S.; Isanta Pomar, C. Adrenal insufficiency in a patient treated with tramadol. Aten Primaria 2019, 51, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, G.; Göhler, K.; Etropolski, M.; Steigerwald, I.; Pergolizzi, J.; Kim, M.; Vorsanger, G. Does tapentadol affect sex hormone concentrations differently from morphine and oxycodone? An initial assessment and possible implications for opioid-induced androgen deficiency. J. Opioid Manag. 2015, 11, 211–227. [Google Scholar] [CrossRef]
- Peng, J.; Sarkar, S.; Chang, S.L. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012, 124, 223–228. [Google Scholar] [CrossRef]
- Stein, C. Peripheral mechanisms of opioid analgesia. Anesth. Analg. 1993, 76, 182–191. [Google Scholar] [CrossRef]
- Stein, C.; Lang, L.J. Peripheral mechanisms of opioid analgesia. Curr. Opin. Pharmacol. 2009, 9, 3–8. [Google Scholar] [CrossRef]
- Tegeder, I.; Meier, S.; Burian, M.; Schmidt, H.; Geisslinger, G.; Lötsch, J. Peripheral opioid analgesia in experimental human pain models. Brain 2003, 126, 1092–1102. [Google Scholar] [CrossRef]
- Schmidt, Y.; Machelska, H. Immunohistochemical Analysis of Opioid Receptors in Peripheral Tissues. Methods Mol. Biol. 2021, 2201, 71–82. [Google Scholar] [CrossRef]
- Estomba, H.; Hoyos, I.M.; Gianzo, M.; Arenaza, I.U.; Casis, L.; Irazusta, J.; Subirán, N. Expression and Localization of Opioid Receptors in Male Germ Cells and the Implication for Mouse Spermatogenesis. PLoS ONE 2016, 11, e0152162. [Google Scholar] [CrossRef]
- He, S.; Jin, S.; Yang, W.; Pan, Y.; Huang, J.; Zhang, S.; Zhang, L.; Zhang, Y. Cardiac μ-opioid receptor contributes to opioid-induced cardioprotection in chronic heart failure. Br. J. Anaesth. 2018, 121, 26–37. [Google Scholar] [CrossRef]
- Hedner, T.; Cassuto, J. Opioids and Opioid Receptors in Peripheral Tissues. Scand. J. Gastroenterol. 1987, 22, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, G.M. Enteric neuropeptides: Role in neuromuscular activity of the gut. Trends Pharmacol. Sci. 1985, 6, 214–218. [Google Scholar] [CrossRef]
- Ninković, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef]
- Brejchova, J.; Holan, V.; Svoboda, P. Expression of Opioid Receptors in Cells of the Immune System. Int. J. Mol. Sci. 2020, 22, 315. [Google Scholar] [CrossRef]
- Mousa, S.A.; Straub, R.H.; Schäfer, M.; Stein, C. Beta-endorphin, Met-enkephalin and corresponding opioid receptors within syn-ovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 871–879. [Google Scholar] [CrossRef]
- Baamonde, A.; Lastra, A.; Juárez, L.; García, V.; Hidalgo, A.; Menéndez, L. Effects of the local administration of selective mu-, del-ta-and kappa-opioid receptor agonists on osteosarcoma-induced hyperalgesia. Naunyn Schmiedeberg Arch. Pharmacol. 2005, 372, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Baamonde, A.; Lastra, A.; Juárez, L.; García-Suárez, O.; Meana, Á.; Hidalgo, A.; Menéndez, L. Endogenous beta-endorphin induces thermal analgesia at the initial stages of a murine osteosarcoma. Peptides 2006, 27, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Pontén, F. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Fricker, L.D.; Margolis, E.B.; Gomes, I.; Devi, L.A. Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions. Mol. Pharmacol. 2020, 98, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.; Hope, P.; Pyle, D. Tissue Distribution of Opioid Receptor Gene Expression in the Rat. Biochem. Biophys. Res. Commun. 1996, 218, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała-Koziec, K.; Dziedzicka-Wasylewska, M.; Oeltgen, P.; Zubel-Łojek, J.; Latacz, A.; Ocłon, E. The Effect of CRH, Dexame-thasone and Naltrexone on the Mu, Delta and Kappa Opioid Receptor Agonist Binding in Lamb Hypothalamic-Pituitary-Adrenal Axis. Folia Biol. 2015, 63, 187–193. [Google Scholar] [CrossRef]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Madison, D.V.; Krasnow, M.A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 2015, 527, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Krazinski, B.; Koziorowski, M.; Brzuzan, P.; Okrasa, S. The expression of genes encoding opioid precursors and the influence of opioid receptor agonists on steroidogenesis in porcine adrenocortical cells in vitro. J. Physiol. Pharmacol. 2011, 62, 461–468. [Google Scholar]
- Kitamura, G.; Ohta, T.; Kai, T.; Kon, Y.; Ito, S. Inhibitory effects of opioids on voltage-dependent Ca2+ channels and catecholamine secretion in cultured porcine adrenal chromaffin cells. Brain Res. 2002, 942, 11–22. [Google Scholar] [CrossRef]
- Kilpatrick, D.L.; Lewis, R.V.; Stein, S.; Udenfriend, S. Release of enkephalins and enkephalin-containing polypeptides from perfused beef adrenal glands. Proc. Natl. Acad. Sci. USA 1980, 77, 7473–7475. [Google Scholar] [CrossRef]
- Sasahara, I. Catecholamine and ATP metabolites released from perfused adrenal glands of guinea-pig. Jpn. J. Vetinary Res. 2000, 48, 63. [Google Scholar]
- Yang, H.-Y.; Hexum, T.; Costa, E. Opioid peptides in adrenal gland. Life Sci. 1980, 27, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Viveros, O.H.; DiLiberto, E.J., Jr.; Hazum, E.; Chang, K.J. Opiate-like materials in the adrenal medulla: Evidence for storage and secretion with catecholamines. Mol. Pharmacol. 1979, 16, 1101–1108. [Google Scholar]
- Barron, B.; Hexum, T. Modulation of bovine adrenal gland secretion by etorphine and diprenorphine. Life Sci. 1986, 38, 935–940. [Google Scholar] [CrossRef] [PubMed]
- North, R.A.; Egan, T.M. Actions and distributions of opioid peptides in peripheral tissues. Br. Med. Bull. 1983, 39, 71–75. [Google Scholar] [CrossRef]
- Hinson, J.P.; Vinson, G.P.; Whitehouse, B.J.; Price, G. Control of zona glomerulosa function in the isolated perfused rat adrenal gland in situ. J. Endocrinol. 1985, 104, 387–395. [Google Scholar] [CrossRef]
- Hinson, J.P.; Cameron, L.A.; Purbrick, A.; Kapas, S. The role of neuropeptides in the regulation of adrenal zona glomerulosa function: Effects of substance P, neuropeptide Y, neurotensin, Met-enkephalin, Leu-enkephalin and corticotrophin-releasing hormone on aldosterone secretion in the intact perfused rat adrenal. J. Endocrinol. 1994, 140, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.; Cameron, L.; Purbrick, A.; Kapas, S. The role of neuropeptides in the regulation of adrenal vascular tone: Effects of vasoactive intestinal polypeptide, substance P, neuropeptide Y, neurotensin, Met-enkephalin, and Leu-enkephalin on perfusion medium flow rate in the intact perfused rat adrenal. Regul. Pept. 1994, 51, 55–61. [Google Scholar] [CrossRef]
- Kapas, S.; Purbrick, A.; Hinson, J.P. Action of opioid peptides on the rat adrenal cortex: Stimulation of steroid secretion through a specific mu opioid receptor. J. Endocrinol. 1995, 144, 503–510. [Google Scholar] [CrossRef]
- Smart, D.; Smith, G.; Lambert, D.G. Mu-opioids activate phospholipase C in SH-SY5Y human neuroblastoma cells via calcium-channel opening. Biochem. J. 1995, 305, 577–581. [Google Scholar] [CrossRef]
- Coiro, V.; Volpi, R.; Stella, A.; Venturi, N.; Chiodera, P. Stimulatory effect of naloxone on plasma cortisol in human: Possible direct stimulatory action at the adrenal cortex. Regul. Pept. 2011, 166, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.; Fearon, U.; Cunningham, S.K.; McKenna, T.J. The steroidogenic effects of beta-endorphin and joining peptide: A potential role in the modulation of adrenal androgen production. J. Endocrinol. 1996, 151, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.A.; Raaen, L.; Chen, C.; Azhar, G.; Shahidinia, N.; Shen, M.; Maksabedian, E.; Shanman, R.M.; Newberry, S.; Hempel, S. Effects of medication assisted treatment (MAT) for opioid use disorder on functional outcomes: A systematic review. J. Subst. Abus. Treat. 2018, 89, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, E.; Singh, D.; Dobariya, V.; Akhigbe, E.J.; Gilkerson, C. Opioid-Induced Hyperalgesia in a Cancer Patient on High-Dose Methadone Maintenance Therapy: A Case for Subspecialty Opioid Use Disorder Primary Care. Cureus 2020, 12, e12345. [Google Scholar] [CrossRef]
- Gudin, J.A.; Laitman, A.; Nalamachu, S. Opioid Related Endocrinopathy: Table 1. Pain Med. 2015, 16, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Scerpa, M.S.; Centanni, M. The Effect of Opiates on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.D.; Edlund, M.J.; Zhang, L.; Unützer, J.; Wells, K.B. Association Between Mental Health Disorders, Problem Drug Use, and Regular Prescription Opioid Use. Arch. Intern. Med. 2006, 166, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).