Recent Advances in the Preparation of Delivery Systems for the Controlled Release of Scents
Abstract
:1. Introduction
2. Polymer- and Gel-Based Release Systems
3. Ionic Liquid-Based Release Systems
4. Metal-Organic Framework-Based Release Systems
5. Organic Salt-Based Release Systems
6. Coumarin-Based Release Systems
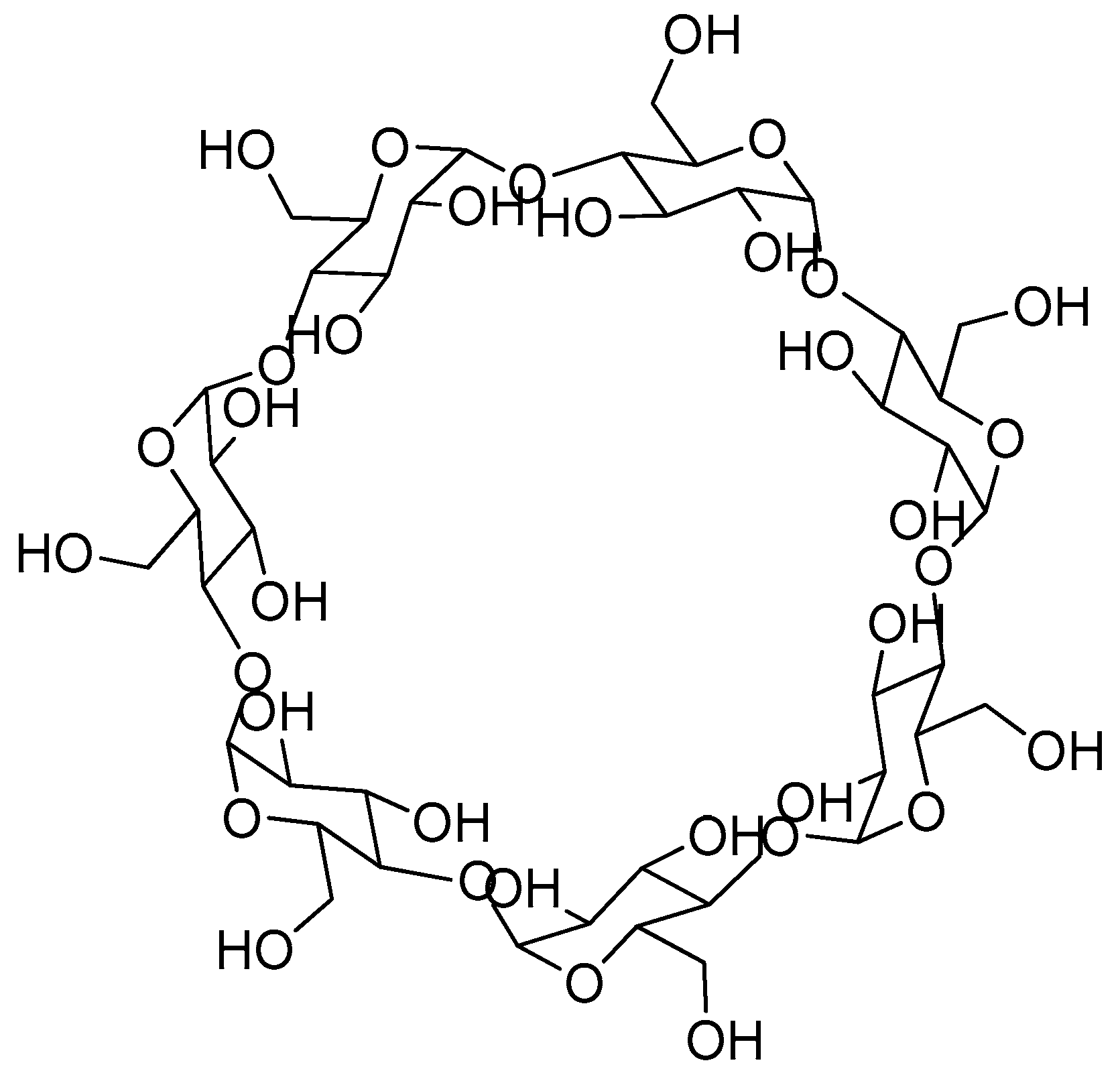
7. Cyclodextrin-Based Release Systems
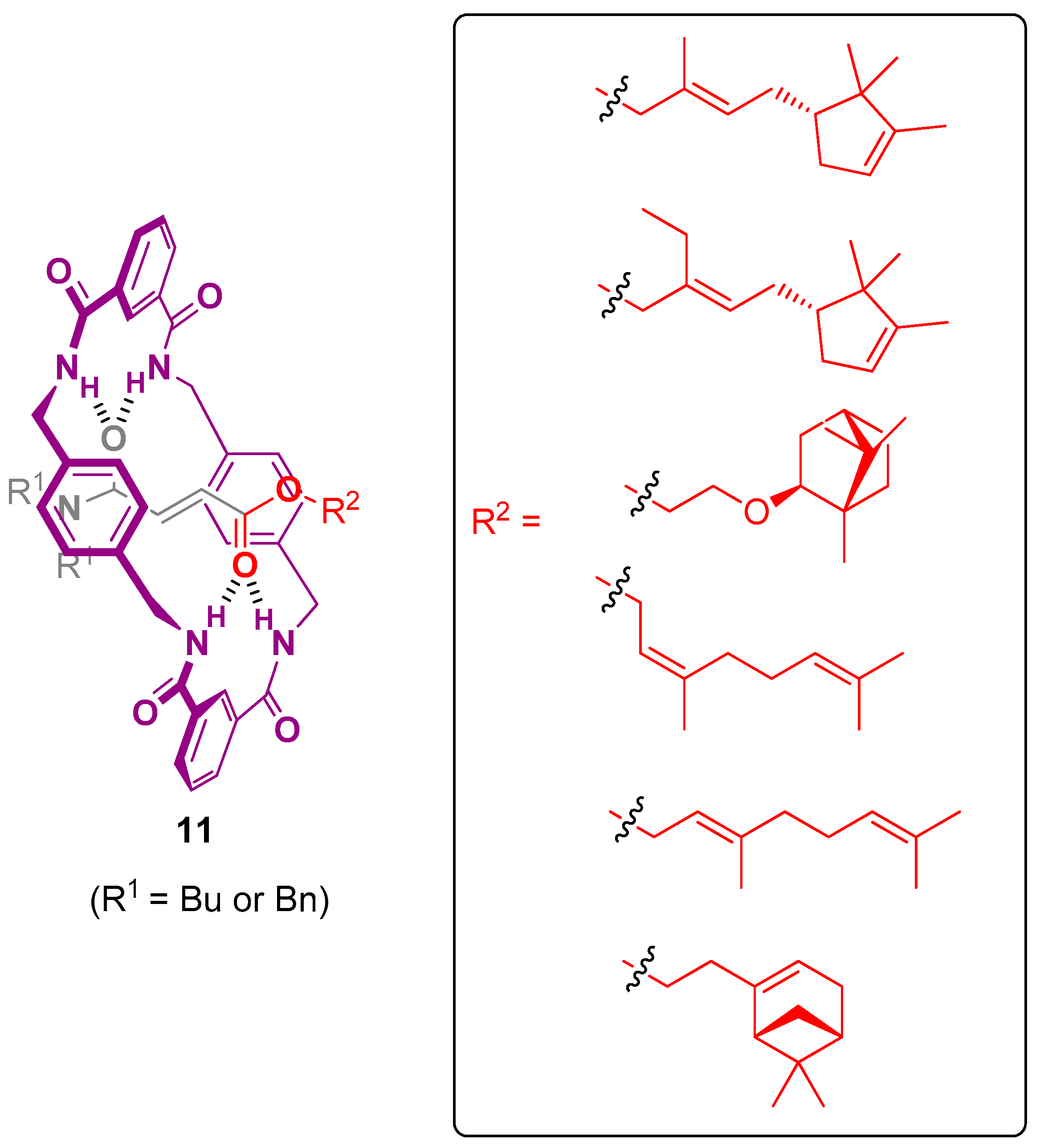
8. Rotaxane-Based Release Systems
9. General Discussion
10. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fortineau, A.-D. Chemistry Perfumes Your Daily Life. J. Chem. Educ. 2004, 81, 45–50. [Google Scholar] [CrossRef]
- Schilling, B.; Kaiser, R.; Natsch, A.; Gautschi, M. Investigation of odors in the fragrance industry. Chemoecology 2009, 20, 135–147. [Google Scholar] [CrossRef]
- Logan, J.L.; Rumbaugh, C.E. The Chemistry of Perfume: A Laboratory Course for Nonscience Majors. J. Chem. Educ. 2012, 89, 613–619. [Google Scholar] [CrossRef]
- Epstein, J.L.; Castaldi, M.; Patel, G.; Telidecki, P.; Karakkatt, K. Using Flavor Chemistry to Design and Synthesize Artificial Scents and Flavors. J. Chem. Educ. 2014, 92, 954–957. [Google Scholar] [CrossRef]
- Francl, M. Scents and sensibility. Nat. Chem. 2015, 7, 265–266. [Google Scholar] [CrossRef]
- Goss, K.-U. The physical chemistry of odors—Consequences for the work with detection dogs. Forensic Sci. Int. 2019, 296, 110–114. [Google Scholar] [CrossRef]
- Quellet, C.; Schudel, M.; Ringgenberg, R. Flavors & Fragrance Delivery Systems. Chimia 2001, 55, 421. [Google Scholar] [CrossRef]
- Herrmann, A. Controlled Release of Volatiles under Mild Reaction Conditions: From Nature to Everyday Products. Angew. Chem. Int. Ed. 2007, 46, 5836–5863. [Google Scholar] [CrossRef]
- Derrer, S.; Flachsmann, F.; Plessis, C.; Stang, M. Applied Photochemistry–Light Controlled Perfume Release. Chimia 2007, 61, 665–669. [Google Scholar] [CrossRef]
- Herrmann, A. Photochemistry of 2-Oxoacetates: From Mechanistic Insights to Profragrances and Bursting Capsules. Chimia 2019, 74, 39–48. [Google Scholar] [CrossRef]
- Shahriari, M.; Torchilin, V.P.; Taghdisi, S.M.; Abnous, K.; Ramezani, M.; Alibolandi, M. “Smart” self-assembled structures: Toward intelligent dual responsive drug delivery systems. Biomater. Sci. 2020, 8, 5787–5803. [Google Scholar] [CrossRef] [PubMed]
- Lugger, S.J.D.; Houben, S.J.A.; Foelen, Y.; Debije, M.G.; Schenning, A.P.H.J.; Mulder, D.J. Hydrogen-Bonded Supramolecular Liquid Crystal Polymers: Smart Materials with Stimuli-Responsive, Self-Healing, and Recyclable Properties. Chem. Rev. 2021, 122, 4946–4975. [Google Scholar] [CrossRef] [PubMed]
- Saura-Sanmartin, A. Photoresponsive Metal-Organic Frameworks as Adjustable Scaffolds in Reticular Chemistry. Int. J. Mol. Sci. 2022, 23, 7121. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Q.; Yin, Y. Colloidal Self-Assembly Approaches to Smart Nanostructured Materials. Chem. Rev. 2021, 122, 4976–5067. [Google Scholar] [CrossRef] [PubMed]
- Seale, J.S.W.; Feng, Y.; Feng, L.; Astumian, R.D.; Stoddart, J.F. Polyrotaxanes and the pump paradigm. Chem. Soc. Rev. 2022, 51, 8450–8475. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Filipczak, N.; Torchilin, V.P. Cell penetrating peptides: A versatile vector for co-delivery of drug and genes in cancer. J. Control. Release 2020, 330, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Vahidi, O.; Moghbeli, M.R.; Ahmadi, S.; Asadnia, M.; Akhavan, O.; Seidi, F.; Rabiee, M.; Saeb, M.R.; Webster, T.J.; et al. Customizing nano-chitosan for sustainable drug delivery. J. Control. Release 2022, 350, 175–192. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Zarebkohan, A.; Salehi, R.; Rahimi, F.; Torchilin, V.P.; Hamblin, M.R.; Seifalian, A. An update on dual targeting strategy for cancer treatment. J. Control. Release 2022, 349, 67–96. [Google Scholar] [CrossRef]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef]
- Madani, F.; Esnaashari, S.S.; Webster, T.J.; Khosravani, M.; Adabi, M. Polymeric nanoparticles for drug delivery in glioblastoma: State of the art and future perspectives. J. Control. Release 2022, 349, 649–661. [Google Scholar] [CrossRef]
- Kaur, R.; Kukkar, D.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Potential use of polymers and their complexes as media for storage and delivery of fragrances. J. Control. Release 2018, 285, 81–95. [Google Scholar] [CrossRef]
- Manfredini, N.; Ilare, J.; Invernizzi, M.; Polvara, E.; Mejia, D.C.; Sironi, S.; Moscatelli, D.; Sponchioni, M. Polymer Nanoparticles for the Release of Fragrances: How the Physicochemical Properties Influence the Adsorption on Textile and the Delivery of Limonene. Ind. Eng. Chem. Res. 2020, 59, 12766–12773. [Google Scholar] [CrossRef]
- Wei, M.; Pan, X.; Rong, L.; Dong, A.; He, Y.; Song, X.; Li, J. Polymer carriers for controlled fragrance release. Mater. Res. Express 2020, 7, 082001. [Google Scholar] [CrossRef]
- Pithanthanakul, U.; Vatanyoopaisarn, S.; Thumthanaruk, B.; Puttanlek, C.; Uttapap, D.; Kietthanakorn, B.; Rungsardthong, V. Encapsulation of fragrances in zein nanoparticles and use as fabric softener for textile application. Flavour Fragr. J. 2021, 36, 365–373. [Google Scholar] [CrossRef]
- Ali, M.; Meaney, S.; Abedin, J.; Holt, P.; Majumder, M.; Tabor, R.F. Graphene oxide–silica hybrid capsules for sustained fragrance release. J. Colloid Interface Sci. 2019, 552, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Afonso, C.; Fernandes, I.; Barreiro, M.-F.; Costa, P.; Rodrigues, A.E. Chitosan-cellulose particles as delivery vehicles for limonene fragrance. Ind. Crops Prod. 2019, 139, 111407. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, K.; Zhao, M.; Wang, S.; Zhou, Z.; Shen, Y.; Jiang, L. A pH-responsive fragrance release system based on pseudopeptide polymeric micelles. React. Funct. Polym. 2018, 132, 138–144. [Google Scholar] [CrossRef]
- Liu, M.; Yan, C.; Han, J.; Guo, Z.; Zhu, W.; Xiao, Z.; Wu, Y.; Huang, J. pH-activated polymeric profragrances for dual-controllable perfume release. AIChE J. 2021, 67, e17265. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, T.; Lu, Z.; Shen, J.; Xiao, Z.; Zhang, X. Synthesis and characterization of novel biocompatible nanocapsules encapsulated lily fragrance. Chin. Chem. Lett. 2018, 30, 739–742. [Google Scholar] [CrossRef]
- Tchakalova, V.; Lutz, E.; Lamboley, S.; Moulin, E.; Benczédi, D.; Giuseppone, N.; Herrmann, A. Design of Stimuli-Responsive Dynamic Covalent Delivery Systems for Volatile Compounds (Part 2): Fragrance-Releasing Cleavable Surfactants in Functional Perfumery Applications. Chem.—A Eur. J. 2021, 27, 13468–13476. [Google Scholar] [CrossRef]
- Trachsel, A.; Paret, N.; Berthier, D.L.; Herrmann, A. Light-Induced Fragrance Release from 2-Oxoacetates: Impact of Compound Mixtures on the Efficiency of the Norrish Type II Photoreaction in Solution and in Encapsulation Systems. Chemphotochem 2022, 6, e202200045. [Google Scholar] [CrossRef]
- Lamboley, S.; Trachsel, A.; Herrmann, A. Polystyrene-Based 2-Oxoacetates for the Light-Induced Release of Fragrances Under Realistic Application Conditions. Macromol. Chem. Phys. 2020, 221, 2000196. [Google Scholar] [CrossRef]
- Paret, N.; Trachsel, A.; Berthier, D.L.; Herrmann, A. Developing Multi Stimuli-Responsive Core/Shell Microcapsules to Control the Release of Volatile Compounds. Macromol. Mater. Eng. 2018, 304, 1800599. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, K.; Lee, Y.; Yang, K.; Choe, D.; Roh, Y.H. Thermoresponsive semi-interpenetrating gelatin-alginate networks for encapsulation and controlled release of scent molecules. Int. J. Biol. Macromol. 2022, 208, 1096–1105. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Z.; Wang, X.; Shen, J.; Wang, J.; Niu, Y.; Xiao, Z.; Zhang, X. Zwitterionic comb-like lipid polymers encapsulating linalool for increasing the fragrance retention time. Chin. Chem. Lett. 2020, 32, 573–576. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Zhang, D. Stimuli responsive gels based on low molecular weight gelators. J. Mater. Chem. 2011, 22, 38–50. [Google Scholar] [CrossRef]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Draper, E.R.; Adams, D.J. Controlling the Assembly and Properties of Low-Molecular-Weight Hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Luo, Y.; Li, F.; Jian, X.; Liu, Y. Development of Natural-Drugs-Based Low-Molecular-Weight Supramolecular Gels. Gels 2021, 7, 105. [Google Scholar] [CrossRef]
- Adams, D.J. Personal Perspective on Understanding Low Molecular Weight Gels. J. Am. Chem. Soc. 2022, 144, 11047–11053. [Google Scholar] [CrossRef]
- Nicastro, G.; Black, L.M.; Ravarino, P.; D’Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.; Buggy, M. Hydrogen-bonded supramolecular polymers: A literature review. J. Mater. Sci. 2005, 40, 547–559. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Subramanian, V.; Sathyamurthy, N. Hydrogen Bonding without Borders: An Atoms-in-Molecules Perspective. J. Phys. Chem. A 2006, 110, 3349–3351. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Hunter, C.A.; Low, C.M.R.; Perez-Velasco, A.; Vinter, J.G. Solvent Effects on Hydrogen Bonding. Angew. Chem. Int. Ed. 2007, 46, 3706–3709. [Google Scholar] [CrossRef]
- Bertrand, A.; Lortie, F.; Bernard, J. Routes to Hydrogen Bonding Chain-End Functionalized Polymers. Macromol. Rapid Commun. 2012, 33, 2062–2091. [Google Scholar] [CrossRef]
- Sobczyk, L.; Chudoba, D.; Tolstoy, P.M.; Filarowski, A. Some Brief Notes on Theoretical and Experimental Investigations of Intramolecular Hydrogen Bonding. Molecules 2016, 21, 1657. [Google Scholar] [CrossRef] [Green Version]
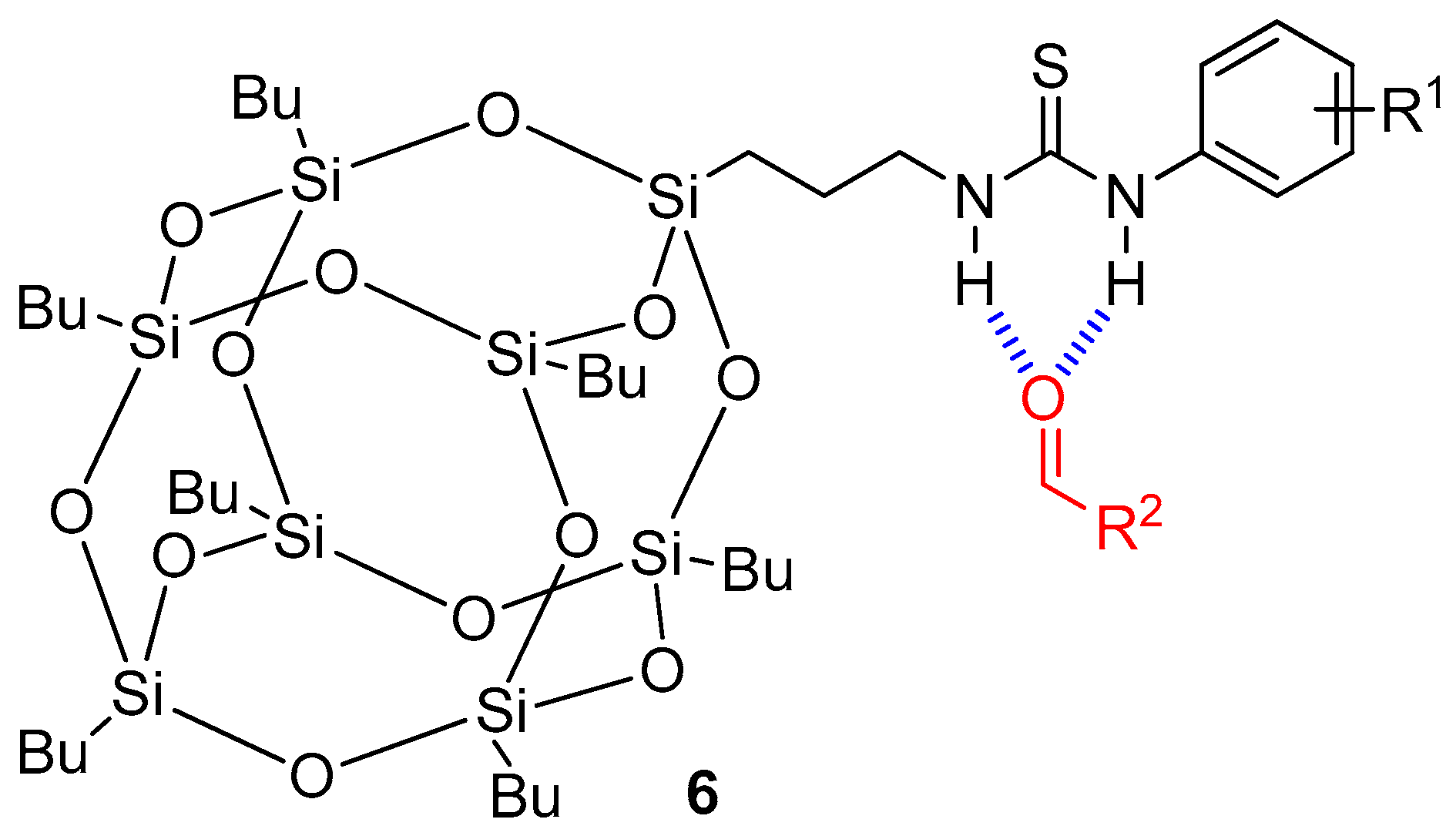
- Xue, C.; Liu, M.; Zhang, Z.-A.; Han, J.; Wang, C.; Wang, L.; Xiao, Z.; Zhu, W.-H. Controllable Fragrance Release Mediated by Spontaneous Hydrogen Bonding with POSS–Thiourea Derivatives. CCS Chem. 2020, 2, 478–487. [Google Scholar] [CrossRef]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. USA 2018, 115, 7296–7301. [Google Scholar] [CrossRef] [Green Version]
- Tanner, E.; Curreri, A.M.; Balkaran, J.P.R.; Selig-Wober, N.C.; Yang, A.; Kendig, C.; Fluhr, M.P.; Kim, N.; Mitragotri, S. Design Principles of Ionic Liquids for Transdermal Drug Delivery. Adv. Mater. 2019, 31, e1901103. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.M.; Silva, S.S.; Reis, R.L. Biocompatible ionic liquids: Fundamental behaviours and applications. Chem. Soc. Rev. 2019, 48, 4317–4335. [Google Scholar] [CrossRef]
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2019, 25, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Greer, A.; Jacquemin, J.; Hardacre, C. Industrial Applications of Ionic Liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2019, 297, 112038. [Google Scholar] [CrossRef]
- Pedro, S.; Freire, C.R.; Silvestre, A.; Freire, M. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Philippi, F.; Welton, T. Targeted modifications in ionic liquids–from understanding to design. Phys. Chem. Chem. Phys. 2021, 23, 6993–7021. [Google Scholar] [CrossRef]
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E.L. Recent Advances in Ionic Liquids in Biomedicine. Adv. Sci. 2021, 8, e2004819. [Google Scholar] [CrossRef]
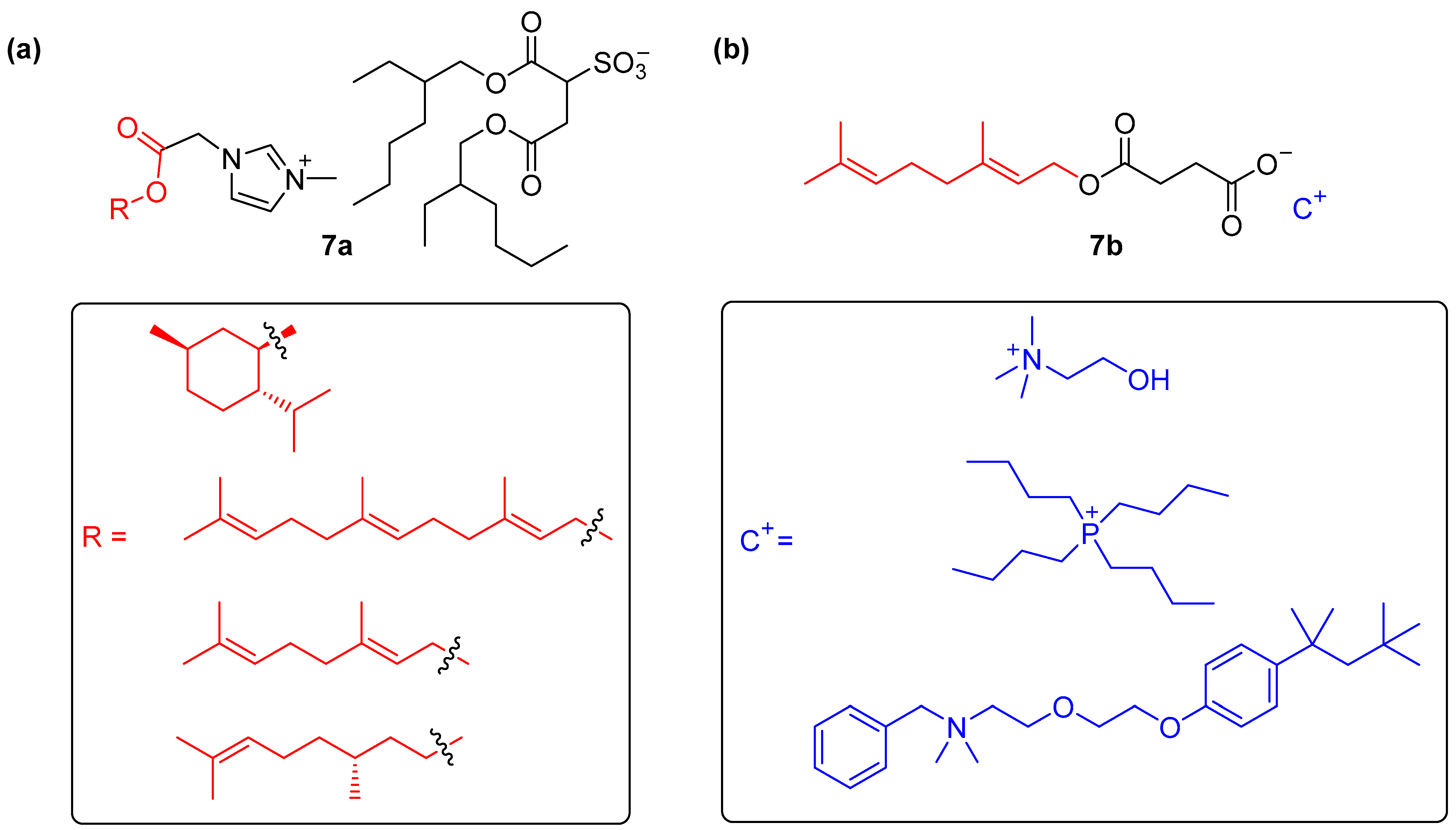
- Berton, P.; Shamshina, J.L.; Bica, K.; Rogers, R.D. Ionic Liquids as Fragrance Precursors: Smart Delivery Systems for Volatile Compounds. Ind. Eng. Chem. Res. 2018, 57, 16069–16076. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.-L.; Xu, Q. Metal-Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2017, 30, e1703663. [Google Scholar] [CrossRef]
- He, Y.; Chen, F.; Li, B.; Qian, G.; Zhou, W.; Chen, B. Porous metal–organic frameworks for fuel storage. Co-ord. Chem. Rev. 2018, 373, 167–198. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, e1705189. [Google Scholar] [CrossRef]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal–Organic Frameworks for Food Safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Lin, W. Nanoscale metal–organic frameworks for phototherapy of cancer. Co-ord. Chem. Rev. 2017, 379, 65–81. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, e1906846. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jana, D.; Zhao, Y. Metal–Organic Framework Derived Nanozymes in Biomedicine. Accounts Chem. Res. 2020, 53, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air, Anywhere, Anytime. ACS Cent. Sci. 2020, 6, 1348–1354. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.-K.; Falcaro, P.; Doonan, C.J. Metal–Organic Framework-Based Enzyme Biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef]
- Saura-Sanmartin, A.; Pastor, A.; Martinez-Cuezva, A.; Cutillas-Font, G.; Alajarin, M.; Berna, J. Mechanically interlocked molecules in metal–organic frameworks. Chem. Soc. Rev. 2022, 51, 4949–4976. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Tang, Y.; Shen, H.; Li, J.; Yi, Z.; Ke, Q.; Xu, H. Copper-Based Metal–Organic Framework as a Controllable Nitric Oxide-Releasing Vehicle for Enhanced Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 18319–18331. [Google Scholar] [CrossRef]
- Javanbakhta, S.; Hemmatia, A.; Namaziab, H.; Heydari, A. [Protected], Carboxymethylcellulose-coated 5-fluorouracil@MOF-5 nano-hybrid as a bio-nanocomposite carrier for the anticancer oral delivery. Int. J. Biol. Macromol. 2019, 155, 876–882. [Google Scholar] [CrossRef]
- Saura-Sanmartin, A.; Martinez-Cuezva, A.; Bautista, D.; Marzari, M.R.B.; Martins, M.A.P.; Alajarin, M.; Berna, J. Copper-Linked Rotaxanes for the Building of Photoresponsive Metal Organic Frameworks with Controlled Cargo Delivery. J. Am. Chem. Soc. 2020, 142, 13442–13449. [Google Scholar] [CrossRef]
- Pinto, R.V.; Wang, S.; Tavares, S.R.; Pires, J.; Antunes, F.; Vimont, A.; Clet, G.; Daturi, M.; Maurin, G.; Serre, C.; et al. Tuning Cellular Biological Functions Through the Controlled Release of NO from a Porous Ti-MOF. Angew. Chem. Int. Ed. 2020, 59, 5135–5143. [Google Scholar] [CrossRef]
- Kathuria, A.; Harding, T.; Auras, R.; Kivy, M.B. Encapsulation of hexanal in bio-based cyclodextrin metal organic framework for extended release. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 121–130. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Asl, E.A.; Namazi, H. A new pH-sensitive CS/Zn-MOF@GO ternary hybrid compound as a biofriendly and implantable platform for prolonged 5-Fluorouracil delivery to human breast cancer cells. J. Alloys Compd. 2021, 885, 160992. [Google Scholar] [CrossRef]
- Saura-Sanmartin, A.; Martinez-Cuezva, A.; Marin-Luna, M.; Bautista, D.; Berna, J. Effective Encapsulation of C 60 by Metal–Organic Frameworks with Polyamide Macrocyclic Linkers. Angew. Chem. Int. Ed. 2021, 60, 10814–10819. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ren, X.; Guo, T.; Cao, Z.; Sun, H.; Wang, C.; Wang, F.; Shu, Z.; Hao, J.; Gui, S.; et al. Controlled release of iodine from cross-linked cyclodextrin metal-organic frameworks for prolonged periodontal pocket therapy. Carbohydr. Polym. 2021, 267, 118187. [Google Scholar] [CrossRef]
- Chen, X.-X.; Hou, M.-J.; Mao, G.-J.; Wang, W.-X.; Xu, F.; Li, Y.; Li, C.-Y. ATP-responsive near-infrared fluorescence MOF nanoprobe for the controlled release of anticancer drug. Microchim. Acta 2021, 188, 287. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, G.; Xiong, C.; Wang, C.; Lian, X.; Hu, L.; Li, Z.; Dalgarno, S.J.; Yang, Y.-W.; Tian, J. One-Pot Fabrication of Hollow Porphyrinic MOF Nanoparticles with Ultrahigh Drug Loading toward Controlled Delivery and Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2021, 13, 3679–3693. [Google Scholar] [CrossRef]
- Yang, J.; Ma, S.; Xu, R.; Wei, Y.; Zhang, J.; Zuo, T.; Wang, Z.; Deng, H.; Yang, N.; Shen, Q. Smart biomimetic metal organic frameworks based on ROS-ferroptosis-glycolysis regulation for enhanced tumor chemo-immunotherapy. J. Control. Release 2021, 334, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Ye, L.; Lv, Y.; Zhou, Z.; Shen, Y.; He, Y.; Jiang, L. Encapsulation of Highly Volatile Fragrances in Y Zeolites for Sustained Release: Experimental and Theoretical Studies. ACS Omega 2020, 5, 31925–31935. [Google Scholar] [CrossRef]
- Costa, S.P.; Soares, O.S.; Aguiar, C.A.; Neves, I.C. Fragrance carriers obtained by encapsulation of volatile aromas into zeolite structures. Ind. Crop. Prod. 2022, 187, 115397. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Liu, K.; Zhou, Z.; Jiang, L.; Shen, Y.; Zhao, D. Biocompatible Cyclodextrin-Based Metal–Organic Frameworks for Long-Term Sustained Release of Fragrances. Ind. Eng. Chem. Res. 2019, 58, 19767–19777. [Google Scholar] [CrossRef]
- Smaldone, R.A.; Forgan, R.; Furukawa, H.; Gassensmith, J.J.; Slawin, A.M.Z.; Yaghi, O.M.; Stoddart, J.F. Metal-Organic Frameworks from Edible Natural Products. Angew. Chem. Int. Ed. 2010, 49, 8630–8634. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Huang, J.; Zhou, Z.; Zhao, D.; Jiang, L.; Shen, Y. Encapsulation and controlled release of fragrances from functionalized porous metal-organic frameworks. AIChE J. 2018, 65, 491–499. [Google Scholar] [CrossRef]
- Hu, Z.; Peng, Y.; Kang, Z.; Qian, Y.; Zhao, D. A Modulated Hydrothermal (MHT) Approach for the Facile Synthesis of UiO-66-Type MOFs. Inorg. Chem. 2015, 54, 4862–4868. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Farooq, S.; Zhao, D. A highly stable metal-organic framework with optimum aperture size for CO 2 capture. AIChE J. 2017, 63, 4103–4114. [Google Scholar] [CrossRef]
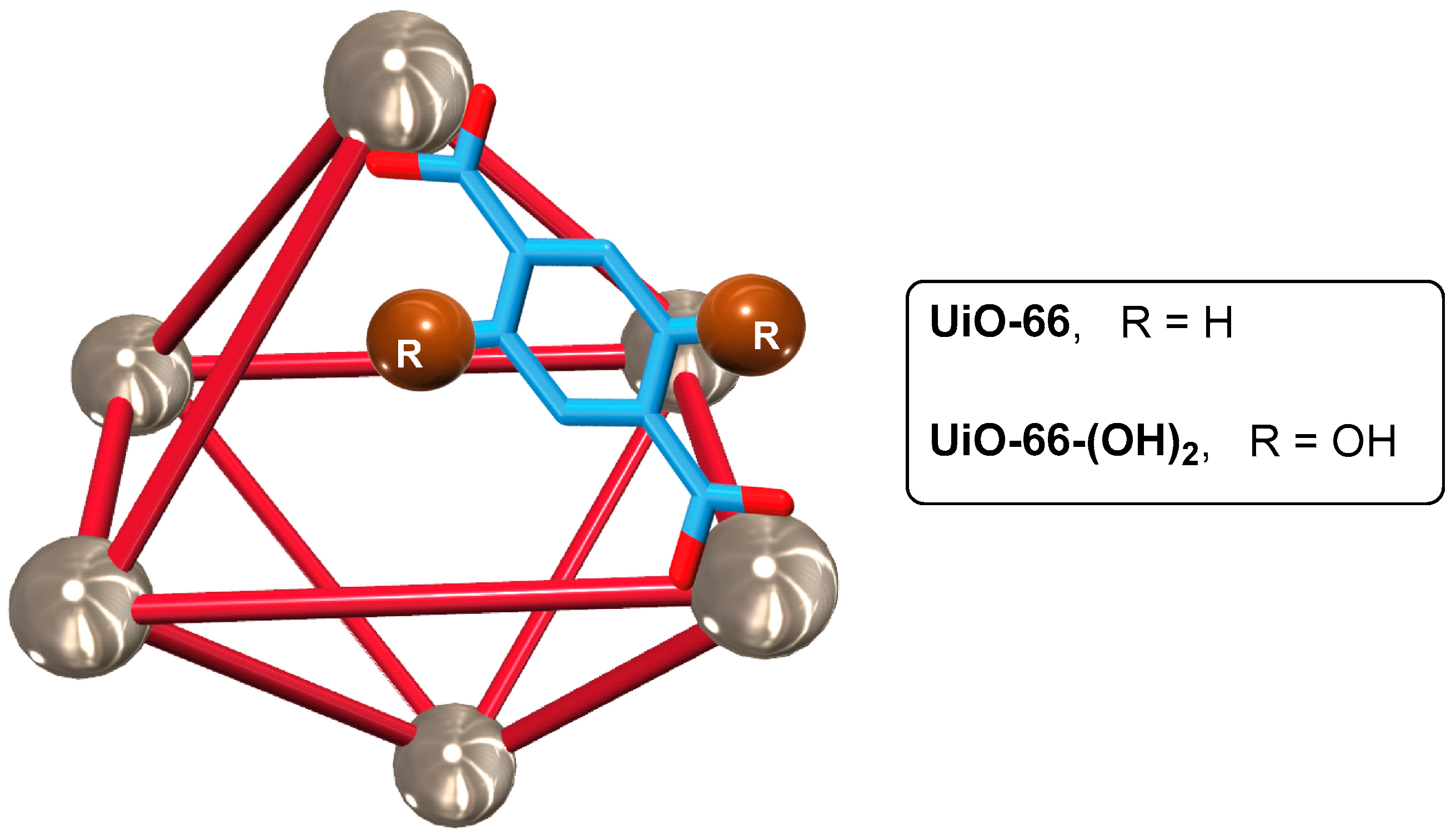
- Mao, D.; Xie, C.; Li, Z.; Hong, L.; Qu, R.; Gao, Y.; He, J.; Wang, J. Adsorption and controlled release of three kinds of flavors on UiO-66. Food Sci. Nutr. 2020, 8, 1914–1922. [Google Scholar] [CrossRef]
- Silva, C.G.; Luz, I.; Llabres i Xamena, F.X.; Corma, A.; García, H. Water Stable Zr-Benzenedicarboxylate Metal-Organic Frameworks as Photocatalysts for Hydrogen Generation. Chem.—A Eur. J. 2010, 16, 11133–11138. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H.; Zhang, B.; Jiang, L.; Shen, Y.; Fu, E.; Zhao, D.; Zhou, Z. Tuning the release rate of volatile molecules by pore surface engineering in metal-organic frameworks. Chin. Chem. Lett. 2020, 32, 1988–1992. [Google Scholar] [CrossRef]
- Wu, C.-J.; Liu, Y.-F.; Zhang, W.-F.; Zhang, C.; Chai, G.-B.; Zhang, Q.-D.; Mao, J.; Ahmad, I.; Zhang, S.-S.; Xie, J.-P. Encapsulation and controlled release of fragrances from MIL-101(Fe)-based recyclable magnetic nanoporous carbon. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 640, 128453. [Google Scholar] [CrossRef]
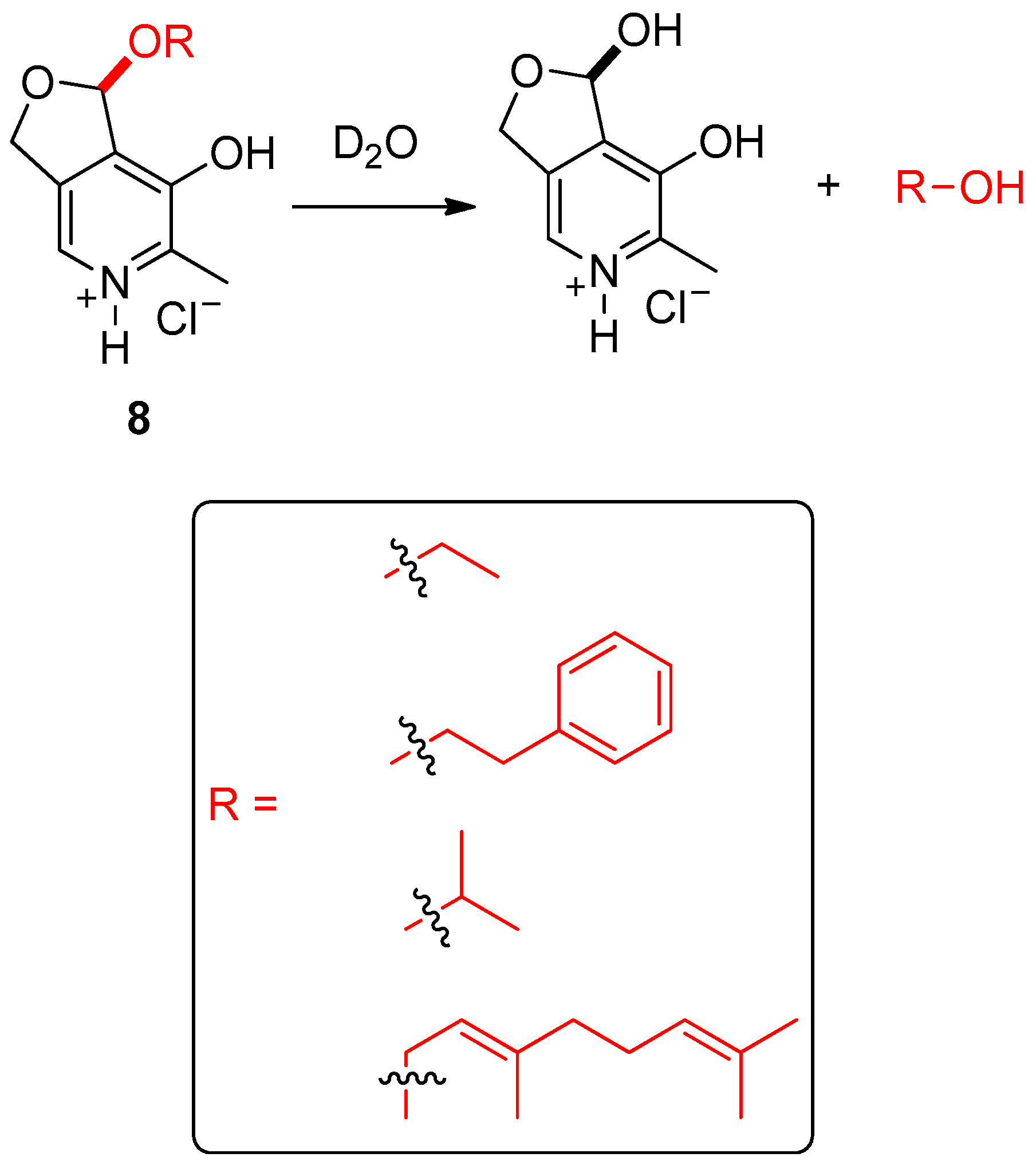
- Weeks, K.L.; Rutkowski, K.R.; Loyola, A.A.M.; Boyce, G.R. Utilization of pyridoxal acetal salts as water-triggered, slow-release pro-fragrances. New J. Chem. 2018, 42, 15538–15540. [Google Scholar] [CrossRef]
- Narayan, S.; Metaxas, A.E.; Bachnak, R.; Neumiller, T.; Dutcher, C.S. Zooming in on the role of surfactants in droplet coalescence at the macroscale and microscale. Curr. Opin. Colloid Interface Sci. 2020, 50, 101385. [Google Scholar] [CrossRef]
- Siyal, A.A.; Shamsuddin, M.R.; Low, A.; Rabat, N.E. A review on recent developments in the adsorption of surfactants from wastewater. J. Environ. Manag. 2019, 254, 109797. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Rakshit, A.; Acharjee, A.; Saha, B. Biodegradability and biocompatibility: Advancements in synthetic surfactants. J. Mol. Liq. 2020, 324, 115105. [Google Scholar] [CrossRef]
- Tsarkova, L.A.; Gurkov, T.D. Volatile surfactants: Characterization and areas of application. Curr. Opin. Colloid Interface Sci. 2022, 60, 101592. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, M.; Han, J.; Wang, L.; Xiao, Z.; Zhu, W.-H. Hydrolyzable Quaternary Pyridinium Surfactants: Antimicrobial Profragrances for Controllable Perfume Release. Ind. Eng. Chem. Res. 2022, 61, 4202–4211. [Google Scholar] [CrossRef]
- Wang, W.; Camenisch, G.; Sane, D.C.; Zhang, H.; Hugger, E.; Wheeler, G.L.; Borchardt, R.T.; Wang, B. A coumarin-based prodrug strategy to improve the oral absorption of RGD peptidomimetics. J. Control. Release 2000, 65, 245–251. [Google Scholar] [CrossRef]
- Lin, H.-M.; Wang, W.-K.; Hsiung, P.-A.; Shyu, S.-G. Light-sensitive intelligent drug delivery systems of coumarin-modified mesoporous bioactive glass. Acta Biomater. 2010, 6, 3256–3263. [Google Scholar] [CrossRef]
- Rahimi, S.; Khoee, S.; Ghandi, M. Development of photo and pH dual crosslinked coumarin-containing chitosan nanoparticles for controlled drug release. Carbohydr. Polym. 2018, 201, 236–245. [Google Scholar] [CrossRef]
- Wang, H.; Miao, W.; Wang, F.; Cheng, Y. A Self-Assembled Coumarin-Anchored Dendrimer for Efficient Gene Delivery and Light-Responsive Drug Delivery. Biomacromolecules 2018, 19, 2194–2201. [Google Scholar] [CrossRef]
- Arjmand, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Preparation of photolabile nanoparticles by coumarin-based crosslinker for drug delivery under light irradiation. J. Phys. Chem. Solids 2021, 154, 110102. [Google Scholar] [CrossRef]
- Liu, M.; Han, J.; Yan, C.; Guo, Z.; Xiao, Z.; Zhu, W.-H. Photocontrollable Release with Coumarin-Based Profragrances. ACS Appl. Bio Mater. 2019, 2, 4002–4009. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yan, C.; Han, J.; Guo, Z.; Wu, Y.; Huang, J.; Xiao, Z.; Zhu, W.-H. Engineering photo-controllable fragrance release with flash nanoprecipitation. Green Chem. Eng. 2021, 2, 301–308. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Loftsson, T.; Saokham, P.; Couto, A.R.S. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.D.S.; Quintans-Júnior, L.J.; Veiga Júnior, V.F.D.; De Lima, A.N. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, Y.; Liu, Y. Cyclodextrin-Based Multistimuli-Responsive Supramolecular Assemblies and Their Biological Functions. Adv. Mater. 2019, 32, e1806158. [Google Scholar] [CrossRef]
- Matencio, A.; Pedrazzo, A.R.; Difalco, A.; Navarro-Orcajada, S.; Monfared, Y.K.; Conesa, I.; Rezayat, A.; López-Nicolás, J.M.; Trotta, F. Advances and Classification of Cyclodextrin-Based Polymers for Food-Related Issues. Polymers 2021, 13, 4226. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.; Prieto, M.; Gandara, J.S. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science. A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Xiao, Z.; Deng, J.; Niu, Y.; Zhu, G.; Zhu, J.; Liu, M.; Liu, S. Preparation of sustained-release fragrance based on the cavity structure of β-cyclodextrin and its application in cotton fabric. Text. Res. J. 2018, 89, 3466–3474. [Google Scholar] [CrossRef]
- Ishiguro, T.; Sakata, Y.; Arima, H.; Iohara, D.; Anraku, M.; Uekama, K.; Hirayama, F. Release control of fragrances by complexation with β-cyclodextrin and its derivatives. J. Incl. Phenom. Macrocycl. Chem. 2018, 92, 147–155. [Google Scholar] [CrossRef]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Lucas-Abellán, C.; Pellicer, J.A.; Pérez-Garrido, A.; Pérez-Sánchez, H.; Yáñez-Gascón, M.J.; Gabaldón, J.A.; Núñez-Delicado, E. Comprehensive Characterization of Linalool-HP-β-Cyclodextrin Inclusion Complexes. Molecules 2020, 25, 5069. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Deng, J.; Xiao, Z.; Kou, X.; Zhu, G.; Liu, M.; Liu, S. Preparation and slow release kinetics of apple fragrance/β-cyclodextrin inclusion complex. J. Therm. Anal. Calorim. 2020, 143, 3775–3781. [Google Scholar] [CrossRef]
- Fourtaka, K.; Christoforides, E.; Tzamalis, P.; Bethanis, K. Inclusion of citral isomers in native and methylated cyclodextrins: Structural insights by X-ray crystallography and molecular dynamics simulation analysis. J. Mol. Struct. 2021, 1234, 130169. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z.; Zhu, G. Fabrication and characterization of ethyl acetate–hydroxypropyl-β-cyclodextrin inclusion complex. J. Food Sci. 2021, 86, 3589–3597. [Google Scholar] [CrossRef]
- Xing, C.; Xu, X.; Song, L.; Wang, X.; Li, B.; Guo, K. β-Cyclodextrin-Based Poly (Vinyl Alcohol) Fibers for Sustained Release of Fragrances. Polymers 2022, 14, 2002. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yu, S.; Shen, Y.; Wu, H. Facile synthesis of self-dispersed β-cyclodextrin-coupled cellulose microgel for sustained release of vanillin. Int. J. Biol. Macromol. 2022, 208, 70–79. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, W.; Kang, Y.; Niu, Y.; Kou, X. Encapsulation and sustained release properties of watermelon flavor and its characteristic aroma compounds from γ-cyclodextrin inclusion complexes. Food Hydrocoll. 2019, 97, 105202. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of Pseudorotaxanes and Rotaxanes: From Synthesis to Stimuli-Responsive Motions to Applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-Y.; Zong, Q.-S.; Han, Y.; Chen, C.-F. Recent advances in higher order rotaxane architectures. Chem. Commun. 2020, 56, 9916–9936. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, B.T.; Maghsoudi, S.; Fatahi, Y.; Rabiee, N.; Bahadorikhalili, S.; Dinarvand, R.; Bagherzadeh, M.; Verpoort, F. The flowering of Mechanically Interlocked Molecules: Novel approaches to the synthesis of rotaxanes and catenanes. Coord. Chem. Rev. 2020, 423, 213484. [Google Scholar] [CrossRef]
- Qiu, Y.; Song, B.; Pezzato, C.; Shen, D.; Liu, W.; Zhang, L.; Feng, Y.; Guo, Q.-H.; Cai, K.; Li, W.; et al. A precise polyrotaxane synthesizer. Science 2020, 368, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Wang, W.; Wang, X.-Q.; Li, M.; Ke, Y.; Yao, R.; Wen, J.; Yin, G.-Q.; Jiang, B.; Li, X.; et al. Daisy Chain Dendrimers: Integrated Mechanically Interlocked Molecules with Stimuli-Induced Dimension Modulation Feature. J. Am. Chem. Soc. 2020, 142, 8473–8482. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Qiu, Y.; Guo, Q.-H.; Chen, Z.; Seale, J.S.W.; He, K.; Wu, H.; Feng, Y.; Farha, O.K.; Astumian, R.D.; et al. Active mechanisorption driven by pumping cassettes. Science 2021, 374, 1215–1221. [Google Scholar] [CrossRef]
- Wilson, B.H.; Vojvodin, C.S.; Gholami, G.; Abdulla, L.M.; O’Keefe, C.A.; Schurko, R.W.; Loeb, S.J. Precise Spatial Arrangement and Interaction between Two Different Mobile Components in a Metal-Organic Framework. Chem 2020, 7, 202–211. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhang, D.; Hu, Y.; Xu, W.; Xu, L.; Wang, W.; Yang, H. Artificial Light-Harvesting Systems Based on AIEgen-branched Rotaxane Dendrimers for Efficient Photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 18761–18768. [Google Scholar] [CrossRef]
- Li, M.; Chia, X.L.; Tian, C.; Zhu, Y. Mechanically planar chiral rotaxanes through catalytic desymmetrization. Chem 2022, 8, 2843–2855. [Google Scholar] [CrossRef]
- Geng, J.-S.; Mei, L.; Liang, Y.-Y.; Yuan, L.-Y.; Yu, J.-P.; Hu, K.-Q.; Feng, W.; Chai, Z.-F.; Shi, W.-Q. Controllable photomechanical bending of metal-organic rotaxane crystals facilitated by regioselective confined-space photodimerization. Nat. Commun. 2022, 13, 2030. [Google Scholar] [CrossRef]
- Lopez-Leonardo, C.; Saura-Sanmartin, A.; Marin-Luna, M.; Alajarin, M.; Martinez-Cuezva, A.; Berna, J. Ring-to-Thread Chirality Transfer in [2]Rotaxanes for the Synthesis of Enantioenriched Lactams. Angew. Chem. Int. Ed. 2022, 61, e202209904. [Google Scholar] [CrossRef] [PubMed]
- Binks, L.; Tian, C.; Fielden, S.D.P.; Vitorica-Yrezabal, I.J.; Leigh, D.A. Transamidation-Driven Molecular Pumps. J. Am. Chem. Soc. 2022, 144, 15838–15844. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jamagne, R.; Tetlow, D.J.; Leigh, D.A. A tape-reading molecular ratchet. Nature 2022, 612, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Barat, R.; Legigan, T.; Tranoy-Opalinski, I.; Renoux, B.; Péraudeau, E.; Clarhaut, J.; Poinot, P.; Fernandes, A.E.; Aucagne, V.; Leigh, D.A.; et al. A mechanically interlocked molecular system programmed for the delivery of an anticancer drug. Chem. Sci. 2015, 6, 2608–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kench, T.; Summers, P.A.; Kuimova, M.K.; Lewis, J.E.M.; Vilar, R. Rotaxanes as Cages to Control DNA Binding, Cytotoxicity, and Cellular Uptake of a Small Molecule**. Angew. Chem. Int. Ed. 2021, 60, 10928–10934. [Google Scholar] [CrossRef]
- D’Orchymont, F.; Holland, J.P. A rotaxane-based platform for tailoring the pharmacokinetics of cancer-targeted radiotracers. Chem. Sci. 2022, 13, 12713–12725. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.; Alajarin, M.; Pastor, A.; Berna, J. Mechanically Interlocked Profragrances for the Controlled Release of Scents. J. Org. Chem. 2021, 86, 15045–15054. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P.; Ga, L.; Ai, J. Advances in Applications of Molecular Logic Gates. ACS Omega 2021, 6, 30189–30204. [Google Scholar] [CrossRef]
- Popplewell, L.M.; Lee, K.; Johan, J.; Brain, J.; Zhen, Y. Polymer Encapsulated Fragrance Chemicals. U.S. Patent EP1407754, 14 March 2004. [Google Scholar]
- Winton, B.C.D.; Popplewell, L.M.; Lewis, M.; Semoff, S.; Lindauer, J.I.; Jerome, I.; Lee, K.; Zhen, Y.; Prol, M.B.; Pluyter, P.J.G. Polymer-Encapsulated Fragrances for Skin and Hair Compositions. U.S. Patent US20050226900, 13 October 2005. [Google Scholar]
- Wagenheim, L. Packaging Unit Having a Polymer Fragrance Carrier. U.S. Patent US20090155505, 13 December 2009. [Google Scholar]
- Wei, M.; Wan, Y.; Zhang, X. Metal-Organic Framework-Based Stimuli-Responsive Polymers. J. Compos. Sci. 2021, 5, 101. [Google Scholar] [CrossRef]
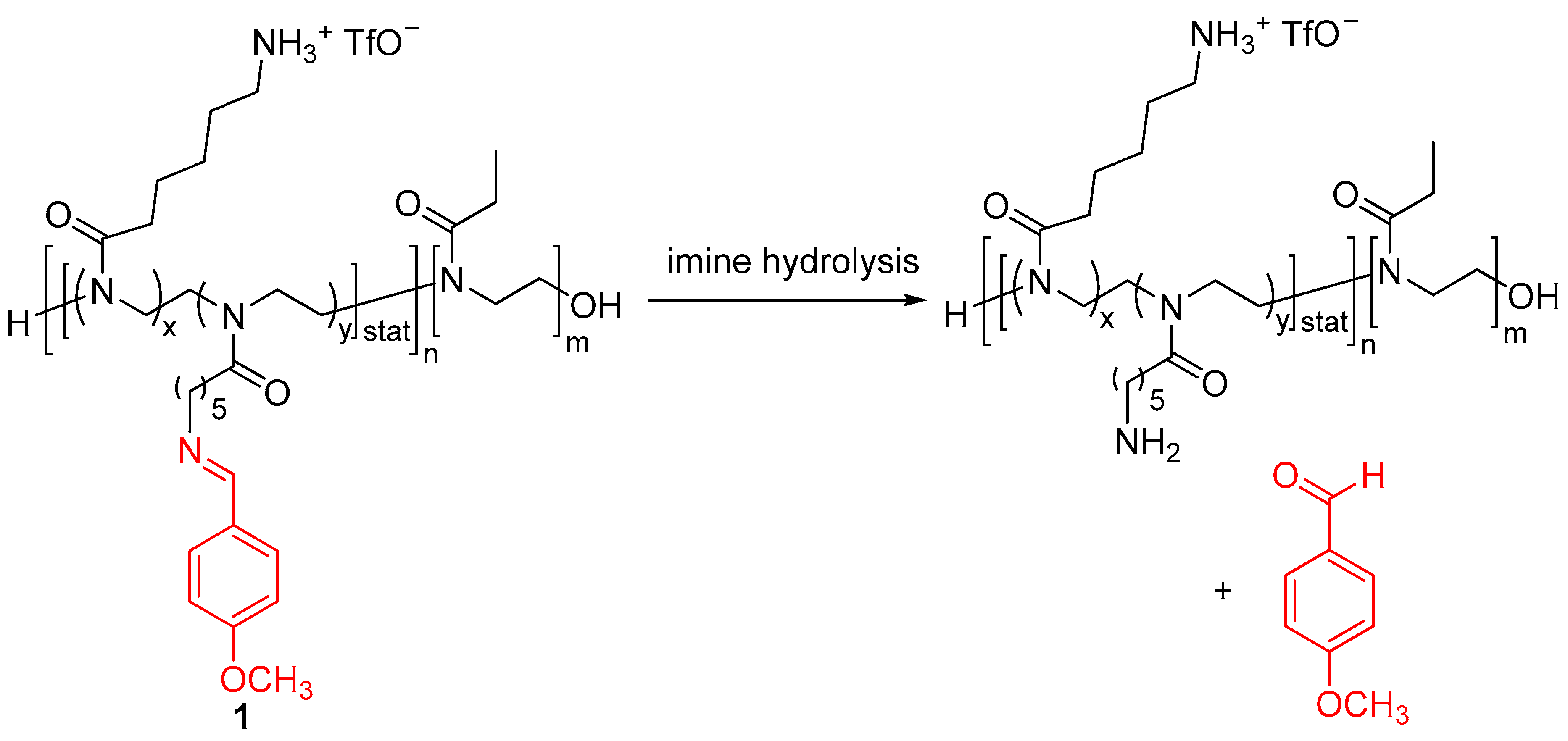
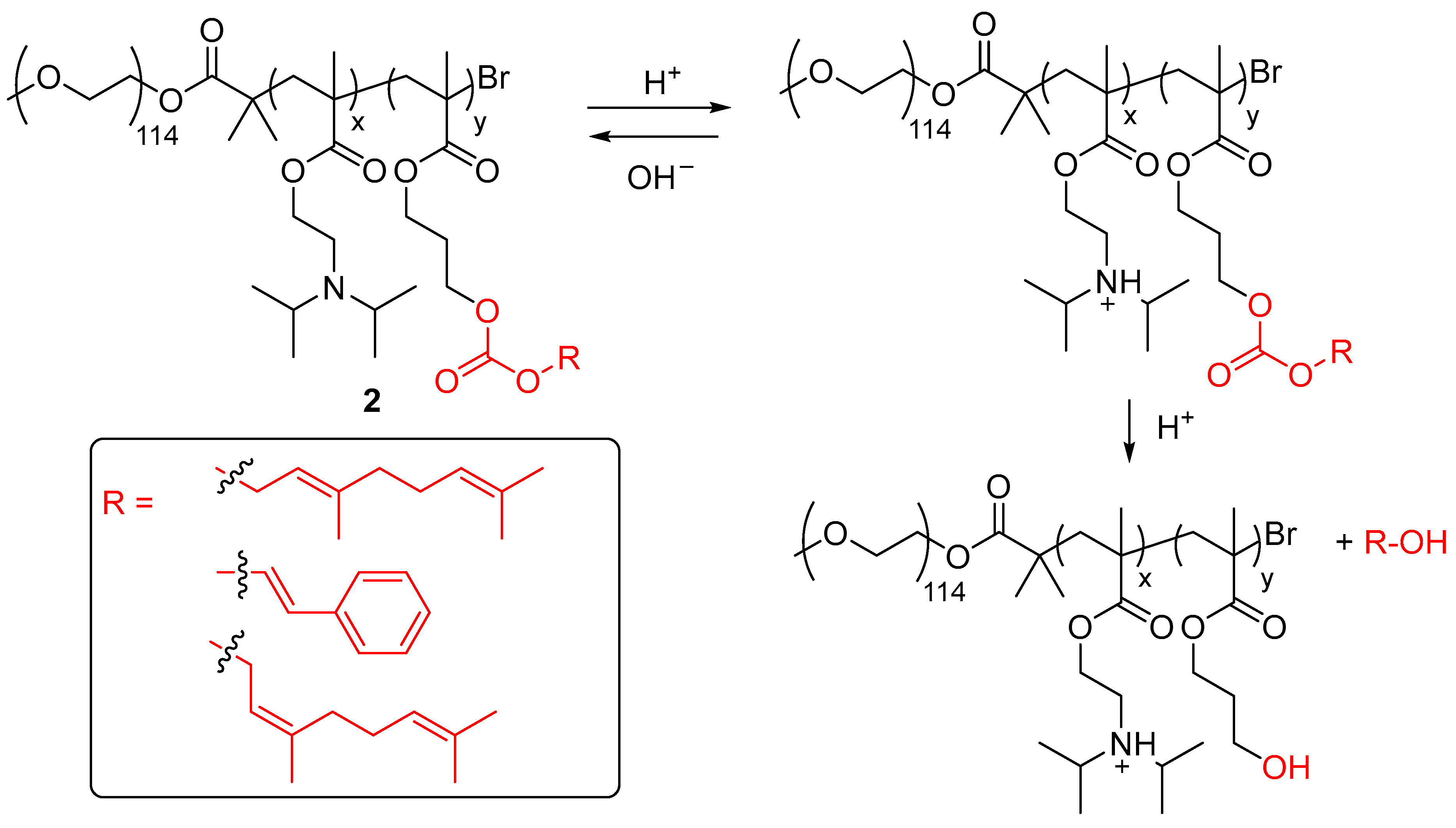
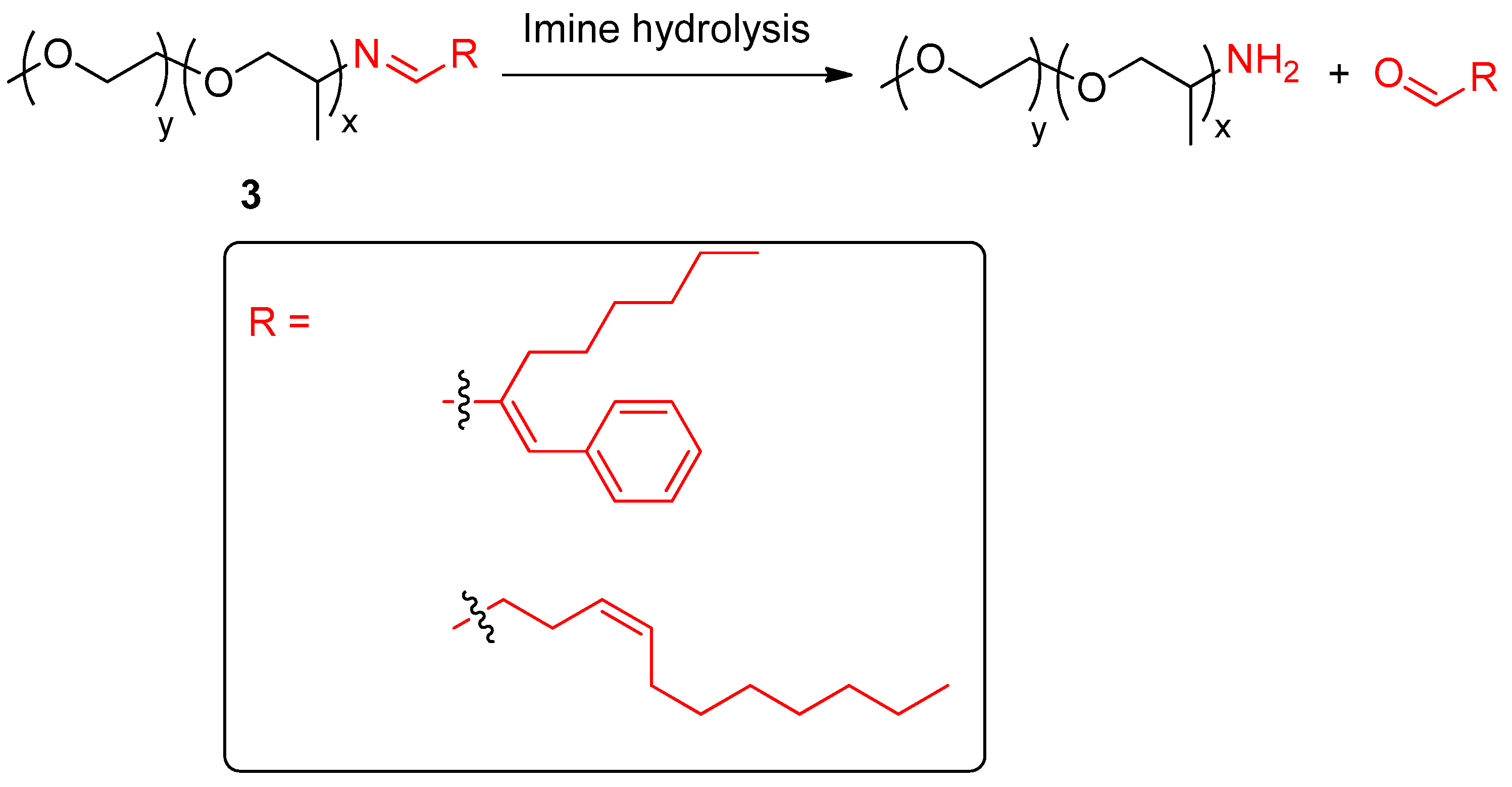

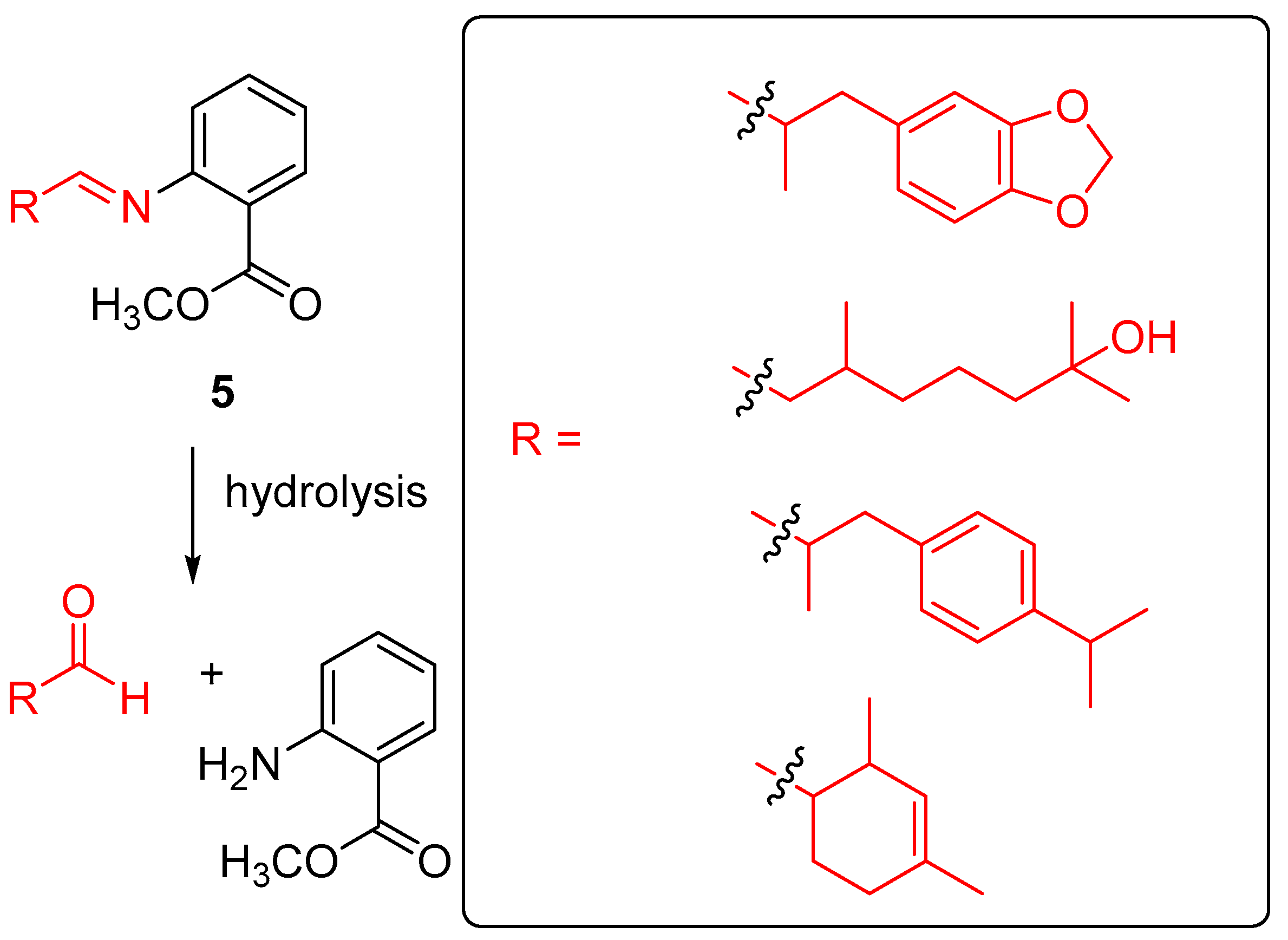



| Entry | Type of Controlled Release System | Available Stimuli | Principal Property |
|---|---|---|---|
| 1 | Polymers | pH, light, humidity, temperature | Great structural range |
| 2 | Ionic liquids | pH, temperature | Potential development of dual systems |
| 3 | MOFs | Sustained release | Long release times |
| 4 | Organic salts | pH, humidity | Fine-tuning of release rate |
| 5 | Coumarins | Light | Sunlight-responsiveness |
| 6 | Cyclodextrins | Sustained release | Biocompatibility |
| 7 | Rotaxanes | Light, temperature, enzyme | Precise control over release |
| Entry | Type of Controlled Release System | Release Time Range |
|---|---|---|
| 1 | Polymers | Hours to months |
| 2 | Ionic liquids | Hours to days |
| 3 | MOFs | Hours to years |
| 4 | Organic salts | Hours to days |
| 5 | Coumarins | Hours to weeks |
| 6 | Cyclodextrins | Hours to months |
| 7 | Rotaxanes | Hours to days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saura-Sanmartin, A.; Andreu-Ardil, L. Recent Advances in the Preparation of Delivery Systems for the Controlled Release of Scents. Int. J. Mol. Sci. 2023, 24, 4685. https://doi.org/10.3390/ijms24054685
Saura-Sanmartin A, Andreu-Ardil L. Recent Advances in the Preparation of Delivery Systems for the Controlled Release of Scents. International Journal of Molecular Sciences. 2023; 24(5):4685. https://doi.org/10.3390/ijms24054685
Chicago/Turabian StyleSaura-Sanmartin, Adrian, and Laura Andreu-Ardil. 2023. "Recent Advances in the Preparation of Delivery Systems for the Controlled Release of Scents" International Journal of Molecular Sciences 24, no. 5: 4685. https://doi.org/10.3390/ijms24054685
APA StyleSaura-Sanmartin, A., & Andreu-Ardil, L. (2023). Recent Advances in the Preparation of Delivery Systems for the Controlled Release of Scents. International Journal of Molecular Sciences, 24(5), 4685. https://doi.org/10.3390/ijms24054685







