Design, Synthesis and Bioactivity of Novel Pyrimidine Sulfonate Esters Containing Thioether Moiety
Abstract
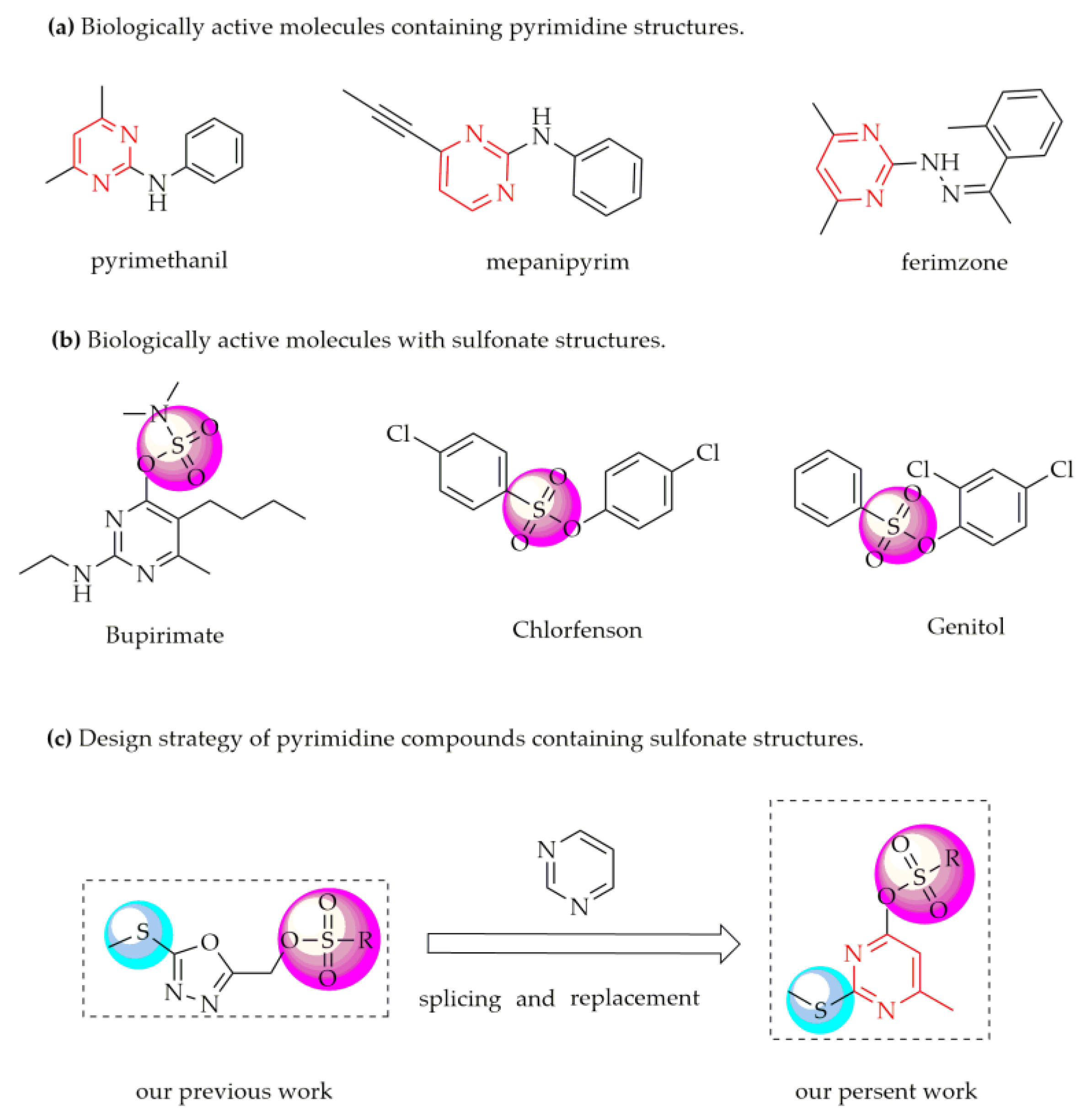
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties Analysis
2.2. Chemistry
2.2.1. Synthesis
2.2.2. Crystal Structure of Compound A10
2.3. In Vitro Antibacterial Activity
2.4. Structure-Activity Relationship
2.5. In Vivo Activity Analysis
2.6. Scanning Electron Microscopy (SEM) Characterization Analysis
2.7. The Cytoplasmic Content of Xoo Treated with Compound A5
2.8. Changes in Xoo Defense Enzyme Activity by Compound A5
2.9. Molecular Docking Study
2.10. Insecticidal Activity Analysis
3. Materials and Methods
3.1. Physicochemical Properties Prediction
3.2. Chemicals and Instruments
3.3. Synthetic Procedures of the Target Compounds
3.3.1. Preparation of Intermediate 1 [31,32]
3.3.2. Preparation of Intermediate 2, 3 and 4 [33,34]
3.3.3. Preparation of Title Compound A1–A29 [23,35]
3.3.4. Preparation of Title Compound A30–A31
3.3.5. Preparation of Title Compound A32–A33
3.4. Crystallographic Analysis
3.5. Antibacterial Activity Test
3.5.1. Antibacterial Bioassay In Vitro
3.5.2. Antibacterial Activity Bioassay In Vivo
3.6. Mechanism Analysis
3.6.1. Antibacterial Bioassay by Scanning Electron Microscopy
3.6.2. The cytoplasmic Content Leakage Assay
3.6.3. Test the Defense Enzyme Activity
3.7. Molecular Docking
3.8. Insecticidal Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thimmegowda, P.R.; Ambika, D.S.; Manjunatha, L.; Arun, R.S.; Prasad, P.S.; Chandrashekar, M. Screening germplasm for resistance to bacterial blight of rice caused by Xanthomonas oryzae pv. oryzae. Int. J. Sci. Nat. 2011, 2, 498–500. [Google Scholar]
- Kumar, D.; Jarial, K.; Jarial, R.S.; Banyal, S.K.; Jandaik, S. Prevalence of citrus canker caused by Xanthomonas axonopodis pv. citri in subtropical zone of himachal pradesh. Int. J. Bio-Resour. Stress Manag. 2020, 11, 89–94. [Google Scholar] [CrossRef]
- Bastas, K.K.; Karakaya, A. First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae in Turkey. Plant Dis. 2012, 96, 452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, Y.; Tian, X.; Xu, Q.; Chen, Z.; Lin, W. Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil Ecol. 2017, 112, 90–96. [Google Scholar] [CrossRef]
- Wu, S.; Shi, J.; Chen, J.; Hu, D.; Zang, L.; Song, B. Synthesis, antibacterial activity, and mechanisms of novel 6-sulfonyl-1,2,4-triazolo[3,4-b][1,3,4]thiadiazole derivatives. J. Agric. Food Chem. 2021, 69, 4645–4654. [Google Scholar] [CrossRef]
- Liu, T.; Peng, F.; Cao, X.i.; Liu, F.; Wang, Q.; Liu, L.; Xue, W. Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega 2021, 6, 30826–30833. [Google Scholar] [CrossRef]
- Li, X.; Tu, M.; Yang, B.; Zhang, Q.; Li, H.; Ma, W. Chlorantraniliprole in foods: Determination, dissipation and decontamination. Food Chem. 2022, 406, 135030. [Google Scholar] [CrossRef]
- Bhat, A.R.; Dongre, R.S.; Almalki, F.A.; Berredjem, M.; Aissaoui, M.; Touzani, R.; Hadda, T.B.; Akhter, M.S. Synthesis, biological activity and POM/DFT/docking analyses of annulated pyrano[2,3-d]pyrimidine derivatives: Identification of antibacterial and antitumor pharmacophore sites. Bioorganic Chem. 2021, 106, 104480. [Google Scholar] [CrossRef]
- Wu, W.; Lan, W.; Wu, C.; Fei, Q. Synthesis and antifungal activity of pyrimidine derivatives containing an amide moiety. Front. Chem. 2021, 9, 695628. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Sun, Z.H.; Wedge, D.E.; Becnel, J.J.; Estep, A.S.; Tan, C.X.; Weng, J.Q. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manag. Sci. 2016, 73, 953–959. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Y.; He, L.; Fan, Y.; Wang, T.; Luo, J. Synthesis and herbicidal activity of novel 1,2,4-triazole derivatives containing fluorine, phenyl sulfonyl and pyrimidine moieties. J. Mol. Struct. 2022, 1259, 132722. [Google Scholar] [CrossRef]
- Kimura, H.; Katoh, T.; Kajimoto, T.; Node, M.; Hisaki, M.; Sugimoto, Y.; Majima, T.; Uehara, Y.; Yamori, T. Modification of pyrimidine derivatives from antiviral agents to antitumor agents. Anticancer Res. 2006, 26, 91–97. [Google Scholar] [PubMed]
- Kumar, S.; Deep, A.; Narasimhan, B. A review on synthesis, anticancer and antiviral potentials of pyrimidine derivatives. Curr. Bioact. Compd. 2019, 15, 289–303. [Google Scholar] [CrossRef]
- Thirumurugan, K.; Lakshmanan, S.; Govindaraj, D.; Daniel Prabu, D.S.; Ramalakshmi, N.; Arul Antony, S. Design, synthesis and anti-inflammatory activity of pyrimidine scaffold benzamide derivatives as epidermal growth factor receptor tyrosine kinase inhibitors. J. Mol. Struct. 2018, 1171, 541–550. [Google Scholar] [CrossRef]
- Kotb, E.R.; Morsy, E.M.H.; Anwar, M.; Mohi, E.M.; Awad, H. New pyrimidine derivatives: Synthesis, antitumor and antioxidant evaluation. Int. J. Pharm. Technol. 2015, 7, 8061–8085. [Google Scholar]
- Bhat, A.R. Biological activity of pyrimidine derivativies: A review. Org. Med. Chem. Int. J. 2017, 2, 23–26. [Google Scholar] [CrossRef]
- Sharma, V.; Chitranshi, N.; Agarwal, A.K. Significance and biological importance of pyrimidine in the microbial world. Int. J. Med. Chem. 2014, 2014, 202784. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T.; Innocenti, A.; Mastrolorenzo, A.; Scozzafava, A. Antiviral sulfonamide derivatives. Mini Rev. Med. Chem. 2004, 4, 189–200. [Google Scholar] [CrossRef]
- Wahid, S.; Hanif, M.; Jahangir, S.; Shafique, M.; Shahid, H.A.; Muhammad, H.; Shah, S.A.A.; Versiani, M.A.; Khan, K.M.; Tahiri, I.A. Secnidazole-sulfonates: Synthesis, physical, electrochemical, antibacterial & antifungal characteristics. J. Mol. Struct. 2019, 1184, 569–575. [Google Scholar] [CrossRef]
- Ghazzali, M.; Khattab, S.A.N.; Elnakady, Y.A.; Al-Mekhlafi, F.A.; Al-Farhan, K.; El-Faham, A. Synthesis, structure, theoretical calculations and biological activity of sulfonate active ester new derivatives. J. Mol. Struct. 2013, 1046, 147–152. [Google Scholar] [CrossRef]
- Selvi, G.; Özdemir, F.A.; Aykutoglu, G.; Özdemir, N.; Şerbetçi, Z.; Çetinkaya, B.; Dayan, O. A neutral arene ruthenium (II) complex with a sulfonated N,O-chelating ligand: Synthesis, characterization, in vitro cytotoxicity and antibacterial activity. Polyhedron 2020, 176, 114300. [Google Scholar] [CrossRef]
- Torti, E.; Protti, S.; Bollanti, S.; Flora, F.; Torre, A.; Brusatin, G.; Gerardino, A.; Businaro, L.; Fagnoni, M.; Giustina, G.d.; et al. Aryl sulfonates as initiators for extreme ultraviolet lithography: Applications in epoxy-based hybrid materials. Chemphotochem 2018, 2, 425–432. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Lu, H.; Mu, X.; Jin, L. Synthesis and antibacterial evaluation of novel 1, 3, 4-Oxadiazole derivatives containing sulfonate/carboxylate moiety. Molecules 2020, 25, 1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karami, T.K.; Hailu, S.; Feng, S.X.; Graham, R.; Gukasyan, H.J. Eyes on lipinski’s rule of five: A new “Rule of Thumb” for physicochemical design space of ophthalmic drugs. J. Ocul. Pharmacol. Ther. 2022, 38, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2023 update. Pharmacol. Res. 2023, 187, 106552. [Google Scholar] [CrossRef]
- Azad, I.; Jafri, A.; Khan, T.; Akhter, Y.; Arshad, M.; Hassan, F.; Ahmad, N.; Khan, A.R.; Nasibullah, M. Evaluation of pyrrole-2,3-dicarboxylate derivatives: Synthesis, DFT analysis, molecular docking, virtual screening and in vitro anti-hepatic cancer study. J. Mol. Struct. 2019, 1176, 314–334. [Google Scholar] [CrossRef]
- Horishny, V.Y.; Arshad, M.; Matiychuk, V.S. Synthesis and anticancer activity of 2-cyano-N-(furan-2-ylmethyl)-2-(4-oxo-3-arylthiazolidin-2-ylidene)acetamide derivatives. Russ. J. Org. Chem. 2021, 57, 212–218. [Google Scholar] [CrossRef]
- Myrko, I.; Chaban, T.; Matiichuk, Y.; Arshad, M.; Matiychuk, V. Approaches for the synthesis, chemical modification and biological properties of N-acylphenothiazines. Curr. Chem. Lett. 2021, 10, 377–392. [Google Scholar] [CrossRef]
- Arshad, M.; Ahmad, D.; Akhter, R. 1, 3-thiazolidin-4-one derivatives bearing pyrimidine moieties: Design, computational studies, synthesis, characterization, antimicrobial, MTT assessment and molecular docking assessment. Chem. Data Collect. 2020, 28, 100405. [Google Scholar] [CrossRef]
- Arshad, M. Substituted pyrimidine-sulfonamide derivatives: Computational, synthesis, characterization, anti-bacterial, MTT and molecular docking assessment. Biointerface Res. Appl. Chem. 2022, 13, 239. [Google Scholar] [CrossRef]
- Barmaki, M.; Valiyeva, G.; Maharramovm, A.A.; Allaverdiyev, M.M. Synthesis of 2, 3-dihydro-6-methyl-2-thiopyrimidin-4 (1H)-one (6-methylthiouracil) derivatives and their reactions. J. Chem. 2013, 2013, 176213. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-J.; Yang, A.; Gu, Y.-F.; Zhang, X.-S.; Shao, K.-P.; Xue, D.-Q.; He, P.; Jiang, T.-F.; Zhang, Q.-R.; Liu, H.-M. Synthesis, in vitro antimicrobial and cytotoxic activities of novel pyrimidine–benzimidazol combinations. Bioorganic Med. Chem. Lett. 2014, 24, 2741–2743. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-X.; Yang, S.-Q.; He, Q.-Q.; Ma, X.-D.; Chen, F.-E.; Dai, H.-F.; De Clercq, E.; Balzarini, J.; Pannecouque, C. Design, synthesis and biological evaluation of cycloalkyl arylpyrimidines (CAPYs) as HIV-1 NNRTIs. Bioorganic Med. Chem. 2011, 19, 7093–7099. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shi, J.; Chen, J.; Hu, D.; Song, B. Design, synthesis, antibacterial activity, and mechanisms of novel 1, 3, 4-thiadiazole derivatives containing an amide moiety. J. Agric. Food Chem. 2021, 69, 8660–8670. [Google Scholar] [CrossRef] [PubMed]
- Che, Z.; Guo, X.; Li, Y.; Zhang, S.; Zhu, L.; He, J.; Sun, D.; Guo, Y.; Liu, Y.; Wei, R. Synthesis of paeonol ester derivatives and their insecticidal, nematicidal, and anti-oomycete activities. Pest Manag. Sci. 2022, 78, 3442–3455. [Google Scholar] [CrossRef]
- Liu, T.; Ren, X.; Cao, G.; Zhou, X.; Jin, L. Transcriptome analysis on the mechanism of ethylicin inhibiting Pseudomonas syringae pv. actinidiae on kiwifruit. Microorganisms 2021, 9, 724. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.; Zhang, L.; Liu, S.; Zhang, J.; Zhou, X.; Wang, P.; Yang, S. Discovery of novel rost-4-ene derivatives as potential plant activators for preventing phytopathogenic bacterial infection: Design, synthesis and biological studies. Pest Manag. Sci. 2022, 78, 3404–3415. [Google Scholar] [CrossRef]
- Qi, P.-Y.; Zhang, T.-H.; Feng, Y.-M.; Wang, M.-W.; Shao, W.-B.; Zeng, D.; Jin, L.-H.; Wang, P.-Y.; Zhou, X.; Yang, S. Exploring an innovative strategy for suppressing bacterial plant disease: Excavated novel isopropanolamine-tailored pterostilbene derivatives as potential antibiofilm agents. J. Agric. Food Chem. 2022, 70, 4899–4911. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.; Long, Z.; Xiang, S.; Zhang, J.; Li, Z.; Wu, Y.; Shao, W.; Zhou, X.; Liu, L.; et al. Design, synthesis, and biological profiles of novel 1,3,4-oxadiazole-2-carbohydrazides with molecular diversity. J. Agric. Food Chem. 2022, 70, 2825–2838. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, T.; Zhou, X.; Wang, P.; Gan, J.; Song, B.; Yang, S.; Yang, C.-G. Dysregulation of clpP by small-molecule activators used against Xanthomonas oryzae pv. oryzae infections. J. Agric. Food Chem. 2021, 69, 7545–7553. [Google Scholar] [CrossRef]
- Liu, H.; Ji, Q.; Ren, G.; Wang, F.; Su, F.; Wang, P.; Zhou, X.; Wu, Z.; Li, Z.; Yang, S. Antibacterial functions and proposed modes of action of novel 1,2,3,4-tetrahydro-β-carboline derivatives that possess an attractive 1,3-diaminopropan-2-ol pattern against rice bacterial blight, kiwifruit bacterial canker, and citrus bacterial canker. J. Agric. Food Chem. 2020, 68, 12558–12568. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xiang, M.; Luo, M.; Liu, H.; Zhou, X.; Wu, Z.; Liu, L.; Li, Z.; Yang, S. Novel piperazine-tailored ursolic acid hybrids as significant antibacterial agents targeting phytopathogens Xanthomonas oryzae pv. oryzae and X. axonopodis pv. citri probably directed by activation of apoptosis. Pest Manag. Sci. 2020, 76, 2746–2754. [Google Scholar] [CrossRef]
- Feng, Y.; Qi, P.; Xiao, W.; Zhang, T.-H.; Zhou, X.; Liu, L.; Yang, S. Fabrication of isopropanolamine-decorated coumarin derivatives as novel quorum sensing inhibitors to suppress plant bacterial disease. J. Agric. Food Chem. 2022, 70, 6037–6049. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, L.; Shi, H.; Niu, X.; Zhang, H.; Zhang, K.; Wu, Z. Design, synthesis and antifungal mechanism of novel acetophenone derivatives containing 1,3,4-thiadiazole-2-thioethers. New J. Chem. 2022, 46, 9017–9023. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Yang, X.; He, H.; Wu, Z.; Song, B.; Song, R. Synthesis, antibacterial activity, and mechanisms of novel indole derivatives containing pyridinium moieties. J. Agric. Food Chem. 2022, 70, 12341–12354. [Google Scholar] [CrossRef]
- Wu, R.; Liu, T.; Wu, S.; Li, H.; Song, R.; Song, B. Synthesis, antibacterial activity, and action mechanism of novel sulfonamides containing oxyacetal and pyrimidine. J. Agric. Food Chem. 2022, 70, 9305–9318. [Google Scholar] [CrossRef]
- Wang, M.L.; Du, Y.; Ling, C.; Yang, Z.K.; Jiang, B.B.; Duan, H.X.; An, J.; Li, X.H.; Yang, X.L. Design, synthesis and antifungal/anti-oomycete activity of pyrazolyl oxime ethers as novel potential succinate dehydrogenase inhibitors. Pest Manag. Sci. 2021, 77, 3910–3920. [Google Scholar] [CrossRef]
- Peng, J.W.; Yin, X.D.; Li, H.; Ma, K.Y.; Zhang, Z.J.; Zhou, R.; Wang, Y.L.; Hu, G.F.; Liu, Y.Q. Design, synthesis, and structure-activity relationship of quinazolinone derivatives as potential fungicides. J. Agric. Food Chem. 2021, 69, 4604–4614. [Google Scholar] [CrossRef]
- Koo, Y.; Ka, D.; Kim, E.J.; Suh, N.; Bae, E. Conservation and variability in the structure and function of the cas5d endoribonuclease in the CRISPR-mediated microbial immune system. J. Mol. Biol. 2013, 425, 3799–3810. [Google Scholar] [CrossRef]
- Zhang, A.G.; Zhou, J.Y.; Tao, K.; Hou, T.P.; Jin, H. Design, synthesis and antifungal evaluation of novel pyrazole carboxamides with diarylamines scaffold as potent succinate dehydrogenase inhibitors. Bioorganic Med. Chem. Lett. 2018, 28, 3042–3045. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, Y.; Guo, S.; Dai, A.; Wu, J. Diamide derivatives containing a trifluoromethylpyridine skeleton: Design, synthesis, and insecticidal activity. J. Integr. Agric. 2022, 21, 2995–3003. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Xiong, L.; Wang, Q. Design, synthesis and insecticidal activity of novel phenylpyrazoles containing a 2,2,2-trichloro-1-alkoxyethyl moiety. J. Agric. Food Chem. 2010, 58, 4992–4998. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; Pan, S.; Zhu, M.; Guan, A.; Sun, X.; Zhang, J.; Song, Y.; Liu, C.; Yang, X. A new potential aphicide against Myzus persicae: Design, synthesis and 3D-QSAR of novel phenoxypyridine derivatives containing 4-aminopyrimidine. J. Mol. Struct. 2022, 1262, 132949. [Google Scholar] [CrossRef]













| Compd. | Physicochemical Property Score | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| milogP | TPSA | Natoms | MW | nON | nOHNH | Nviolations | Nrotb | Volume | |
| A1 | 3.016 | 69.16 | 19 | 296.373 | 5 | 0 | 0 | 4 | 238.806 |
| A2 | 3.131 | 69.16 | 20 | 314.363 | 5 | 0 | 0 | 4 | 243.737 |
| A3 | 3.179 | 69.16 | 20 | 314.363 | 5 | 0 | 0 | 4 | 243.737 |
| A4 | 3.271 | 69.16 | 21 | 332.353 | 5 | 0 | 0 | 4 | 248.669 |
| A5 | 3.546 | 69.16 | 24 | 386.323 | 5 | 0 | 0 | 4 | 263.462 |
| A6 | 3.645 | 69.16 | 20 | 330.818 | 5 | 0 | 0 | 4 | 252.342 |
| A7 | 3.669 | 69.16 | 20 | 330.818 | 5 | 0 | 0 | 4 | 252.342 |
| A8 | 3.694 | 69.16 | 20 | 330.818 | 5 | 0 | 0 | 4 | 252.342 |
| A9 | 4.906 | 69.16 | 22 | 399.708 | 5 | 0 | 0 | 4 | 279.413 |
| A10 | 3.761 | 69.16 | 21 | 348.808 | 5 | 0 | 0 | 4 | 257.273 |
| A11 | 3.801 | 69.16 | 20 | 375.269 | 5 | 0 | 0 | 4 | 256.691 |
| A12 | 3.825 | 69.16 | 20 | 375.269 | 5 | 0 | 0 | 4 | 256.691 |
| A13 | 3.916 | 69.16 | 21 | 393.259 | 5 | 0 | 0 | 4 | 261.623 |
| A14 | 2.746 | 92.952 | 21 | 321.383 | 6 | 0 | 0 | 4 | 255.665 |
| A15 | 2.77 | 92.95 | 21 | 321.38 | 6 | 0 | 0 | 4 | 255.66 |
| A16 | 2.974 | 114.984 | 22 | 341.37 | 8 | 0 | 0 | 5 | 262.14 |
| A17 | 3.072 | 78.394 | 21 | 326.399 | 6 | 0 | 0 | 5 | 264.352 |
| A18 | 3.911 | 69.16 | 23 | 364.37 | 5 | 0 | 0 | 5 | 270.103 |
| A19 | 4.722 | 69.16 | 23 | 352.481 | 5 | 0 | 0 | 5 | 304.993 |
| A20 | 2.963 | 69.16 | 18 | 302.402 | 5 | 0 | 0 | 4 | 229.518 |
| A21 | 3.765 | 69.16 | 19 | 336.847 | 5 | 0 | 0 | 4 | 243.053 |
| A22 | 3.896 | 69.16 | 19 | 381.298 | 5 | 0 | 0 | 4 | 247.403 |
| A23 | 2.361 | 69.16 | 16 | 262.356 | 5 | 0 | 0 | 5 | 217.562 |
| A24 | 1.848 | 69.16 | 16 | 260.34 | 5 | 0 | 0 | 4 | 206.987 |
| A25 | 1.487 | 72.398 | 16 | 263.344 | 6 | 0 | 0 | 4 | 213.303 |
| A26 | 3.041 | 78.394 | 22 | 338.41 | 6 | 0 | 0 | 4 | 270.552 |
| A27 | 2.28 | 82.052 | 19 | 297.361 | 6 | 0 | 0 | 4 | 234.65 |
| A28 | 4.175 | 69.16 | 23 | 346.433 | 5 | 0 | 0 | 4 | 282.798 |
| A29 | 4.199 | 69.16 | 23 | 346.433 | 5 | 0 | 0 | 4 | 282.798 |
| A30 | 3.391 | 69.16 | 20 | 310.4 | 5 | 0 | 0 | 5 | 255.608 |
| A31 | 3.922 | 69.16 | 25 | 400.35 | 5 | 0 | 0 | 5 | 280.264 |
| A32 | 4.61 | 69.16 | 25 | 372.471 | 5 | 0 | 0 | 6 | 310.455 |
| A33 | 5.141 | 69.16 | 30 | 462.421 | 5 | 0 | 1 | 6 | 335.112 |
| BT | 0.277 | 81.42 | 15 | 278.413 | 6 | 4 | 0 | 4 | 195.864 |
| TC | −0.84 | 49.46 | 13 | 261.82 | 4 | 0 | 0 | 4 | 187.46 |
| Compd. | Bioactivity Score | |||||
|---|---|---|---|---|---|---|
| GPCR Ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor | |
| A1 | −0.41 | −0.39 | −0.64 | −0.86 | −0.47 | −0.04 |
| A2 | −0.32 | −0.44 | −0.51 | −0.72 | −0.46 | −0.06 |
| A3 | −0.34 | −0.38 | −0.53 | −0.74 | −0.44 | −0.05 |
| A4 | −0.27 | −0.36 | −0.49 | −0.67 | −0.36 | −0.02 |
| A5 | −0.22 | −0.35 | −0.42 | −0.55 | −0.21 | 0.10 |
| A6 | −0.35 | −0.44 | −0.62 | −0.80 | −0.43 | −0.13 |
| A7 | −0.33 | −0.38 | −0.60 | −0.78 | −0.46 | −0.06 |
| A8 | −0.28 | −0.36 | −0.40 | −0.57 | −0.32 | 0.02 |
| A9 | −0.23 | −0.32 | −0.45 | −0.60 | −0.38 | −0.07 |
| A10 | −0.40 | −0.40 | −0.48 | −0.75 | −0.51 | −0.12 |
| A11 | −0.49 | −0.50 | −0.66 | −0.94 | −0.54 | −0.12 |
| A12 | −0.47 | −0.46 | −0.62 | −0.90 | −0.55 | −0.12 |
| A13 | −0.45 | −0.63 | −0.51 | −0.80 | −0.58 | −0.15 |
| A14 | −0.29 | −0.40 | −0.44 | −0.58 | −0.33 | 0.01 |
| A15 | −0.28 | −0.36 | −0.40 | −0.57 | −0.32 | −0.02 |
| A16 | −0.44 | −0.40 | −0.62 | −0.75 | −0.47 | −0.16 |
| A17 | −0.35 | −0.44 | −0.55 | −0.70 | −0.41 | −0.08 |
| A18 | −0.16 | −0.23 | −0.37 | −0.42 | −0.22 | −0.02 |
| A19 | −0.18 | −0.25 | −0.44 | −0.46 | −0.25 | −0.01 |
| A20 | −0.45 | −0.63 | −0.86 | −0.96 | −0.51 | −0.15 |
| A21 | −0.31 | −0.46 | −0.84 | −0.76 | −0.20 | −0.02 |
| A22 | −0.52 | −0.57 | −0.95 | −0.90 | −0.49 | −0.17 |
| A23 | −0.59 | −0.61 | −0.97 | −0.93 | −0.67 | 0.07 |
| A24 | −0.36 | −0.51 | −0.81 | −0.76 | −0.47 | 0.29 |
| A25 | −1.01 | −0.84 | −1.19 | −1.02 | −0.67 | 0.10 |
| A26 | −0.12 | −0.31 | −0.39 | −0.50 | −0.24 | 0.07 |
| A27 | −0.33 | −0.40 | −0.56 | −0.75 | −0.32 | 0.01 |
| A28 | −0.08 | −0.24 | −0.39 | −0.54 | −0.14 | 0.11 |
| A29 | −0.19 | −0.32 | −0.38 | −0.57 | −0.17 | 0.01 |
| A30 | −0.42 | −0.47 | −0.73 | −0.89 | −0.50 | −0.09 |
| A31 | −0.27 | −0.42 | −0.54 | −0.63 | −0.28 | 0.04 |
| A32 | −0.25 | −0.58 | −0.47 | −0.61 | −0.22 | −0.02 |
| A33 | −0.21 | −0.52 | −0.41 | −0.52 | −0.18 | 0.08 |
| BT | −1.20 | −1.27 | −0.76 | −1.50 | −0.76 | −0.20 |
| TC | −0.95 | −0.53 | −1.10 | −1.09 | −0.97 | −0.48 |
| Treatment | Protection Activity (14 Days after Spraying) | Curative Activity (14 Days after Spraying) | ||
|---|---|---|---|---|
| Lesion Length (mm ± SE) | Control Efficiency (%) α | Lesion Length (mm ± SE) | Control Efficiency (%) α | |
| A33 | 3.09 ± 0.01 b | 27.68 ± 0.19 | 2.81 ± 0.20 b | 26.86 ± 3.15 |
| BT | 3.15 ± 0.10 b | 26.24 ± 2.36 | 3.05 ± 0.13 b | 21.03 ± 3.13 |
| CK b | 4.27 ± 0.27 a | - | 3.86 ± 0.17 a | - |
| Compd. | P. xylostella | Myzus persicae | Compd. | P. xylostella | Myzus persicae | ||
|---|---|---|---|---|---|---|---|
| 500 μg/mL | 50 μg/mL | 500 μg/mL | 500 μg/mL | 50 μg/mL | 500 μg/mL | ||
| A1 | 23 ± 5.77 | - | 90 ± 0.00 | A19 | 83 ± 5.77 | - | 17 ± 5.77 |
| A2 | 27 ± 5.77 | - | 83 ± 5.77 | A20 | 80 ± 0.00 | - | 47 ± 5.77 |
| A3 | 100 ± 0.00 | 37 ± 5.77 | 90 ± 0.00 | A21 | 93 ± 5.77 | 57 ± 5.77 | 27 ± 5.77 |
| A4 | 57 ± 5.77 | - | 37 ± 5.77 | A22 | 100 ± 0.00 | 20 ± 0.00 | 57 ± 5.77 |
| A5 | 27 ± 5.77 | - | 73 ± 5.77 | A23 | 100 ± 0.00 | 40 ± 0.00 | 37 ± 5.77 |
| A6 | 83 ± 5.77 | - | 13 ± 5.77 | A24 | 100 ± 0.00 | 27 ± 5.77 | 43 ± 5.77 |
| A7 | 20 ± 0.00 | - | 37 ± 5.77 | A25 | 77 ± 5.77 | - | 93 ± 5.77 |
| A8 | 100 ± 0.00 | 67 ± 5.77 | 27 ± 5.77 | A26 | 77 ± 5.77 | - | 20 ± 0.00 |
| A9 | 27 ± 5.77 | - | 87 ± 5.77 | A27 | 100 ± 0.00 | 30 ± 0.00 | 17 ± 5.77 |
| A10 | 7 ± 5.77 | - | 83 ± 5.77 | A28 | 83 ± 5.77 | - | 40 ± 0.00 |
| A11 | 100 ± 0.00 | 37 ± 5.77 | 70 ± 0.00 | A29 | 83 ± 5.77 | - | 57 ± 5.77 |
| A12 | 100 ± 0.00 | 27 ± 5.77 | 27 ± 5.77 | A30 | 77 ± 5.77 | - | 33 ± 5.77 |
| A13 | 17 ± 5.77 | - | 47 ± 5.77 | A31 | 90 ± 0.00 | 27 ± 5.77 | 47 ± 5.77 |
| A14 | 37 ± 5.77 | - | 20 ± 0.00 | A32 | 83 ± 5.77 | ± | 60 ± 0.00 |
| A15 | 47 ± 5.77 | - | 30 ± 0.00 | A33 | 100 ± 0.00 | 40 ± 0.00 | 77 ± 5.77 |
| A16 | 27 ± 5.77 | - | 27 ± 5.77 | CK | 0 ± 0.00 | 0 ± 0.00 | 0 ± 0.00 |
| A17 | 27 ± 5.77 | - | 60 ± 0.00 | Chlorantr Aniliprole b | 100 ± 0.00 | 100 ± 0.00 | 100 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, Y.; Ren, X.; Tan, Y.; Jin, L.; Zhou, X. Design, Synthesis and Bioactivity of Novel Pyrimidine Sulfonate Esters Containing Thioether Moiety. Int. J. Mol. Sci. 2023, 24, 4691. https://doi.org/10.3390/ijms24054691
Li C, Liu Y, Ren X, Tan Y, Jin L, Zhou X. Design, Synthesis and Bioactivity of Novel Pyrimidine Sulfonate Esters Containing Thioether Moiety. International Journal of Molecular Sciences. 2023; 24(5):4691. https://doi.org/10.3390/ijms24054691
Chicago/Turabian StyleLi, Changkun, Youhua Liu, Xiaoli Ren, Yanni Tan, Linhong Jin, and Xia Zhou. 2023. "Design, Synthesis and Bioactivity of Novel Pyrimidine Sulfonate Esters Containing Thioether Moiety" International Journal of Molecular Sciences 24, no. 5: 4691. https://doi.org/10.3390/ijms24054691






