Polydeoxyribonucleotide in the Treatment of Tendon Disorders, from Basic Science to Clinical Practice: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Literature Search and Study Eligibility
2.2. Study Quality Assessment and Risk of Bias of the Included Studies
3. Results
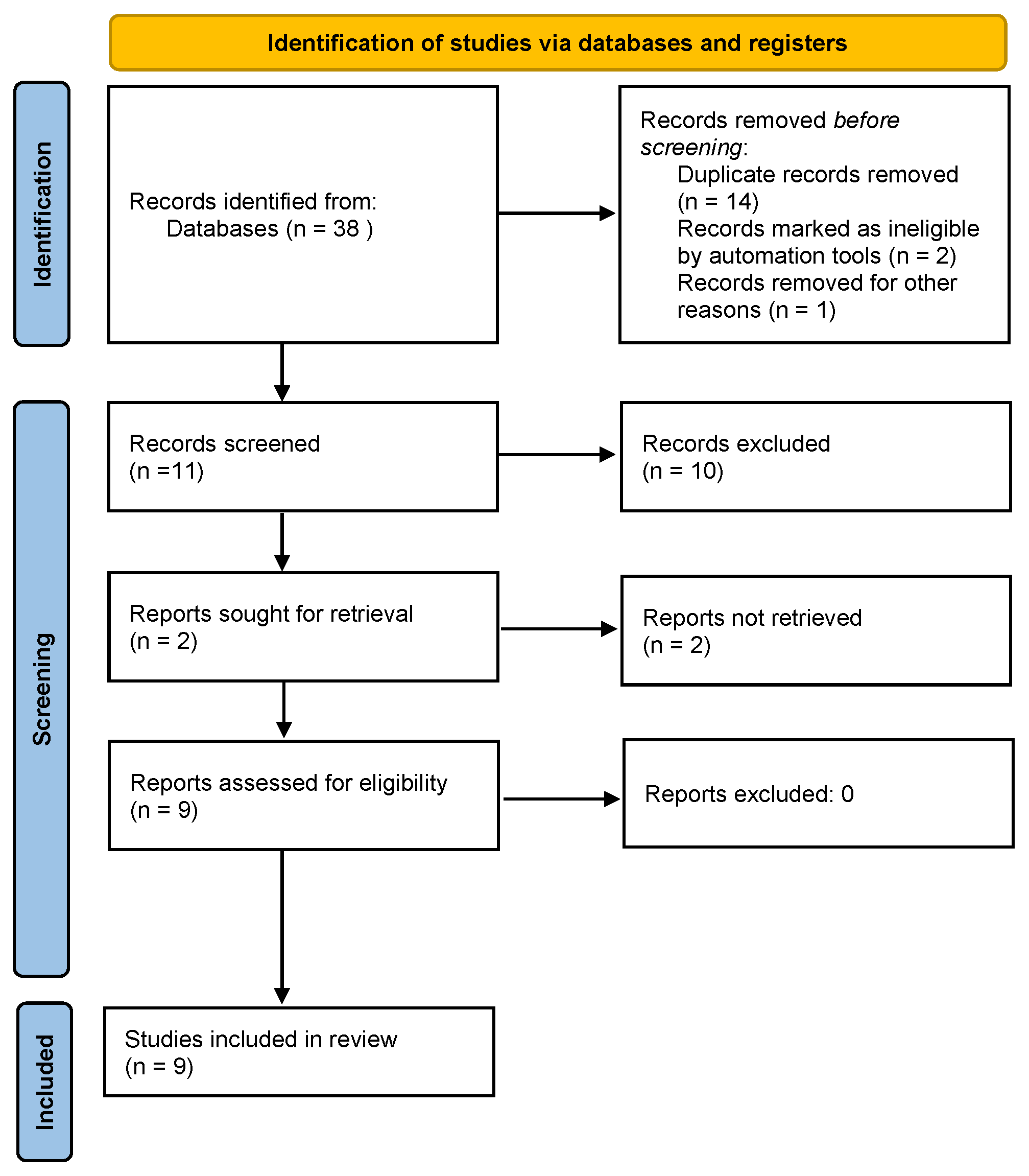
3.1. Study Selection
3.2. Study Quality and Characteristics
3.3. Effectiveness of PDRN in the Management of Tendinopathies
3.3.1. Achilles Tendinopathy
3.3.2. Plantar Fasciitis
3.3.3. Rotator Cuff Tendinopathy
3.3.4. Epicondylitis
3.3.5. Pes Anserine Tendinopathy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, A.; Squier, K.; Alfredson, H.; Bahr, R.; Cook, J.L.; Coombes, B.; de Vos, R.-J.; Fu, S.N.; Grimaldi, A.; Lewis, J.S.; et al. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br. J. Sports Med. 2020, 54, 260–262. [Google Scholar] [CrossRef]
- van der Windt, D.A.; Koes, B.W.; de Jong, B.A.; Bouter, L.M. Shoulder Disorders in General Practice: Incidence, Patient Characteristics, and Management. Ann. Rheum. Dis. 1995, 54, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Linsell, L.; Dawson, J.; Zondervan, K.; Rose, P.; Randall, T.; Fitzpatrick, R.; Carr, A. Prevalence and Incidence of Adults Consulting for Shoulder Conditions in UK Primary Care; Patterns of Diagnosis and Referral. Rheumatology 2006, 45, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Riel, H.; Lindstrøm, C.F.; Rathleff, M.S.; Jensen, M.B.; Olesen, J.L. Prevalence and Incidence Rate of Lower-Extremity Tendinopathies in a Danish General Practice: A Registry-Based Study. BMC Musculoskelet. Disord. 2019, 20, 239. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Murrell, G.A.C.; Trickett, A.; Wang, M.-X. Involvement of Cytochrome c Release and Caspase-3 Activation in the Oxidative Stress-Induced Apoptosis in Human Tendon Fibroblasts. Biochim. Biophys. Acta 2003, 1641, 35–41. [Google Scholar] [CrossRef]
- Burry, H.C. Pathogenesis of Some Traumatic and Degenerative Disorders of Soft Tissue. Aust. N. Z. J. Med. 1978, 8 (Suppl. S1), 163–167. [Google Scholar] [CrossRef]
- Khan, K.M.; Cook, J.L.; Bonar, F.; Harcourt, P.; Astrom, M. Histopathology of Common Tendinopathies. Update and Implications for Clinical Management. Sports Med. 1999, 27, 393–408. [Google Scholar] [CrossRef]
- Mallows, A.; Debenham, J.; Walker, T.; Littlewood, C. Association of Psychological Variables and Outcome in Tendinopathy: A Systematic Review. Br. J. Sports Med. 2017, 51, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Feng, Z.; Cao, J.; Fu, W. Efficacy of Extracorporeal Shock Wave Therapy for Achilles Tendinopathy: A Meta-Analysis. Orthop. J. Sports Med. 2020, 8, 2325967120903430. [Google Scholar] [CrossRef]
- Hang, D.W.; Bach, B.R.; Bojchuk, J. Partial Achilles Tendon Rupture Following Corticosteroid Injection. Phys. Sportsmed. 1995, 23, 57–66. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Caruso, I.; Nappi, V.S.; Giancaspro, G.; Carrozzo, M.; Moretti, B. New insights into the treatment of non-healing diabetic foot ulcers. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. S1), 15–21. [Google Scholar] [PubMed]
- Rubegni, P.; De Aloe, G.; Mazzatenta, C.; Cattarini, L.; Fimiani, M. Clinical Evaluation of the Trophic Effect of Polydeoxyribonucleotide (PDRN) in Patients Undergoing Skin Explants. A Pilot Study. Curr. Med. Res. Opin. 2001, 17, 128–131. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattani, G.; Schettini, G. Polydeoxyribonucleotides Enhance the Proliferation of Human Skin Fibroblasts: Involvement of A2 Purinergic Receptor Subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.C.; Desai, A.; Chen, J.-F.; Yee, H.; Schwarzschild, M.A.; Fink, J.S.; Cronstein, B.N. Adenosine Promotes Wound Healing and Mediates Angiogenesis in Response to Tissue Injury Via Occupancy of A2A Receptors. Am. J. Pathol. 2002, 160, 2009–2018. [Google Scholar] [CrossRef]
- Sini, P.; Denti, A.; Cattarini, G.; Daglio, M.; Tira, M.; Balduini, C. Effect of Polydeoxyribonucleotides on Human Fibroblasts in Primary Culture. Cell Biochem. Funct. 1999, 17, 107–114, Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/%28SICI%291099-0844%28199906%2917%3A2%3C107%3A%3AAID-CBF815%3E3.0.CO%3B2-%23 (accessed on 25 October 2022). [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) Promotes Human Osteoblast Proliferation: A New Proposal for Bone Tissue Repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Gennero, L.; Denysenko, T.; Calisti, G.F.; Vercelli, A.; Vercelli, C.M.; Amedeo, S.; Mioletti, S.; Parino, E.; Montanaro, M.; Melcarne, A.; et al. Protective Effects of Polydeoxyribonucleotides on Cartilage Degradation in Experimental Cultures. Cell Biochem. Funct. 2013, 31, 214–227. [Google Scholar] [CrossRef]
- Avantaggiato, A.; Palmieri, A.; Carinci, F.; Trapella, G.; Sollazzo, V.; Lauritano, D. Effects of Glucosamine and Nucleotide Association on Fibroblast: Extracellular Matrix Gene Expression. Int. J. Immunopathol. Pharmacol. 2014, 27, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Altavilla, D.; Minutoli, L.; Migliorato, A.; Squadrito, F. Polydeoxyribonucleotide (PDRN) Restores Blood Flow in an Experimental Model of Peripheral Artery Occlusive Disease. J. Vasc. Surg. 2008, 48, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine Receptor Stimulation by Polynucleotides (PDRN) Reduces Inflammation in Experimental Periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef]
- Minutoli, L.; Arena, S.; Antonuccio, P.; Romeo, C.; Bitto, A.; Magno, C.; Rinaldi, M.; Micali, A.; Irrera, N.; Pizzino, G.; et al. Role of Inhibitors of Apoptosis Proteins in Testicular Function and Male Fertility: Effects of Polydeoxyribonucleotide Administration in Experimental Varicocele. Biomed. Res. Int. 2015, 2015, 248976. [Google Scholar] [CrossRef]
- Arena, S.; Minutoli, L.; Arena, F.; Nicotina, P.A.; Romeo, C.; Squadrito, F.; Altavilla, D.; Morgia, G.; Magno, C. Polydeoxyribonucleotide Administration Improves the Intra-Testicular Vascularization in Rat Experimental Varicocele. Fertil. Steril. 2012, 97, 165–168. [Google Scholar] [CrossRef]
- Jeong, E.K.; Jang, H.J.; Kim, S.S.; Lee, S.Y.; Oh, M.Y.; Kim, H.J.; Eom, D.W.; Ham, J.Y.; Han, D.J. Protective Effect of Polydeoxyribonucleotide Against Renal Ischemia-Reperfusion Injury in Mice. Transplant. Proc. 2016, 48, 1251–1257. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Lo Cascio, P.; Stagno d’Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide Stimulates Angiogenesis and Wound Healing in the Genetically Diabetic Mouse. Wound Repair Regen 2008, 16, 208–217. [Google Scholar] [CrossRef]
- AAOS Clinical Practice Guideline and Systematic Review Methodology. 2017. Available online: https://www.mendeley.com/catalogue/aaos-clinical-practice-guideline-systematic-review-methodology/ (accessed on 1 December 2022).
- Kang, S.H.; Choi, M.S.; Kim, H.K.; Kim, W.S.; Bae, T.H.; Kim, M.K.; Chang, S.H. Polydeoxyribonucleotide Improves Tendon Healing Following Achilles Tendon Injury in Rats. J. Orthop. Res. 2018, 36, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.H.; Ko, I.-G.; Jin, J.-J.; Hwang, L.; Kim, S.-H.; Chung, J.-Y.; Hwang, T.-J.; Han, J.H. Polydeoxyribonucleotide Ameliorates Inflammation and Apoptosis in Achilles Tendon-Injury Rats. Int. Neurourol. J. 2020, 24, 79–87. [Google Scholar] [CrossRef]
- Lim, T.-H.; Cho, H.R.; Kang, K.N.; Rhyu, C.J.; Chon, S.W.; Lim, Y.S.; Yoo, J.I.; Kim, J.-W.; Kim, Y.U. The Effect of Polydeoxyribonucleotide Prolotherapy on Posterior Tibial Tendon Dysfunction after Ankle Syndesmotic Surgery: A Case Report. Medicine 2016, 95, e5346. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Pak, C.S.; Park, J.H.; Jeong, J.H.; Heo, C.Y. Effects of Polydeoxyribonucleotide in the Treatment of Pressure Ulcers. J. Korean Med. Sci. 2014, 29 (Suppl. S3), S222–S227. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-O.; Yoo, J.-H.; Cho, H.-I.; Cho, S.; Cho, H.R. Comparing Effectiveness of Polydeoxyribonucleotide Injection and Corticosteroid Injection in Plantar Fasciitis Treatment: A Prospective Randomized Clinical Study. Foot Ankle Surg. 2020, 26, 657–661. [Google Scholar] [CrossRef]
- Ryu, K.; Ko, D.; Lim, G.; Kim, E.; Lee, S.H. Ultrasound-Guided Prolotherapy with Polydeoxyribonucleotide for Painful Rotator Cuff Tendinopathy. Pain Res. Manag. 2018, 2018, 8286190. [Google Scholar] [CrossRef]
- Shim, B.J.; Seo, E.-M.; Hwang, J.-T.; Kim, D.-Y.; Yang, J.-S.; Seo, S.-J.; Hong, M.S. Comparison of the Effectiveness of Extensor Muscle Strengthening Exercise by Itself, Exercise with Polydeoxyribonucleotide Injection, and Exercise with Extracorporeal Shockwave Therapy in Lateral Epicondylitis: A Randomized Controlled Trial. Clin. Shoulder Elb. 2021, 24, 231–238. [Google Scholar] [CrossRef]
- Lee, G.J.; Park, D. Usefulness of polydeoxyribonucleotide as an alternative to corticosteroids in patients with lateral epicondylitis: A case series. Medicine 2018, 97, e10809. [Google Scholar] [CrossRef] [PubMed]
- Mun, J.-U.; Cho, H.R.; Bae, S.M.; Park, S.K.; Choi, S.L.; Seo, M.S.; Lim, Y.S.; RN, S.H.W.; Kim, Y.U. Effect of Polydeoxyribonucleotide Injection on Pes Anserine Bursitis. Medicine 2017, 96, e8330. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Hong, G.; Zhang, C. Gene Expression of Transforming Growth Factor Beta1 in Zone II Flexor Tendon Wound Healing of Rabbit. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi Zhongguo Xiufu Chongjian Waike Zazhi Chin. J. 2007, 21, 975–978. [Google Scholar]
- James, R.; Kesturu, G.; Balian, G.; Chhabra, A.B. Tendon: Biology, Biomechanics, Repair, Growth Factors, and Evolving Treatment Options. J. Hand. Surg. Am. 2008, 33, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Liu, H.; Fernandez, P.; Luna, A.; Perez-Aso, M.; Bujor, A.M.; Trojanowska, M.; Cronstein, B.N. Adenosine A2A Receptors Promote Collagen Production by a Fli1- and CTGF-Mediated Mechanism. Arthritis. Res. Ther. 2013, 15, R58. [Google Scholar] [CrossRef]
- Pearce, C.J.; Ismail, M.; Calder, J.D. Is Apoptosis the Cause of Noninsertional Achilles Tendinopathy? Am. J. Sports Med. 2009, 37, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224, Erratum in Front. Pharmacol. 2022, 13, 1073510. [Google Scholar] [CrossRef]
- Kim, D.H.; Kwon, D.R.; Park, G.Y.; Moon, Y.S. Synergetic effects of shock waves with polydeoxyribonucleotides on rotator cuff tendon tear in a rabbit model. Biocell 2021, 45, 527–536. [Google Scholar] [CrossRef]
- Kwon, D.R.; Moon, Y.S. Synergic regenerative effects of polydeoxyribonucleotide and microcurrent on full-thickness rotator cuff healing in a rabbit model. Ann. Phys. Rehabil. Med. 2020, 63, 474–482. [Google Scholar] [CrossRef]
- Solarino, G.; Bizzoca, D.; Moretti, L.; Vicenti, G.; Piazzolla, A.; Moretti, B. What’s New in the Diagnosis of Periprosthetic Joint Infections: Focus on Synovial Fluid Biomarkers. Trop. Med. Infect. Dis. 2022, 7, 355. [Google Scholar] [CrossRef]
- Bizzoca, D.; Moretti, L.; Gnoni, A.; Moretti, F.L.; Scacco, S.; Banfi, G.; Piazzolla, A.; Solarino, G.; Moretti, B. The Usefulness of Synovial Fluid Proteome Analysis in Orthopaedics: Focus on Osteoarthritis and Periprosthetic Joint Infections. J. Funct. Morphol. Kinesiol. 2022, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Moretti, L.; Bizzoca, D.; Giancaspro, G.A.; Cassano, G.D.; Moretti, F.; Setti, S.; Moretti, B. Biophysical Stimulation in Athletes’ Joint Degeneration: A Narrative Review. Medicina 2021, 57, 1206. [Google Scholar] [CrossRef]
- Vicenti, G.; Bizzoca, D.; Nappi, V.S.; Moretti, F.; Carrozzo, M.; Belviso, V.; Moretti, B. Biophysical stimulation of the knee with PEMFs: From bench to bedside. J. Biol. Regul. Homeost. Agents 2018, 32 (Suppl. S1), 23–28. [Google Scholar]
- Vicenti, G.; Bizzoca, D.; Solarino, G.; Moretti, F.; Ottaviani, G.; Simone, F.; Zavattini, G.; Maccagnano, G.; Noia, G.; Moretti, B. The role of biophysical stimulation with pemfs in fracture healing: From bench to bedside. J. Biol. Regul. Homeost. Agents 2020, 34 (Suppl. S1), 131–135, IORS Special Issue on Orthopedics. [Google Scholar]
- Bizzoca, D.; Rocchetti, M.T.; Scacco, S.; Taurino, F.; Vicenti, G.; Spadaccino, F.; Moretti, L.; Ranieri, E.; Gesualdo, L.; Moretti, F.; et al. Beyond pre-analytical and analytical concerns in the study of synovial fluid proteome: Description of an optimized Gel-based protocol. J. Biol. Regul. Homeost. Agents 2021, 35, 827–832. [Google Scholar] [CrossRef]
| Study | Design | Treated Tendinopathy | Number of Patients | PDRN Protocol | Main Findings |
|---|---|---|---|---|---|
| Kang et al.—2018 [26] | In vivo randomized controlled study | Achilles tendon injury | 36 male rats | The rats received daily intraperitoneal administration of polydeoxyribonucleotide (8 mg/kg/day for 1, 2, or 4 weeks) | ↑ Resistant to mechanical stress ↑ Stored energy |
| Rho et al.—2020 [27] | In vivo randomized controlled study | Achilles tendon injury | 40 rats | ↑ The tactile threshold for the von Frey filament test tactile withdrawal latency ↓ The concentration of IL-6 and ↓ TNF-α ↑ Expression of cAMP ↑ Phosphorylation of CREB and PKA ↓ Expression ratio of Bax/Bcl-2 |
| Study | Design and Quality Assessment | Treated Tendinopathy | Number of Patients | PDRN Protocol | Main Findings |
|---|---|---|---|---|---|
| Lim et al.—2016 [28] | Case report (moderate quality) | Posterior Tibial Tendon Dysfunction (PTTD) | 1 Female patient (age: 67) | PDRN injection repeated 4 times at 1-week intervals | ↓ NRS score and pain, swelling and tenderness, complications |
| Kim et al.—2015 [29] | Prospective randomized trial (High quality) | Chronic plantar fasciitis | 20 patients (male: 7) | PDRN injection performed weekly for three weeks | ↓ VAS and MOXFQ scores No complications |
| Lee et al.—2020 [30] | Prospective randomized trial (High quality) | Plantar fasciitis | 44 male patients | PDRN injection performed weekly for three weeks | ↓ VAS and MOXFQ scores No complications |
| Ryu et al.—2018 [31] | Retrospective Study (High quality) | Chronic rotator cuff disease | 32 patients (17 males) | PDRN injection performed weekly for four weeks | ↓ VAS score, pain, SPADI ↑ Function and SANE No complications |
| Shim et al.—2021 [32] | Randomized controlled trial (High quality) | Lateral epicondylitis | 69 patients (Male: 33; Mean age: 51.07) | Group 1: brace only Group 2: brace +PDRN injection Group 3: brace + ESWT | PDRN+ Brace: ↓ VAS score, ↑ MEPS, ↑ ultrasonographic findings, compared to control groups |
| Lee et al.—2018 [33] | Case series (High quality) | Lateral epicondylitis | 2 patients (Male: 2; mean age: 62) | US-guided PDRN injections were made into the common extensor tendons | ↓ Pain with decreased NRS ↓ Hypervascularity of common extensor tendon Improvement in the LE symptoms without any complications |
| Mun et al.—2017 [34] | Case report (Moderate quality) | Pes anserine bursitis | 1 female patient (Age: 50) | US-guided PDRN injections were made into PA bursa | ↓ Pain with decreased NRS ↑ ROM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzoca, D.; Brunetti, G.; Moretti, L.; Piazzolla, A.; Vicenti, G.; Moretti, F.L.; Solarino, G.; Moretti, B. Polydeoxyribonucleotide in the Treatment of Tendon Disorders, from Basic Science to Clinical Practice: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 4582. https://doi.org/10.3390/ijms24054582
Bizzoca D, Brunetti G, Moretti L, Piazzolla A, Vicenti G, Moretti FL, Solarino G, Moretti B. Polydeoxyribonucleotide in the Treatment of Tendon Disorders, from Basic Science to Clinical Practice: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(5):4582. https://doi.org/10.3390/ijms24054582
Chicago/Turabian StyleBizzoca, Davide, Giovanni Brunetti, Lorenzo Moretti, Andrea Piazzolla, Giovanni Vicenti, Francesco Luca Moretti, Giuseppe Solarino, and Biagio Moretti. 2023. "Polydeoxyribonucleotide in the Treatment of Tendon Disorders, from Basic Science to Clinical Practice: A Systematic Review" International Journal of Molecular Sciences 24, no. 5: 4582. https://doi.org/10.3390/ijms24054582
APA StyleBizzoca, D., Brunetti, G., Moretti, L., Piazzolla, A., Vicenti, G., Moretti, F. L., Solarino, G., & Moretti, B. (2023). Polydeoxyribonucleotide in the Treatment of Tendon Disorders, from Basic Science to Clinical Practice: A Systematic Review. International Journal of Molecular Sciences, 24(5), 4582. https://doi.org/10.3390/ijms24054582








