A Closer Look at Opioid-Induced Adrenal Insufficiency: A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Results
4. Epidemiology of OIAI
5. Risk Factors for OIAI
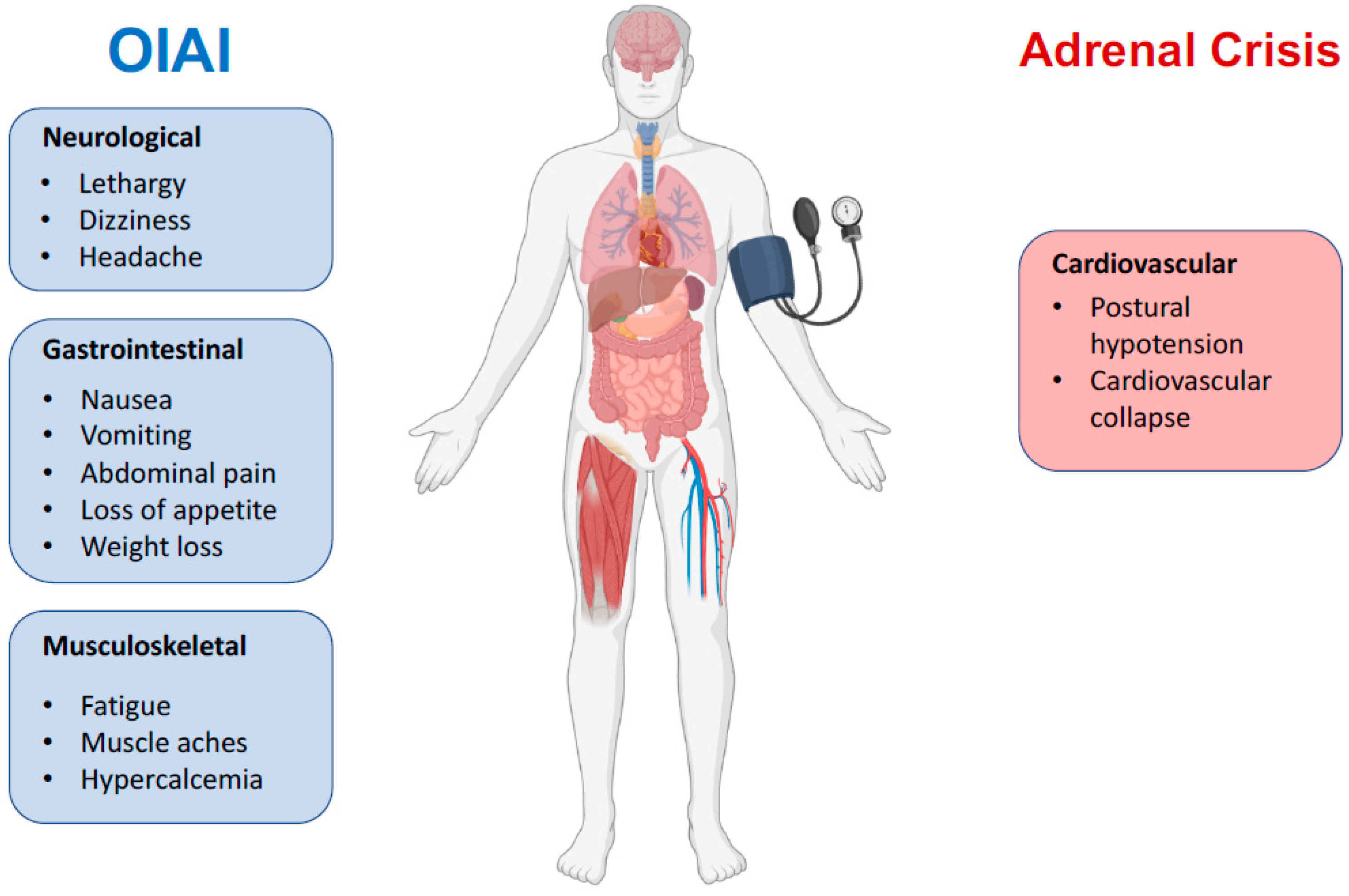
6. Diagnosis
7. The Clinical Course
8. Management of OIAI
9. Adrenal Insufficiency in the COVID Era
10. Adrenal Androgens
11. Adrenal Crisis
12. Clinical Considerations for Daily Practice
13. Experimental Evidence of the Peripheral Effects of Opioids
14. Discussion
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boudreau, D.; Von Korff, M.; Rutter, C.M.; Saunders, K.; Ray, G.T.; Sullivan, M.D.; Campbell, C.I.; Merrill, J.O.; Silverberg, M.J.; Banta-Green, C.; et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol. Drug Saf. 2009, 18, 1166–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donegan, D.; Bancos, I. Opioid-Induced Adrenal Insufficiency. Mayo Clin. Proc. 2018, 93, 937–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Cunningham, J.L.; Gilliam, W.P.; Loukianova, L.; Donegan, D.M.; Bancos, I. Prevalence of Opioid-Induced Adrenal Insufficiency in Patients Taking Chronic Opioids. J. Clin. Endocrinol. Metab. 2020, 105, e3766–e3775. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Rhodin, A.; Stridsberg, M.; Gordh, T. Opioid Endocrinopathy: A Clinical Problem in Patients with Chronic Pain and Long-term Oral Opioid Treatment. Clin. J. Pain 2010, 26, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Fountas, A.; Van Uum, S.; Karavitaki, N. Opioid-induced endocrinopathies. Lancet Diabetes Endocrinol. 2020, 8, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Nenke, M.A.; Haylock, C.L.; Rankin, W.; Inder, W.J.; Gagliardi, L.; Eldridge, C.; Rolan, P.; Torpy, D.J. Low-dose hydrocortisone replacement improves wellbeing and pain tolerance in chronic pain patients with opioid-induced hypocortisolemic responses. A pilot randomized, placebo-controlled trial. Psychoneuroendocrinology 2015, 56, 157–167. [Google Scholar] [CrossRef]
- Husebye, E.S.; Pearce, S.H.; Krone, N.P.; Kämpe, O. Adrenal insufficiency. Lancet 2021, 397, 613–629. [Google Scholar] [CrossRef]
- Hahner, S.; Ross, R.J.; Arlt, W.; Bancos, I.; Burger-Stritt, S.; Torpy, D.J.; Husebye, E.S.; Quinkler, M. Adrenal insufficiency. Nat. Rev. Dis. Primers 2021, 7, 19. [Google Scholar] [CrossRef]
- Rushworth, R.L.; Torpy, D.J.; Falhammar, H. Adrenal Crisis. N. Engl. J. Med. 2019, 381, 852–861. [Google Scholar] [CrossRef]
- Price, S.M.; O’Donoghue, A.C.; Rizzo, L.; Sapru, S.; Aikin, K.J. Opioid Education and Prescribing Practices. J. Am. Board Fam. Med. 2021, 34, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Mojtabai, R. National trends in long-term use of prescription opioids. Pharmacoepidemiol. Drug Saf. 2018, 27, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Donegan, D. Opioid induced adrenal insufficiency: What is new? Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cunningham, J.; Gilliam, W.; Loukianova, L.; Donegan, D.; Bancos, I. SAT-383 Prevalence of Opioid Induced Adrenal Insuf-ficiency in Patients Taking Chronic Opioids. J. Endocr. Soc. 2019, 3, SAT-383. [Google Scholar] [CrossRef]
- Coluzzi, F.; Billeci, D.; Maggi, M.; Corona, G. Testosterone deficiency in non-cancer opioid-treated patients. J. Endocrinol. Investig. 2018, 41, 1377–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprecht, A.; Sorbello, J.; Jang, C.; Torpy, D.J.; Inder, W.J. Secondary adrenal insufficiency and pituitary dysfunction in oral/transdermal opioid users with non-cancer pain. Eur. J. Endocrinol. 2018, 179, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Gibb, F.W.; Stewart, A.; Walker, B.R.; Strachan, M.W.J. Adrenal insufficiency in patients on long-term opioid analgesia. Clin. Endocrinol. 2016, 85, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Bessesen, D.; Cunningham, J. Secondary adrenal insufficiency: An insidious consequence of the opioid epidemic? Post-Grad. Med. J. 2021, 97, 432–433. [Google Scholar] [CrossRef]
- Gordin, Y.; Le, M.; Quarde, A.; Kinard, S.R.; Gordin, V. Recognizing Adrenal Insufficiency in Substance Use Disorder: A Case Study of Opioid-Related Suppression of Hypothalamo-Pituitary-Adrenal Axis. Pain Med. 2019, 20, 607. [Google Scholar]
- Li, T.; Donegan, D.; Hooten, W.; Bancos, I. Clinical Presentation and Outcomes of Opioid-Induced Adrenal Insufficiency. Endocr. Pract. 2020, 26, 1291–1297. [Google Scholar] [CrossRef]
- Gruber, L.M.; Bancos, I. Secondary Adrenal Insufficiency: Recent Updates and New Directions for Diagnosis and Management. Endocr. Pract. 2022, 28, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, M.R.; Batista, R.L.; Biscotto, I.; Carvalho, L.R. Central adrenal insufficiency: Who, when, and how? From the evidence to the controversies—An exploratory review. Arch. Endocrinol. Metab. 2022, 66, 541–550. [Google Scholar] [CrossRef]
- Bleicken, B.; Ventz, M.; Quinkler, M.; Hahner, S. Delayed Diagnosis of Adrenal Insufficiency Is Common: A Cross-Sectional Study in 216 Patients. Am. J. Med Sci. 2010, 339, 525–531. [Google Scholar] [CrossRef]
- Grossman, A.B. Clinical Review#: The diagnosis and management of central hypoadrenalism. J. Clin. Endocrinol. Metab. 2010, 95, 4855–4863. [Google Scholar]
- Colling, C.; Nachtigall, L.; Biller, B.M.K.; Miller, K.K. The biochemical diagnosis of adrenal insufficiency with modern cortisol assays: Reappraisal in the setting of opioid exposure and hospitalization. Clin. Endocrinol. 2022, 96, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, J.C. Glucocorticoids: Exemplars of multi-tasking. Br. J. Pharmacol. 2006, 147, S258–S268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchinetti, F.; Volpe, A.; Farci, G.; Petraglia, F.; Porro, C.A.; Barbieri, G.; Cioni, A.; Balestrieri, A.; Genazzani, A.R. Hypothalamus-pituitary-adrenal axis of heroin addicts. Drug Alcohol Depend. 1985, 15, 361–366. [Google Scholar] [CrossRef]
- Facchinetti, F.; Grasso, A.; Petraglia, F.; Parrini, D.; Volpe, A.; Genazzani, A. Impaired circadian rhythmicity of beta-lipotropin be-ta-endorphin and ACTH in heroin addicts. Acta Endocrinol. 1984, 105, 149–155. [Google Scholar]
- Krieger, D.T.; Allen, W.; Rizzo, F.; Krieger, H.P. Characterization of the Normal Temporal Pattern of Plasma Corticosteroid Levels. J. Clin. Endocrinol. Metab. 1971, 32, 266–284. [Google Scholar] [CrossRef] [PubMed]
- Fleseriu, M.; Hashim, I.A.; Karavitaki, N.; Melmed, S.; Murad, M.H.; Salvatori, R.; Samuels, M.H. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 3888–3921. [Google Scholar] [CrossRef] [Green Version]
- Ospina, N.S.; Al Nofal, A.; Bancos, I.; Javed, A.; Benkhadra, K.; Kapoor, E.; Lteif, A.N.; Natt, N.; Murad, M.H. ACTH Stimulation Tests for the Diagnosis of Adrenal Insufficiency: Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2016, 101, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, M.; Baid, S.; Calis, K.; Raff, H.; Sinaii, N.; Nieman, L. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur. J. Endocrinol. 2010, 162, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeed, Z.I.; Bancos, I.; Donegan, D. Current knowledge and practices of heath care professionals on opioid-induced adrenal insufficiency. Endocr. Pract. 2019, 25, 1012–1021. [Google Scholar] [CrossRef]
- Lee, A.S.; Twigg, S.M. Opioid-induced secondary adrenal insufficiency presenting as hypercalcaemia. Endocrinol. Diabetes Metab. Case Rep. 2015, 2015, 150035. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Pocket Guide: Tapering Opioids for Chronic Pain. CDC. 2018. Available online: https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf (accessed on 15 April 2019).
- Gadelha, M.R.; Karavitaki, N.; Fudin, J.; Bettinger, J.J.; Raff, H.; Ben-Shlomo, A. Opioids and pituitary function: Expert opinion. Pituitary 2022, 25, 52–63. [Google Scholar] [CrossRef]
- Kondo, A.; Murakami, T.; Fujii, T.; Tatsumi, M.; Ueda-Sakane, Y.; Ueda, Y.; Inagaki, N. Opioid-induced adrenal insufficiency in transdermal fentanyl treatment: A revisited di-agnosis in clinical setting. Endocr. J. 2022, 69, 209–215. [Google Scholar] [CrossRef]
- Arlt, W.; Baldeweg, S.E.; Pearce, S.H.S.; Simpson, H.L. Endocrinology in the time of COVID-19: Management of adrenal insufficiency. Eur. J. Endocrinol. 2020, 183, G25–G32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamazaki, K.; Nishigaki, T.; Kuramoto, N.; Oh, K.; Konishi, H. Secondary Adrenal Insufficiency After COVID-19 Diagnosed by Insulin Tolerance Test and Corticotropin-Releasing Hormone Test. Cureus 2022, 14, e23021. [Google Scholar] [CrossRef]
- Naik, D.; Suryadevara, V.; Merugu, C.; Perumal, N.; Sahoo, J.; Kamalanathan, S. Adrenal crisis in a patient with APS2 due to COVID-19: A case report. J. Fam. Med. Prim. Care 2022, 11, 2228. [Google Scholar] [CrossRef] [PubMed]
- Society for Endocrinology. Adrenal Crisis during COVID-19 Pandemic. Society for Endocrinology. 2022. Available online: https://www.endocrinology.org/clinical-practice/clinical-guidance/adrenal-crisis/covid-19-adrenal-crisis-information/ (accessed on 15 December 2022).
- Beshay, L.; Yang, Q. ODP580 A Case of New Onset Primary Adrenal Insufficiency in COVID Infection. J. Endocr. Soc. 2022, 6, A74. [Google Scholar] [CrossRef]
- Kanczkowski, W.; Beuschlein, F.; Bornstein, S.R. Is there a role for the adrenal glands in long COVID? Nat. Rev. Endocrinol. 2022, 18, 451–452. [Google Scholar] [CrossRef]
- Bornstein, S.R. Predisposing Factors for Adrenal Insufficiency. N. Engl. J. Med. 2009, 360, 2328–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, T.; Ledderose, S.; Bartsch, H.; Sun, N.; Soliman, S.; Märkl, B.; Rudelius, M. Adrenal tropism of SARS-CoV-2 and adrenal findings in a post-mortem case series of patients with severe fatal COVID-19. Nat. Commun. 2022, 13, 1589. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.W. DHEAS deficiency during consumption of sustained-action prescribed opioids: Evidence for opioid-induced in-hibition of adrenal androgen production. J. Pain 2006, 7, 901–907. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Allolio, B.; Arlt, W.; Barthel, A.; Don-Wauchope, A.; Hammer, G.D.; Torpy, D.J. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 364–389. [Google Scholar] [CrossRef] [Green Version]
- Mercadante, S.; Coluzzi, F. Factors Influencing Pain Expression in Patients with Cancer: An Expert Opinion. Pain Ther. 2021, 10, 765–775. [Google Scholar] [CrossRef]
- Coluzzi, F.; Scerpa, M.S.; Pergolizzi, J. Naldemedine: A New Option for OIBD. J. Pain Res. 2020, 13, 1209–1222. [Google Scholar] [CrossRef]
- Coluzzi, F.; Di Bussolo, E.; Mandatori, I.; Mattia, C. Bone metastatic disease: Taking aim at new therapeutic targets. Curr. Med. Chem. 2011, 18, 3093–3115. [Google Scholar] [CrossRef]
- Coluzzi, F.; Mattia, C.; Raffa, R.R.; Pergolizzi, J. The unsolved case of “bone-impairing analgesics”: The endocrine effects of opioids on bone metabolism. Ther. Clin. Risk Manag. 2015, 11, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debono, M.; Chan, S.; Rolfe, C.; Jones, T.H. Tramadol-induced adrenal insufficiency. Eur. J. Clin. Pharmacol. 2011, 67, 865–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morlion, B.J.; Mueller-Lissner, S.A.; Vellucci, R.; Leppert, W.; Coffin, B.C.; Dickerson, S.L.; O’Brien, T. Oral Prolonged-Release Oxycodone/Naloxone for Managing Pain and Opi-oid-Induced Constipation: A Review of the Evidence. Pain Pract. 2018, 18, 647–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coluzzi, F.; Bifulco, F.; Cuomo, A.; Dauri, M.; Leonardi, C.; Melotti, R.M.; Natoli, S.; Romualdi, P.; Savoia, G.; Corcione, A. The challenge of perioperative pain management in opioid-tolerant patients. Ther Clin Risk Manag. 2017, 13, 1163–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalvo Gómez, I.; Ortiz Bescós, V.; Luz Miguel, S.; Isanta Pomar, C. Adrenal insufficiency in a patient treated with tramadol. Aten Primaria 2019, 51, 512–513. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, G.; Göhler, K.; Etropolski, M.; Steigerwald, I.; Pergolizzi, J.; Kim, M.; Vorsanger, G. Does tapentadol affect sex hormone concentrations differently from morphine and oxycodone? An initial assessment and possible implications for opioid-induced androgen deficiency. J. Opioid Manag. 2015, 11, 211–227. [Google Scholar] [CrossRef]
- Peng, J.; Sarkar, S.; Chang, S.L. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend. 2012, 124, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Stein, C. Peripheral mechanisms of opioid analgesia. Anesth. Analg. 1993, 76, 182–191. [Google Scholar] [CrossRef]
- Stein, C.; Lang, L.J. Peripheral mechanisms of opioid analgesia. Curr. Opin. Pharmacol. 2009, 9, 3–8. [Google Scholar] [CrossRef]
- Tegeder, I.; Meier, S.; Burian, M.; Schmidt, H.; Geisslinger, G.; Lötsch, J. Peripheral opioid analgesia in experimental human pain models. Brain 2003, 126, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, Y.; Machelska, H. Immunohistochemical Analysis of Opioid Receptors in Peripheral Tissues. Methods Mol. Biol. 2021, 2201, 71–82. [Google Scholar] [CrossRef]
- Estomba, H.; Hoyos, I.M.; Gianzo, M.; Arenaza, I.U.; Casis, L.; Irazusta, J.; Subirán, N. Expression and Localization of Opioid Receptors in Male Germ Cells and the Implication for Mouse Spermatogenesis. PLoS ONE 2016, 11, e0152162. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Jin, S.; Yang, W.; Pan, Y.; Huang, J.; Zhang, S.; Zhang, L.; Zhang, Y. Cardiac μ-opioid receptor contributes to opioid-induced cardioprotection in chronic heart failure. Br. J. Anaesth. 2018, 121, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Hedner, T.; Cassuto, J. Opioids and Opioid Receptors in Peripheral Tissues. Scand. J. Gastroenterol. 1987, 22, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, G.M. Enteric neuropeptides: Role in neuromuscular activity of the gut. Trends Pharmacol. Sci. 1985, 6, 214–218. [Google Scholar] [CrossRef]
- Ninković, J.; Roy, S. Role of the mu-opioid receptor in opioid modulation of immune function. Amino Acids 2013, 45, 9–24. [Google Scholar] [CrossRef]
- Brejchova, J.; Holan, V.; Svoboda, P. Expression of Opioid Receptors in Cells of the Immune System. Int. J. Mol. Sci. 2020, 22, 315. [Google Scholar] [CrossRef]
- Mousa, S.A.; Straub, R.H.; Schäfer, M.; Stein, C. Beta-endorphin, Met-enkephalin and corresponding opioid receptors within syn-ovium of patients with joint trauma, osteoarthritis and rheumatoid arthritis. Ann. Rheum. Dis. 2007, 66, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Baamonde, A.; Lastra, A.; Juárez, L.; García, V.; Hidalgo, A.; Menéndez, L. Effects of the local administration of selective mu-, del-ta-and kappa-opioid receptor agonists on osteosarcoma-induced hyperalgesia. Naunyn Schmiedeberg Arch. Pharmacol. 2005, 372, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Baamonde, A.; Lastra, A.; Juárez, L.; García-Suárez, O.; Meana, Á.; Hidalgo, A.; Menéndez, L. Endogenous beta-endorphin induces thermal analgesia at the initial stages of a murine osteosarcoma. Peptides 2006, 27, 2778–2785. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Pontén, F. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Fricker, L.D.; Margolis, E.B.; Gomes, I.; Devi, L.A. Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions. Mol. Pharmacol. 2020, 98, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.; Hope, P.; Pyle, D. Tissue Distribution of Opioid Receptor Gene Expression in the Rat. Biochem. Biophys. Res. Commun. 1996, 218, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Pierzchała-Koziec, K.; Dziedzicka-Wasylewska, M.; Oeltgen, P.; Zubel-Łojek, J.; Latacz, A.; Ocłon, E. The Effect of CRH, Dexame-thasone and Naltrexone on the Mu, Delta and Kappa Opioid Receptor Agonist Binding in Lamb Hypothalamic-Pituitary-Adrenal Axis. Folia Biol. 2015, 63, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Madison, D.V.; Krasnow, M.A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 2015, 527, 240–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krazinski, B.; Koziorowski, M.; Brzuzan, P.; Okrasa, S. The expression of genes encoding opioid precursors and the influence of opioid receptor agonists on steroidogenesis in porcine adrenocortical cells in vitro. J. Physiol. Pharmacol. 2011, 62, 461–468. [Google Scholar]
- Kitamura, G.; Ohta, T.; Kai, T.; Kon, Y.; Ito, S. Inhibitory effects of opioids on voltage-dependent Ca2+ channels and catecholamine secretion in cultured porcine adrenal chromaffin cells. Brain Res. 2002, 942, 11–22. [Google Scholar] [CrossRef]
- Kilpatrick, D.L.; Lewis, R.V.; Stein, S.; Udenfriend, S. Release of enkephalins and enkephalin-containing polypeptides from perfused beef adrenal glands. Proc. Natl. Acad. Sci. USA 1980, 77, 7473–7475. [Google Scholar] [CrossRef] [Green Version]
- Sasahara, I. Catecholamine and ATP metabolites released from perfused adrenal glands of guinea-pig. Jpn. J. Vetinary Res. 2000, 48, 63. [Google Scholar]
- Yang, H.-Y.; Hexum, T.; Costa, E. Opioid peptides in adrenal gland. Life Sci. 1980, 27, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Viveros, O.H.; DiLiberto, E.J., Jr.; Hazum, E.; Chang, K.J. Opiate-like materials in the adrenal medulla: Evidence for storage and secretion with catecholamines. Mol. Pharmacol. 1979, 16, 1101–1108. [Google Scholar]
- Barron, B.; Hexum, T. Modulation of bovine adrenal gland secretion by etorphine and diprenorphine. Life Sci. 1986, 38, 935–940. [Google Scholar] [CrossRef] [PubMed]
- North, R.A.; Egan, T.M. Actions and distributions of opioid peptides in peripheral tissues. Br. Med. Bull. 1983, 39, 71–75. [Google Scholar] [CrossRef]
- Hinson, J.P.; Vinson, G.P.; Whitehouse, B.J.; Price, G. Control of zona glomerulosa function in the isolated perfused rat adrenal gland in situ. J. Endocrinol. 1985, 104, 387–395. [Google Scholar] [CrossRef]
- Hinson, J.P.; Cameron, L.A.; Purbrick, A.; Kapas, S. The role of neuropeptides in the regulation of adrenal zona glomerulosa function: Effects of substance P, neuropeptide Y, neurotensin, Met-enkephalin, Leu-enkephalin and corticotrophin-releasing hormone on aldosterone secretion in the intact perfused rat adrenal. J. Endocrinol. 1994, 140, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.; Cameron, L.; Purbrick, A.; Kapas, S. The role of neuropeptides in the regulation of adrenal vascular tone: Effects of vasoactive intestinal polypeptide, substance P, neuropeptide Y, neurotensin, Met-enkephalin, and Leu-enkephalin on perfusion medium flow rate in the intact perfused rat adrenal. Regul. Pept. 1994, 51, 55–61. [Google Scholar] [CrossRef]
- Kapas, S.; Purbrick, A.; Hinson, J.P. Action of opioid peptides on the rat adrenal cortex: Stimulation of steroid secretion through a specific mu opioid receptor. J. Endocrinol. 1995, 144, 503–510. [Google Scholar] [CrossRef]
- Smart, D.; Smith, G.; Lambert, D.G. Mu-opioids activate phospholipase C in SH-SY5Y human neuroblastoma cells via calcium-channel opening. Biochem. J. 1995, 305, 577–581. [Google Scholar] [CrossRef]
- Coiro, V.; Volpi, R.; Stella, A.; Venturi, N.; Chiodera, P. Stimulatory effect of naloxone on plasma cortisol in human: Possible direct stimulatory action at the adrenal cortex. Regul. Pept. 2011, 166, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.; Fearon, U.; Cunningham, S.K.; McKenna, T.J. The steroidogenic effects of beta-endorphin and joining peptide: A potential role in the modulation of adrenal androgen production. J. Endocrinol. 1996, 151, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.A.; Raaen, L.; Chen, C.; Azhar, G.; Shahidinia, N.; Shen, M.; Maksabedian, E.; Shanman, R.M.; Newberry, S.; Hempel, S. Effects of medication assisted treatment (MAT) for opioid use disorder on functional outcomes: A systematic review. J. Subst. Abus. Treat. 2018, 89, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezeh, E.; Singh, D.; Dobariya, V.; Akhigbe, E.J.; Gilkerson, C. Opioid-Induced Hyperalgesia in a Cancer Patient on High-Dose Methadone Maintenance Therapy: A Case for Subspecialty Opioid Use Disorder Primary Care. Cureus 2020, 12, e12345. [Google Scholar] [CrossRef]
- Gudin, J.A.; Laitman, A.; Nalamachu, S. Opioid Related Endocrinopathy: Table 1. Pain Med. 2015, 16, S9–S15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coluzzi, F.; Scerpa, M.S.; Centanni, M. The Effect of Opiates on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.D.; Edlund, M.J.; Zhang, L.; Unützer, J.; Wells, K.B. Association Between Mental Health Disorders, Problem Drug Use, and Regular Prescription Opioid Use. Arch. Intern. Med. 2006, 166, 2087–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Priority | Test | Values | Considerations |
|---|---|---|---|
| 1 | Morning cortisol | <3 to 3.6 µg/dL is suggestive | Cortisol secretion has a circadian pattern and can be difficult to assess. Values after 8 a.m. are less likely to be reliable. |
| 2 | DHEAS | >54.5 µg/dL | Ranges vary by sex and age; low-normal values can be suggestive. |
| 3 | CST test | <500 nmol/L | |
| 4 | Insulin tolerance test or ACTH stimulation test | Cutoff values not entirely established | Applicable when first two tests are inconclusive or there remain doubts. An abnormal ACTH is highly specific for adrenal insufficiency. Low ACTH values indicate central etiology. |
| Others | Metyrapone stimulation test Glucagon stimulation test | Note that these tests are not always highly specific. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluzzi, F.; LeQuang, J.A.K.; Sciacchitano, S.; Scerpa, M.S.; Rocco, M.; Pergolizzi, J. A Closer Look at Opioid-Induced Adrenal Insufficiency: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4575. https://doi.org/10.3390/ijms24054575
Coluzzi F, LeQuang JAK, Sciacchitano S, Scerpa MS, Rocco M, Pergolizzi J. A Closer Look at Opioid-Induced Adrenal Insufficiency: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(5):4575. https://doi.org/10.3390/ijms24054575
Chicago/Turabian StyleColuzzi, Flaminia, Jo Ann K. LeQuang, Salvatore Sciacchitano, Maria Sole Scerpa, Monica Rocco, and Joseph Pergolizzi. 2023. "A Closer Look at Opioid-Induced Adrenal Insufficiency: A Narrative Review" International Journal of Molecular Sciences 24, no. 5: 4575. https://doi.org/10.3390/ijms24054575







