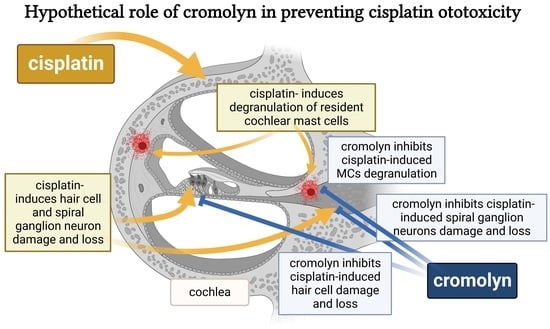
Degranulation of Murine Resident Cochlear Mast Cells: A Possible Factor Contributing to Cisplatin-Induced Ototoxicity and Neurotoxicity
Abstract
:1. Introduction
2. Results
2.1. Cisplatin Affects the Numbers and Morphology of Cochlear Mast Cells
2.1.1. Distribution of Cochlear Mast Cells in the Cochlear Explants
2.1.2. Cisplatin Leads to Degranulation of Cochlear Mast Cells
2.1.3. Cisplatin Decreases the Number of Non-Degranulated Cochlear Mast Cells
2.2. Cromolyn Protects the Auditory Hair Cells from Cisplatin-Induced Ototoxicity
2.2.1. Determination of Biosafety of Cromolyn in the Cochlear Tissues
2.2.2. Determination of the LD50 for Cisplatin
2.2.3. Cromolyn Inhibits the Cisplatin-Induced Degranulation of Cochlear Mast Cells
2.2.4. Cromolyn Protects Auditory Hair Cells from Cisplatin-Induced Damage
2.3. Cromolyn Protects from Cisplatin-Induced Neurotoxicity
3. Discussion
3.1. Brief Overview of Cisplatin-Induced Adverse Effects and Their Mechanisms
3.2. The Effect of Cisplatin on Murine Cochlear Mast Cells
3.3. Effect of Cromolyn on Cisplatin-Induced Cochlear Mast Cell Degranulation
3.4. Effect of Cromolyn on Cisplatin-Induced Hair Cell Damage
3.5. Effect of Cromolyn on Cisplatin-Induced Spiral Ganglion Neuron Loss
3.6. Study Strengths and Limitations
3.7. Future Directions
4. Materials and Methods
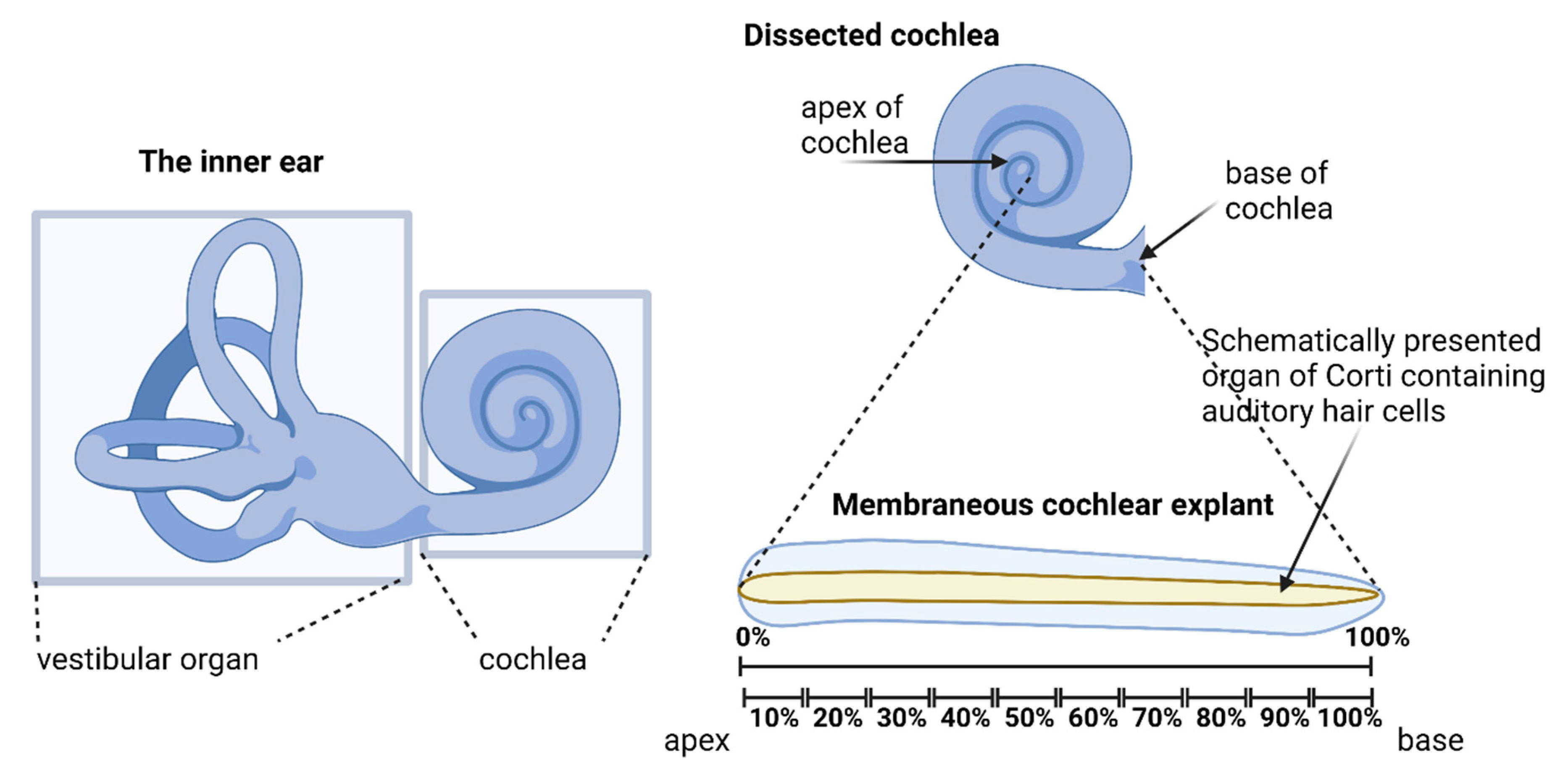
4.1. Cochlear Explants
4.2. Treatments of the Explanted Tissues
4.2.1. Treatment with Cisplatin
4.2.2. Treatment with Cromoglicic Acid
4.3. Immunofluorescent Staining
4.3.1. Hair Cell Staining
4.3.2. SGN Staining
4.3.3. Mast Cell Staining
4.3.4. Fluorescence Microscopy
4.4. Data quantification
4.4.1. Hair Cell Counting
4.4.2. Spiral Ganglion Neuron Counting
4.4.3. Mast Cell Counting
4.5. Statistical analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.H.; Zhang, C.; Frye, M.D. Immune cells and non-immune cells with immune function in mammalian cochleae. Hear. Res. 2018, 362, 14–24. [Google Scholar] [CrossRef]
- Liu, W.; Kämpfe Nordström, C.; Danckwardt-Lillieström, N.; Rask-Andersen, H. Human Inner Ear Immune Activity: A Super-Resolution Immunohistochemistry Study. Front. Neurol. 2019, 10, 728. [Google Scholar] [CrossRef] [Green Version]
- Poulsen, H. Influence of Myxedematous Connective Tissue Changes on the Function of the Labyrinth. Experimental Studies on the Influence of Thyrotrophic Hormone on Mast Cells in the Inner Ear of Guinea Pigs. Ph.D. Thesis, University of Copenhagen, København, Denmark, 1959. [Google Scholar]
- Sleeckx, J.P.; Shea, J.J.; Peremans, J.M. The mast cells of the inner ear. Acta Otorhinolaryngol. Belg. 1976, 30, 443–449. [Google Scholar] [PubMed]
- Miyamura, K.; Kanzaki, Y.; Nagata, M.; Ishikawa, T. Provocation of nystagmus and deviation by type I allergy in the inner ear of the guinea pig. Ann. Allergy 1987, 58, 36–40. [Google Scholar] [PubMed]
- Uno, K.; Miyamura, K.; Kanzaki, Y.; Fukuda, H.; Masuyama, K.; Ishikawa, T. Type I allergy in the inner ear of the guinea pig. Ann. Otol. Rhinol. Laryngol. Suppl. 1992, 157, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Uno, K.; Miyamura, K.; Kanzaki, Y.; Fukuda, H.; Masuyama, K.; Ishikawa, T. Audiological study in guinea pigs with type I allergy induced in the inner ear. Ann. Otol. Rhinol. Laryngol. 1993, 102, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Minoda, R.; Masuyama, K.; Toriya, T.; Uno, K.; Eura, M.; Ishikawa, T. Recurrent hearing impairment and nystagmus induced by repeated antigen exposure in actively sensitized guinea pigs. Int. Arch. Allergy Immunol. 1996, 111, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Szczepek, A.J.; Dudnik, T.; Karayay, B.; Sergeeva, V.; Olze, H.; Smorodchenko, A. Mast Cells in the Auditory Periphery of Rodents. Brain Sci. 2020, 10, 697. [Google Scholar] [CrossRef]
- Sheffield, A.M.; Smith, R.J.H. The Epidemiology of Deafness. Cold Spring Harb. Perspect. Med. 2019, 9, a033258. [Google Scholar] [CrossRef] [Green Version]
- Ina, A.; Altintaş, D.U.; Yilmaz, M.; Uğuz, A.; Tuncer, U.; Kiroğlu, M.; Hergüner, O.; Bicakci, K. Congenital mastocytosis associated with neurosensory deafness. Pediatr. Dermatol. 2007, 24, 460–462. [Google Scholar] [CrossRef]
- Trevisan, G.; Pauluzzi, P.; Gatti, A.; Semeraro, A. Familial mastocytosis associated with neurosensory deafness. J. Eur. Acad. Dermatol. Venereol. JEADV 2000, 14, 119–122. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Kühn, W.; Zuberbier, T.; Maurer, M. Successful treatment of cutaneous mastocytosis and Ménière disease with anti-IgE therapy. J. Allergy Clin. Immunol. 2007, 120, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.A.; Parfitt, R.; Ciarimboli, G. Drug-induced ototoxicity: Mechanisms, Pharmacogenetics, and protective strategies. Clin. Pharm. Ther. 2017, 101, 491–500. [Google Scholar] [CrossRef]
- Schacht, J.; Talaska, A.E.; Rybak, L.P. Cisplatin and aminoglycoside antibiotics: Hearing loss and its prevention. Anat. Rec. 2012, 295, 1837–1850. [Google Scholar] [CrossRef] [Green Version]
- Brock, P.R.; Knight, K.R.; Freyer, D.R.; Campbell, K.C.; Steyger, P.S.; Blakley, B.W.; Rassekh, S.R.; Chang, K.W.; Fligor, B.J.; Rajput, K.; et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. 2012, 30, 2408–2417. [Google Scholar] [CrossRef] [Green Version]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [Green Version]
- Ciarimboli, G.; Deuster, D.; Knief, A.; Sperling, M.; Holtkamp, M.; Edemir, B.; Pavenstadt, H.; Lanvers-Kaminsky, C.; am Zehnhoff-Dinnesen, A.; Schinkel, A.H.; et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010, 176, 1169–1180. [Google Scholar] [CrossRef]
- More, S.S.; Akil, O.; Ianculescu, A.G.; Geier, E.G.; Lustig, L.R.; Giacomini, K.M. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 9500–9509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breglio, A.M.; Rusheen, A.E.; Shide, E.D.; Fernandez, K.A.; Spielbauer, K.K.; McLachlin, K.M.; Hall, M.D.; Amable, L.; Cunningham, L.L. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat. Commun. 2017, 8, 1654. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Camacho, R.; García-Berrocal, J.R.; Buján, J.; Martín-Marero, A.; Trinidad, A. Supporting Cells As a Target of Cisplatin-Induced Inner Ear Damage: Therapeutic Implications. Laryngoscope 2004, 114, 533–537. [Google Scholar] [CrossRef]
- Chen, Y.; Bielefeld, E.C.; Mellott, J.G.; Wang, W.; Mafi, A.M.; Yamoah, E.N.; Bao, J. Early Physiological and Cellular Indicators of Cisplatin-Induced Ototoxicity. J. Assoc. Res. Otolaryngol. 2021, 22, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Ohlemiller, K.K. Age-related loss of spiral ganglion neurons. Hear. Res. 2010, 264, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Dehne, N.; Lautermann, J.; Petrat, F.; Rauen, U.; de Groot, H. Cisplatin ototoxicity: Involvement of iron and enhanced formation of superoxide anion radicals. Toxicol. Appl. Pharm. 2001, 174, 27–34. [Google Scholar] [CrossRef]
- Goncalves, M.S.; Silveira, A.F.; Teixeira, A.R.; Hyppolito, M.A. Mechanisms of cisplatin ototoxicity: Theoretical review. J. Laryngol. Otol. 2013, 127, 536–541. [Google Scholar] [CrossRef] [Green Version]
- Kanat, O.; Ertas, H.; Caner, B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J. Clin. Oncol. 2017, 8, 329–335. [Google Scholar] [CrossRef]
- Yu, X.; Man, R.; Li, Y.; Yang, Q.; Li, H.; Yang, H.; Bai, X.; Yin, H.; Li, J.; Wang, H. Paeoniflorin protects spiral ganglion neurons from cisplatin-induced ototoxicity: Possible relation to PINK1/BAD pathway. J. Cell. Mol. Med. 2019, 23, 5098–5107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ruijven, M.W.; de Groot, J.C.; Klis, S.F.; Smoorenburg, G.F. The cochlear targets of cisplatin: An electrophysiological and morphological time-sequence study. Hear. Res. 2005, 205, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Brzezinska-Blaszczyk, E.; Mincikiewicz, M.; Ochocki, J. Effect of cisplatin and cis-platinum (II) phosphonate complex on murine mast cells. Eur. J. Pharm. 1996, 298, 155–158. [Google Scholar] [CrossRef]
- Benkafadar, N.; Menardo, J.; Bourien, J.; Nouvian, R.; François, F.; Decaudin, D.; Maiorano, D.; Puel, J.L.; Wang, J. Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol. Med. 2017, 9, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Youm, I.; West, M.B.; Li, W.; Du, X.; Ewert, D.L.; Kopke, R.D. siRNA-loaded biodegradable nanocarriers for therapeutic MAPK1 silencing against cisplatin-induced ototoxicity. Int. J. Pharm. 2017, 528, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Liu, Z.; Xie, L.; Lv, L.; Zhu, W.; Liu, J.; Dai, Y.; She, W. Panax notoginseng Saponins protect auditory cells against cisplatin-induced ototoxicity by inducing the AKT/Nrf2 signaling-mediated redox pathway. Mol. Med. Rep. 2020, 22, 3533–3540. [Google Scholar] [CrossRef]
- Ma, W.; Hu, J.; Cheng, Y.; Wang, J.; Zhang, X.; Xu, M. Ginkgolide B protects against cisplatin-induced ototoxicity: Enhancement of Akt-Nrf2-HO-1 signaling and reduction of NADPH oxidase. Cancer Chemother. Pharmacol. 2015, 75, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Ferreira, R.S.; Santos, A.C.D. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 136, 111079. [Google Scholar] [CrossRef] [PubMed]
- Rybak, L.P.; Mukherjea, D.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Prevention. Semin. Hear. 2019, 40, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Paken, J.; Govender, C.D.; Pillay, M.; Sewram, V. A Review of Cisplatin-Associated Ototoxicity. Semin. Hear. 2019, 40, 108–121. [Google Scholar] [CrossRef]
- Heiger-Bernays, W.J.; Essigmann, J.M.; Lippard, S.J. Effect of the antitumor drug cis-diamminedichloroplatinum(II) and related platinum complexes on eukaryotic DNA replication. Biochemistry 1990, 29, 8461–8466. [Google Scholar] [CrossRef]
- Jordan, P.; Carmo-Fonseca, M. Molecular mechanisms involved in cisplatin cytotoxicity. Cell. Mol. Life Sci. 2000, 57, 1229–1235. [Google Scholar] [CrossRef]
- Clerici, W.J.; DiMartino, D.L.; Prasad, M.R. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear. Res. 1995, 84, 30–40. [Google Scholar] [CrossRef]
- Kopke, R.D.; Liu, W.; Gabaizadeh, R.; Jacono, A.; Feghali, J.; Spray, D.; Garcia, P.; Steinman, H.; Malgrange, B.; Ruben, R.J.; et al. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol. 1997, 18, 559–571. [Google Scholar]
- Banfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [CrossRef] [Green Version]
- Sheth, S.; Mukherjea, D.; Rybak, L.P.; Ramkumar, V. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front. Cell. Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Luo, Q.; Zhang, Y.; Jia, F.; Zhao, Y.; Wang, F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem. Res. Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef] [PubMed]
- Kounis, N.G.; Cervellin, G.; Lippi, G. Cisplatin-induced bradycardia: Cardiac toxicity or cardiac hypersensitivity and Kounis syndrome? Int. J. Cardiol. 2016, 202, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.A.; Chan, J.; Gan, P.Y.; Dewage, L.; Nozaki, Y.; Steinmetz, O.M.; Nikolic-Paterson, D.J.; Kitching, A.R.; Holdsworth, S.R. Mast cells mediate acute kidney injury through the production of TNF. J. Am. Soc. Nephrol. JASN 2011, 22, 2226–2236. [Google Scholar] [CrossRef] [Green Version]
- Otani, I.M.; Wong, J.; Banerji, A. Platinum Chemotherapy Hypersensitivity: Prevalence and Management. Immunol. Allergy Clin. N. Am. 2017, 37, 663–677. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478–494. [Google Scholar] [CrossRef]
- Samorapoompichit, P.; Steiner, M.; Lucas, T.; Wachtler, F.; Schedled, A.; Sperr, W.R.; Valent, P. Induction of apoptosis in the human mast cell leukemia cell line HMC-1 by various antineoplastic drugs. Leuk. Lymphoma 2003, 44, 509–515. [Google Scholar] [CrossRef]
- Bergstresser, P.R.; Tigelaar, R.E.; Tharp, M.D. Conjugated avidin identifies cutaneous rodent and human mast cells. J. Investig. Derm. 1984, 83, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Takagi, M.; Koike, K.; Nakahata, T. Antiproliferative effect of IFN-gamma on proliferation of mouse connective tissue-type mast cells. J. Immunol. 1990, 145, 1880–1884. [Google Scholar] [CrossRef]
- Mazzoldi, E.L.; Pavan, S.; Pilotto, G.; Leone, K.; Pagotto, A.; Frezzini, S.; Nicoletto, M.O.; Amadori, A.; Pastò, A. A juxtacrine/paracrine loop between C-Kit and stem cell factor promotes cancer stem cell survival in epithelial ovarian cancer. Cell Death Dis. 2019, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Sodhi, A.; Singh, S.M. Production of interleukin-1 and tumor necrosis factor by cisplatin-treated murine peritoneal macrophages. Nat. Immun. Cell Growth Regul. 1991, 10, 105–116. [Google Scholar] [PubMed]
- Chauhan, P.; Sodhi, A.; Shrivastava, A. Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology 2009, 214, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Hellman, L.; Akula, S.; Fu, Z.; Wernersson, S. Mast Cell and Basophil Granule Proteases-In Vivo Targets and Function. Front. Immunol. 2022, 13, 918305. [Google Scholar] [CrossRef] [PubMed]
- Bankova, L.G.; Lezcano, C.; Pejler, G.; Stevens, R.L.; Murphy, G.F.; Austen, K.F.; Gurish, M.F. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J. Immunol. 2014, 192, 2812–2820. [Google Scholar] [CrossRef] [Green Version]
- Wilcz-Villega, E.M.; McClean, S.; O’Sullivan, M.A. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: Implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 1140–1151. [Google Scholar] [CrossRef]
- Wingard, J.C.; Zhao, H.B. Cellular and Deafness Mechanisms Underlying Connexin Mutation-Induced Hearing Loss-A Common Hereditary Deafness. Front. Cell. Neurosci. 2015, 9, 202. [Google Scholar] [CrossRef] [Green Version]
- Oka, T.; Kalesnikoff, J.; Starkl, P.; Tsai, M.; Galli, S.J. Evidence questioning cromolyn’s effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Lab. Investig. A J. Tech. Methods Pathol. 2012, 92, 1472–1482. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lai, Y.; Bernard, J.J.; MacLeod, D.T.; Cogen, A.L.; Moss, B.; Di Nardo, A. Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides. J. Immunol. 2012, 188, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Vincent, L.; Lapointe, C.; Lo, M.; Gagnon, H.; Pejler, G.; Takai, S.; Day, R.; D’Orléans-Juste, P. Mast Cell Degranulation Increases Mouse Mast Cell Protease 4–Dependent Vasopressor Responses to Big Endothelin-1 But Not Angiotensin I. J. Pharmacol. Exp. Ther. 2021, 376, 213–221. [Google Scholar] [CrossRef]
- Ding, D.; He, J.; Allman, B.L.; Yu, D.; Jiang, H.; Seigel, G.M.; Salvi, R.J. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear. Res. 2011, 282, 196–203. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Monteagudo, A.; Downey, M.A.; Ashton-Rickardt, P.G.; Elmaleh, D.R. Cromolyn inhibits the secretion of inflammatory cytokines by human microglia (HMC3). Sci. Rep. 2021, 11, 8054. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chai, Y.; Liu, H.; Li, G.; Wang, L.; Yang, T.; Wu, H. Postnatal Development of Microglia-Like Cells in Mouse Cochlea. Neural Plast. 2018, 2018, 1970150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Cai, J.; Xu, L.; Wang, H.; Liu, W. Cisplatin-Induced Stria Vascularis Damage Is Associated with Inflammation and Fibrosis. Neural Plast. 2020, 2020, 8851525. [Google Scholar] [CrossRef] [PubMed]
- Gentilin, E.; Simoni, E.; Candito, M.; Cazzador, D.; Astolfi, L. Cisplatin-Induced Ototoxicity: Updates on Molecular Targets. Trends Mol. Med. 2019, 25, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Hashjin, G.; Nijkamp, F.P.; Henricks, P.A.; Folkerts, G. Sodium cromoglycate and doxantrazole are oxygen radical scavengers. Eur. Respir. J. 2002, 20, 867–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, M.R.; Jiang, Y.; Guo, C.; Reed, A.; Meng, H.; Vasko, M.R. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS ONE 2014, 9, e106485. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Silverman, A.J.; Vannucci, S.J. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke 2009, 40, 3107–3112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempuraj, D.; Ahmed, M.E.; Selvakumar, G.P.; Thangavel, R.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Nattanmai Chandrasekaran, P.; Burton, C.; et al. Acute Traumatic Brain Injury-Induced Neuroinflammatory Response and Neurovascular Disorders in the Brain. Neurotox. Res. 2020, 39, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Tokumitsu, H.; Kubota, Y.; Kobayashi, R. Interaction of S100 proteins with the antiallergic drugs, olopatadine, amlexanox, and cromolyn: Identification of putative drug binding sites on S100A1 protein. Biochem. Biophys. Res. Commun. 2002, 292, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Buckiová, D.; Syka, J. Calbindin and S100 protein expression in the developing inner ear in mice. J. Comp. Neurol. 2009, 513, 469–482. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Lu, J.Y.; Wu, X.; Summer, S.; Whoriskey, J.; Saris, C.; Reagan, J.D. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 2010, 86, 1–5. [Google Scholar] [CrossRef]
- Mackenzie, A.E.; Lappin, J.E.; Taylor, D.L.; Nicklin, S.A.; Milligan, G. GPR35 as a Novel Therapeutic Target. Front. Endocrinol. 2011, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Farooq, S.M.; Hou, Y.; Li, H.; O’Meara, M.; Wang, Y.; Li, C.; Wang, J.M. Disruption of GPR35 Exacerbates Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2018, 63, 2910–2922. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Mukherjee, D.; Kao, J.P.Y.; Kanold, P.O. Early peripheral activity alters nascent subplate circuits in the auditory cortex. Sci. Adv. 2021, 7, eabc9155. [Google Scholar] [CrossRef]
- Shnerson, A.; Pujol, R. Age-related changes in the C57BL/6J mouse cochlea. I. Physiological findings. Brain Res. 1981, 254, 65–75. [Google Scholar] [CrossRef]
- Sobkowicz, H.M.; Bereman, B.; Rose, J.E. Organotypic development of the organ of Corti in culture. J. Neurocytol. 1975, 4, 543–572. [Google Scholar] [CrossRef]
- Dulon, D.; Ryan, A.F. The bacterial Neo gene confers neomycin resistance to mammalian cochlear hair cells. Neuroreport 1999, 10, 1189–1193. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, T.; Yu, H.; Hu, B.H.; Hu, B.; Ma, P.; Yang, Y.; Yang, N.; Hu, J.; Cao, T.; et al. Autophagy impairment as a key feature for acetaminophen-induced ototoxicity. Cell Death Dis. 2021, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Gould, H.J.; Sutton, B.J. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008, 8, 205–217. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Orinska, Z.; Bulanova, E.; Budagian, V.; Metz, M.; Maurer, M.; Bulfone-Paus, S. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood 2005, 106, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef]
- Sandig, H.; Bulfone-Paus, S. TLR signaling in mast cells: Common and unique features. Front. Immunol. 2012, 3, 185. [Google Scholar] [CrossRef] [Green Version]
- Supajatura, V.; Ushio, H.; Nakao, A.; Akira, S.; Okumura, K.; Ra, C.; Ogawa, H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Investig. 2002, 109, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Graevskaya, E.E.; Akhalaya, M.Y.; Goncharenko, E.N. Effects of cold stress and epinephrine on degranulation of peritoneal mast cells in rats. Bull Exp. Biol. Med. 2001, 131, 333–335. [Google Scholar] [CrossRef]
- Trunova, G.V. Morphofunctional characteristic of mast cells in BALB/c and C57Bl/6 mice during cold exposure. Bull Exp. Biol. Med. 2004, 138, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Anniko, M.; Lim, D.; Wróblewski, R. Elemental composition of individual cells and tissues in the cochlea. Acta Oto-Laryngol. 1984, 98, 439–453. [Google Scholar] [CrossRef]
- Juhn, S.K.; Rybak, L.P. Labyrinthine barriers and cochlear homeostasis. Acta Oto-Laryngol. 1981, 91, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Lundequist, A.; Pejler, G. Biological implications of preformed mast cell mediators. Cell. Mol. Life Sci. 2011, 68, 965–975. [Google Scholar] [CrossRef]
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.K.; Drescher, M.J.; Hatfield, J.S.; Drescher, D.G. Selective expression of serotonin receptor transcripts in the mammalian cochlea and its subdivisions. Brain Res. Mol. Brain Res. 1999, 70, 135–140. [Google Scholar] [CrossRef]
- Takumida, M.; Takumida, H.; Anniko, M. Localization of histamine (H1, H2, H3 and H4) receptors in mouse inner ear. Acta Oto-Laryngol. 2016, 136, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast cells synthesize, store, and release nerve growth factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaper, S.D. Nerve growth factor: A neuroimmune crosstalk mediator for all seasons. Immunology 2017, 151, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liu, H.; Liu, F.; Dong, Z. Mitochondrial dysregulation and protection in cisplatin nephrotoxicity. Arch. Toxicol. 2014, 88, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxid Med. Cell. Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef]
- Tsushida, K.; Tanabe, K.; Masuda, K.; Tanimura, S.; Miyake, H.; Arata, Y.; Sugiyama, H.; Wada, J. Estrogen-related receptor alpha is essential for maintaining mitochondrial integrity in cisplatin-induced acute kidney injury. Biochem. Biophys. Res. Commun. 2018, 498, 918–924. [Google Scholar] [CrossRef]
- Islam, F.; Islam, M.M.; Khan Meem, A.F.; Nafady, M.H.; Islam, M.R.; Akter, A.; Mitra, S.; Alhumaydhi, F.A.; Emran, T.B.; Khusro, A.; et al. Multifaceted role of polyphenols in the treatment and management of neurodegenerative diseases. Chemosphere 2022, 307, 136020. [Google Scholar] [CrossRef]
- Wood Dos Santos, T.; Cristina Pereira, Q.; Teixeira, L.; Gambero, A.; Villena, A.J.; Lima Ribeiro, M. Effects of Polyphenols on Thermogenesis and Mitochondrial Biogenesis. Int. J. Mol. Sci. 2018, 19, 2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal. 2018, 29, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Haftcheshmeh, S.M.; Mirhafez, S.R.; Abedi, M.; Heydarlou, H.; Shakeri, A.; Mohammadi, A.; Sahebkar, A. Therapeutic potency of curcumin for allergic diseases: A focus on immunomodulatory actions. Biomed. Pharmacother. 2022, 154, 113646. [Google Scholar] [CrossRef]
- Ribatti, D. The Staining of Mast Cells: A Historical Overview. Int. Arch. Allergy Immunol. 2018, 176, 55–60. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karayay, B.; Olze, H.; Szczepek, A.J. Degranulation of Murine Resident Cochlear Mast Cells: A Possible Factor Contributing to Cisplatin-Induced Ototoxicity and Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 4620. https://doi.org/10.3390/ijms24054620
Karayay B, Olze H, Szczepek AJ. Degranulation of Murine Resident Cochlear Mast Cells: A Possible Factor Contributing to Cisplatin-Induced Ototoxicity and Neurotoxicity. International Journal of Molecular Sciences. 2023; 24(5):4620. https://doi.org/10.3390/ijms24054620
Chicago/Turabian StyleKarayay, Betül, Heidi Olze, and Agnieszka J. Szczepek. 2023. "Degranulation of Murine Resident Cochlear Mast Cells: A Possible Factor Contributing to Cisplatin-Induced Ototoxicity and Neurotoxicity" International Journal of Molecular Sciences 24, no. 5: 4620. https://doi.org/10.3390/ijms24054620