Dietary Leucine Improves Fish Intestinal Barrier Function by Increasing Humoral Immunity, Antioxidant Capacity, and Tight Junction
Abstract
1. Introduction
2. Results
2.1. The Humoral Immune-Related Parameters in Intestine
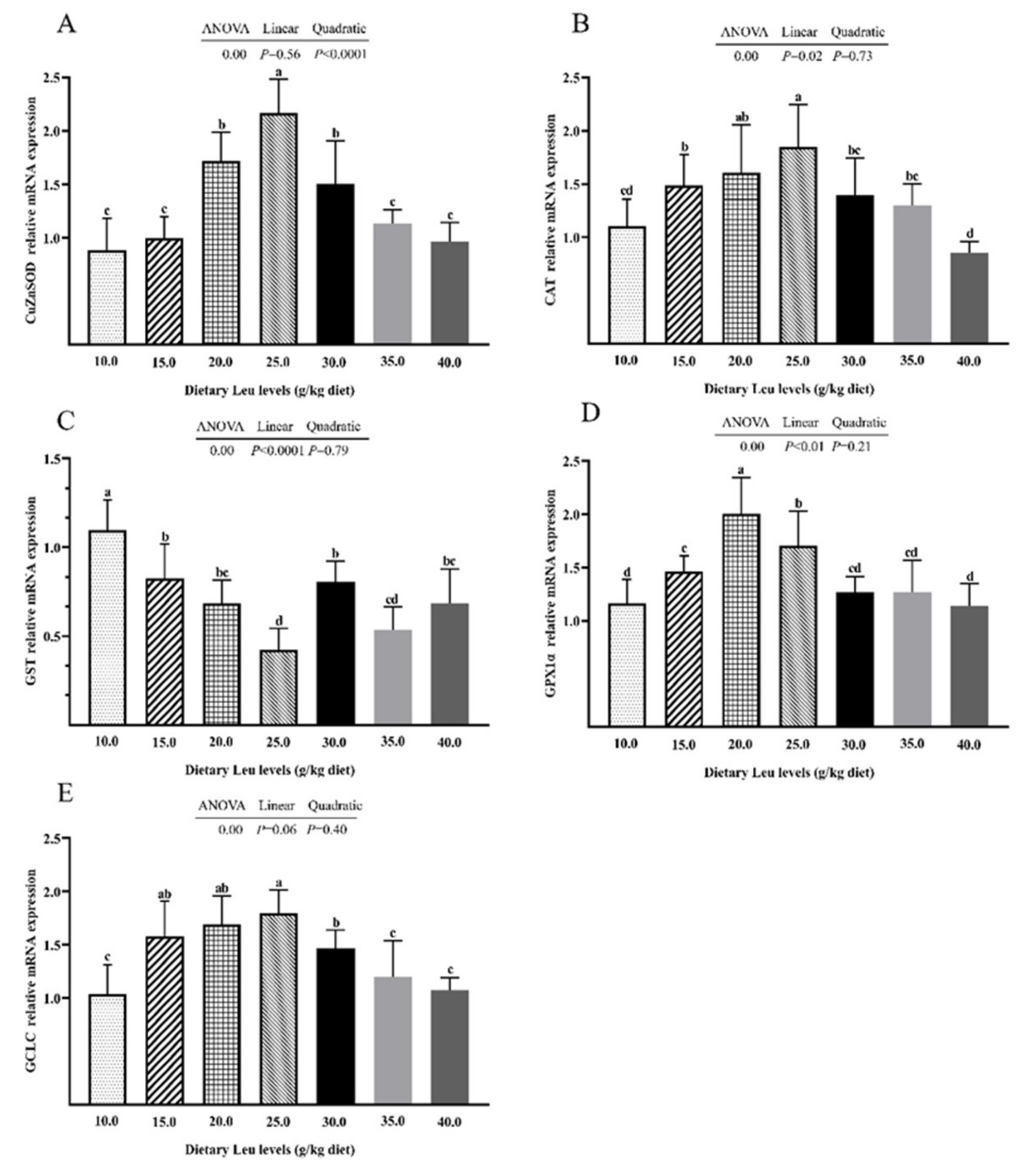
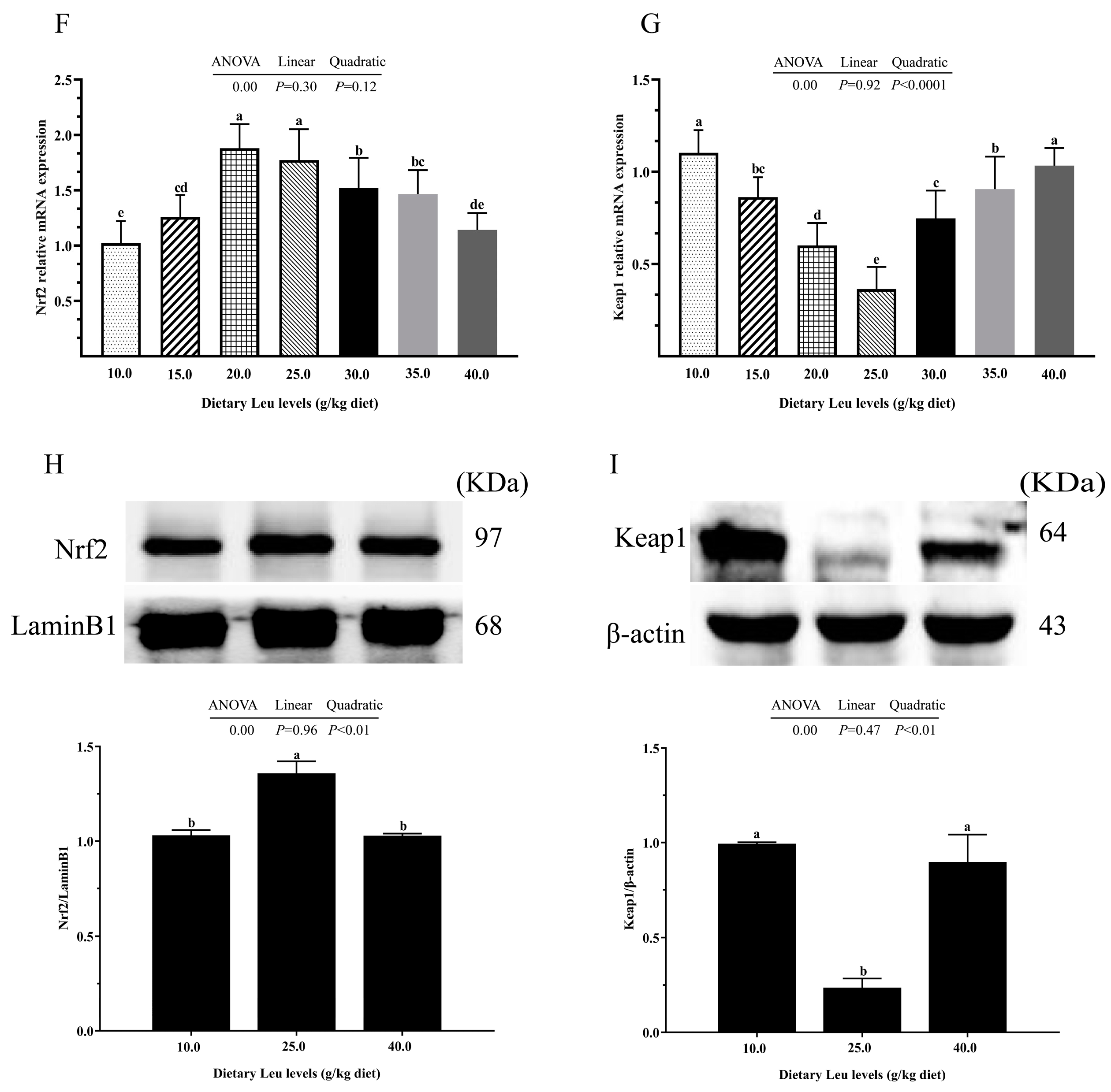
2.2. Antioxidant-Related Parameters in Intestine
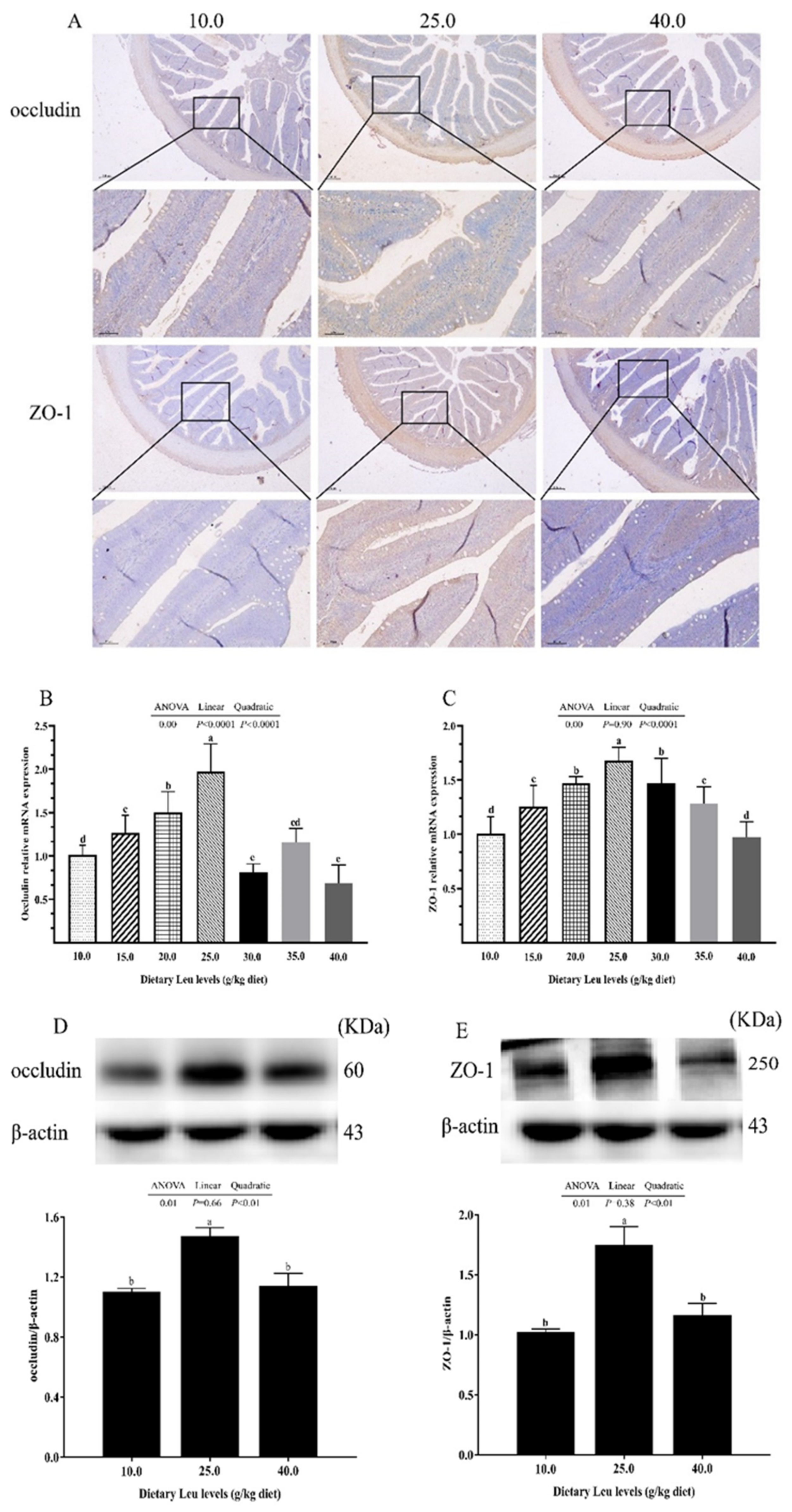
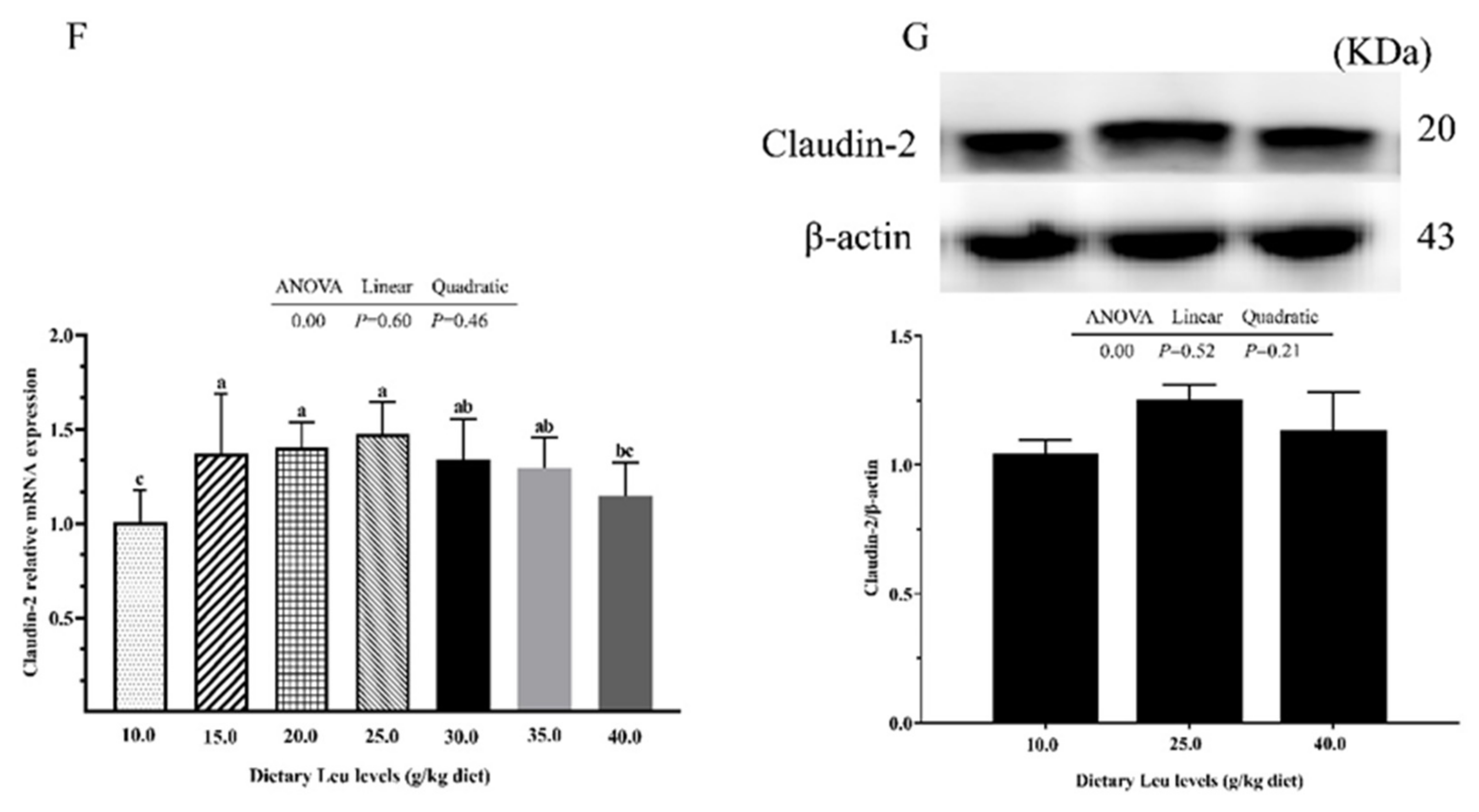
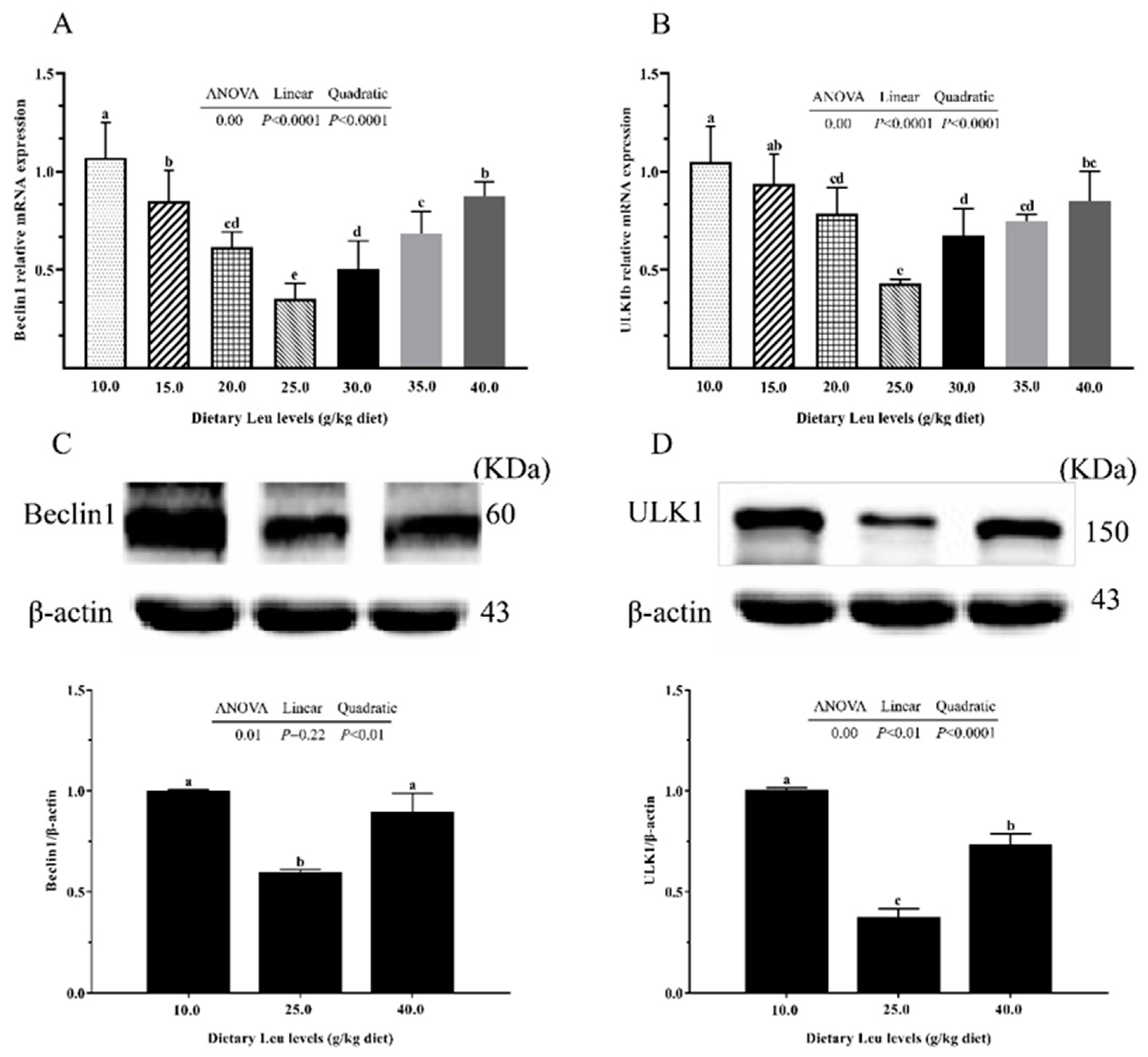
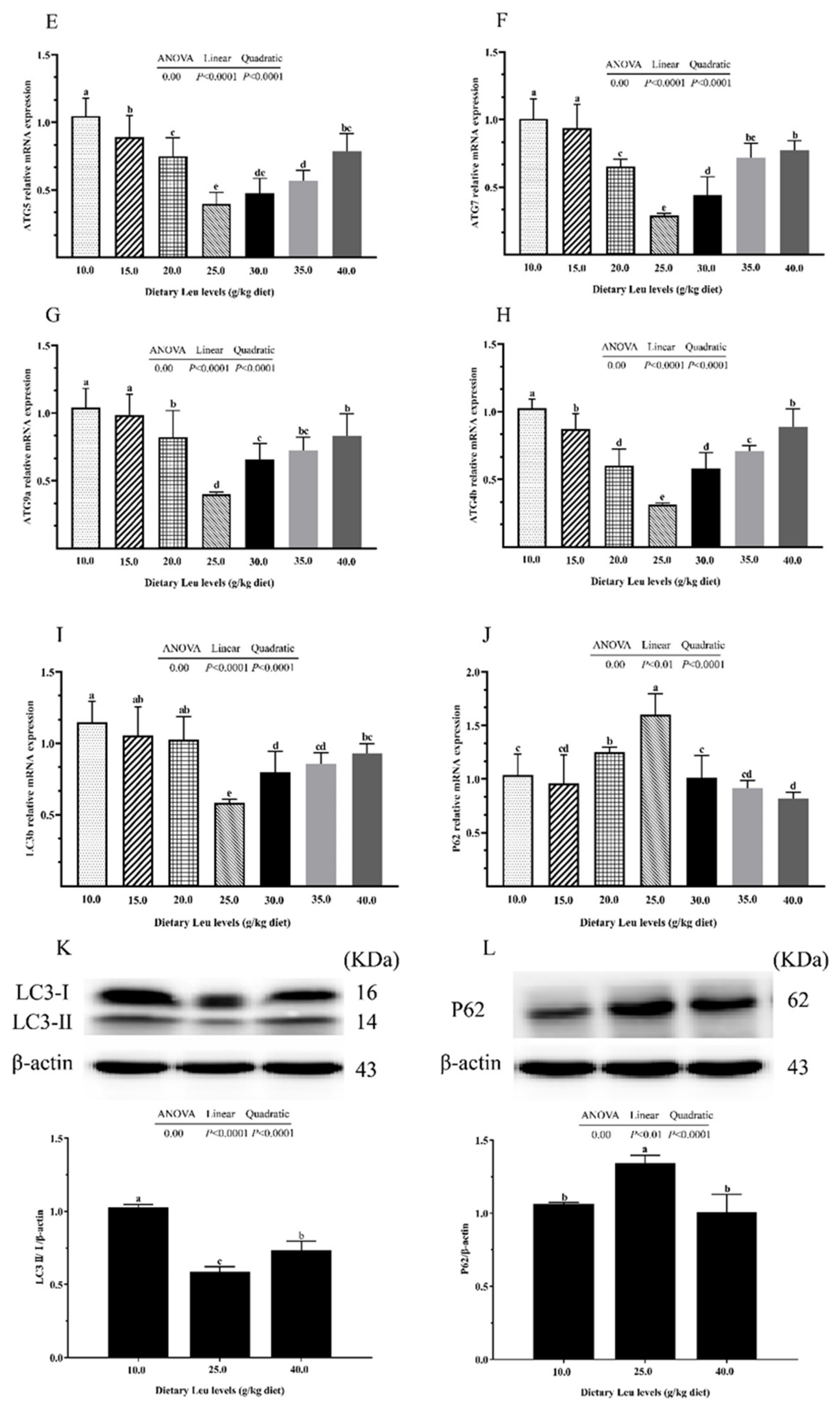
2.3. Tight Junction Protein and Autophagy-Related Parameters in Intestine
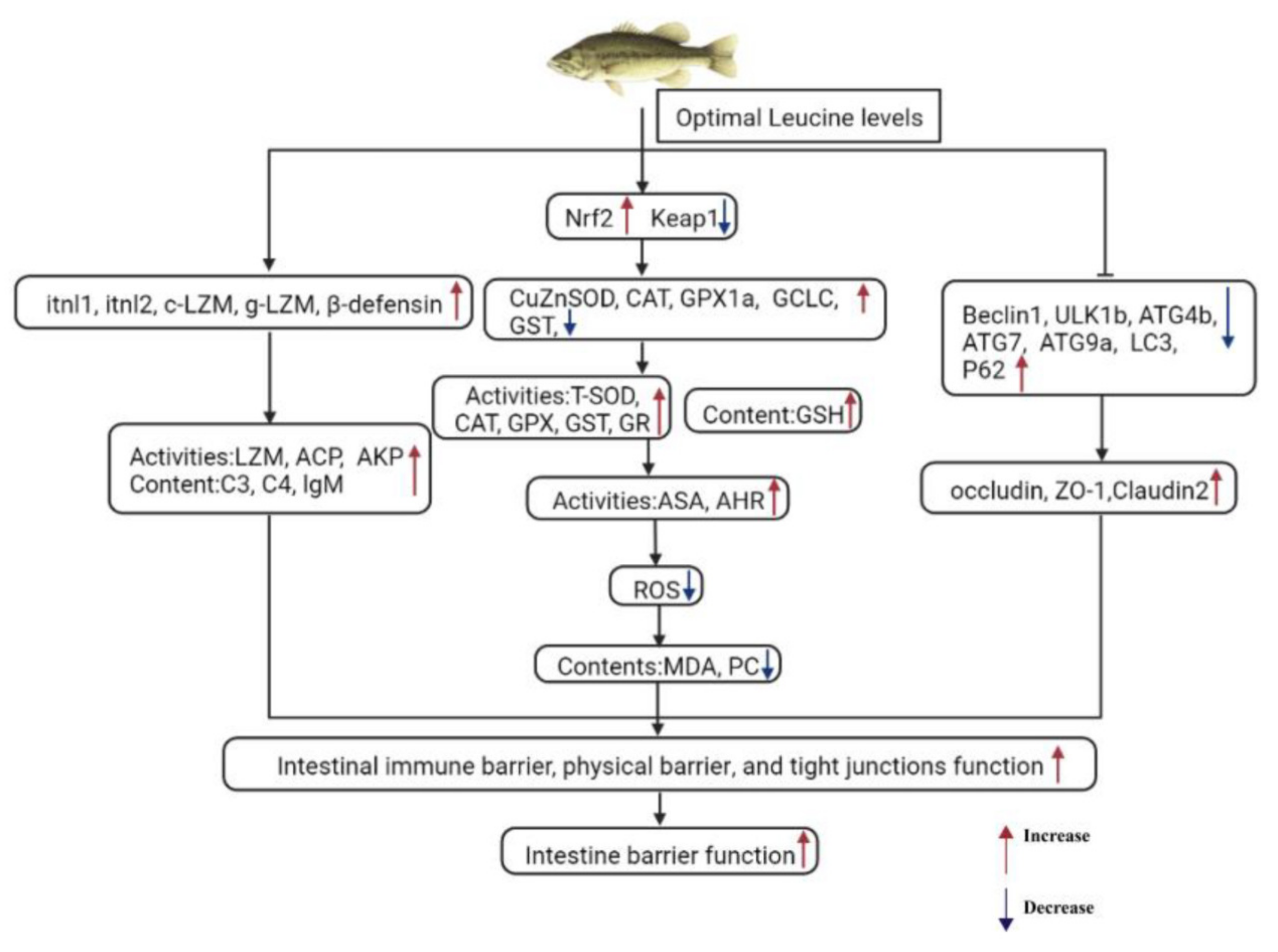
3. Discussion
3.1. Dietary Leu Improved Immune Barrier Function in the Intestine of Fish
3.2. Dietary Leu Enhanced Physical Barrier Function via Up-Regulating Intestinal Antioxidant Capacity in the Intestine of Fish
3.3. Dietary Leu Enhances Paracellular Barrier Functions via Increasing TJ Protein Levels in the Intestine of Fish
4. Materials and Methods
4.1. Experimental Diets, Feeding Trial, and Sampling
4.2. Biochemical Measurements
4.3. Immunohistochemistry (IHC) Staining
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, J.; Yang, Y.; Ma, C.; Liu, X.; Wang, Y.; Chen, F.; Wang, B.; Bian, X.; Yang, C.; Zhang, N. The effects and cell barrier mechanism of main dietary nutrients on intestinal barrier. Curr. Opin. Food Sci. 2022, 48, 100942. [Google Scholar] [CrossRef]
- Beaumont, M.; Blachier, F. Amino acids in intestinal physiology and health. In Amino Acids in Nutrition and Health: Amino Acids in Systems Function and Health; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1265, pp. 1–20. [Google Scholar]
- Li, F.; Yin, Y.; Tan, B.; Kong, X.; Wu, G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 2011, 41, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.B.F.D. Amino Acid Nutrition in Animals: Protein Synthesis and Beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Yao, K.; Liu, Z.; Gong, M.; Ruan, Z.; Deng, D.; Tan, B.; Liu, Z.; Wu, G. Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 2010, 39, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yin, S.; Wang, Y.; Li, L.; Wang, X.; Ding, Y.; Zang, X.; Zhang, H.; Jia, Y.; Hu, Y. The effects of water temperature and stocking density on survival, feeding and growth of the juveniles of the hybrid yellow catfish from Pelteobagrus fulvidraco (♀) × Pelteobagrus vachelli (♂). Aquac. Res. 2016, 47, 2844–2850. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, C.; Fei, S.; Liu, H.; Han, D.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. Arthrospira platensis additive enhances the growth performance and antioxidant response in hybrid yellow catfish (Pelteobagrus fulvidraco♀ × Pelteobagrus vachelli♂). Aquac. Rep. 2021, 20, 100721. [Google Scholar] [CrossRef]
- Fei, S.; Xia, Y.; Chen, Z.; Liu, C.; Liu, H.; Han, D.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. A high-fat diet alters lipid accumulation and oxidative stress and reduces the disease resistance of overwintering hybrid yellow catfish (Pelteobagrus fulvidraco ♀ × P. vachelli ♂). Aquac. Rep. 2022, 23, 101043. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Xu, S.; Xie, J.; Xiang, K.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Zhao, J.; et al. Dietary tryptophan affects growth performance, digestive and absorptive enzyme activities, intestinal antioxidant capacity, and appetite and GH–IGF axis-related gene expression of hybrid catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂). Fish Physiol. Biochem. 2019, 45, 1627–1647. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q.; Zhou, X.Q.; Xu, S.X.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, J.; Jiang, J. Effect of dietary threonine on growth performance and muscle growth, protein synthesis and antioxidant-related signalling pathways of hybrid catfish Pelteobagrus vachelli female symbol x Leiocassis longirostris male symbol. Br. J. Nutr. 2020, 123, 121–134. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, M.Y.; Jiang, Q.; Yin, L.; Jiang, J. Isoleucine improved growth performance, and intestinal immunological and physical barrier function of hybrid catfish Pelteobagrus vachelli × Leiocassis longirostris. Fish Shellfish Immunol. 2020, 109, 20–33. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.Y.; Jiang, Q.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhou, J.; Zhao, J.; et al. Leucine Improved growth performance, muscle growth, and muscle protein deposition through AKT/TOR and AKT/FOXO3a signaling pathways in hybrid catfish Pelteobagrus vachelli × Leiocassis longirostris. Cells 2020, 9, 327. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Heo, J.; Balasundaram, C.; Kim, M.; Kim, J.; Han, Y.; Heo, M. Effect of traditional Korean medicinal (TKM) triherbal extract on the innate immune system and disease resistance in Paralichthys olivaceus against Uronema marinum. Vet. Parasitol. 2010, 170, 1–7. [Google Scholar] [CrossRef]
- Kim, J.; Harikrishnan, R.; Kim, M.; Balasundaram, C.; Heo, M. Dietary administration of Zooshikella sp. enhance the innate immune response and disease resistance of Paralichthys olivaceus against Sreptococcus iniae. Fish Shellfish Immunol. 2010, 29, 104–110. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Heo, J.; Balasundaram, C.; Kim, M.; Kim, J.; Han, Y.; Heo, M. Effect of Punica granatum solvent extracts on immune system and disease resistance in Paralichthys olivaceus against lymphocystis disease virus (LDV). Fish Shellfish Immunol. 2010, 29, 668–673. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Paray, B.A.; Al-Sadoon, M.K.; Hoseinifar, S.H.; Gokul, E. Study the immunomodulation of anthracenedione in striped dwarf catfish, Mystus vittatus against pathogenic bacteria, Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 95, 117–127. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Esteban, M.Á.; Abdel-Tawwab, M. Impact of grape pomace flour (GPF) on immunity and immune-antioxidant-anti-inflammatory genes expression in Labeo rohita against Flavobacterium columnaris. Fish Shellfish Immunol. 2021, 111, 69–82. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Devi, G.; Van Doan, H.; Balasundaram, C.; Thamizharasan, S.; Hoseinifar, S.H.; Abdel-Tawwab, M. Effect of diet enriched with Agaricus bisporus polysaccharides (ABPs) on antioxidant property, innate-adaptive immune response and pro-antiinflammatory genes expression in Ctenopharyngodon idella against Aeromonas hydrophila. Fish Shellfish Immunol. 2021, 114, 238–252. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effects of dietary leucine levels on intestinal antioxidant status and immune response for juvenile golden pompano (Trachinotus ovatus) involved in Nrf2 and NFkB signaling pathway. Fish Shellfish Immunol. 2020, 107, 336–345. [Google Scholar] [CrossRef]
- Liang, H.; Mokrani, A.; Ji, K.; Ge, X.; Ren, M.; Xie, J.; Liu, B.; Xi, B.; Zhou, Q. Dietary leucine modulates growth performance, Nrf2 antioxidant signaling pathway and immune response of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2018, 73, 57–65. [Google Scholar] [CrossRef]
- Hoeven, R.; McCallum, K.C.; Cruz, M.R.; Garsin, D.A. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 2011, 7, e1002453. [Google Scholar]
- Jiang, W.D.; Zhou, X.Q.; Zhang, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Vitamin A deficiency impairs intestinal physical barrier function of fish. Fish Shellfish Immunol. 2019, 87, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Xiang, J.; Xiong, F.; Wang, G.; Zou, H.; Li, W.; Li, M.; Wu, S. Effects of Bacillus licheniformis on the growth, antioxidant capacity, intestinal barrier and disease resistance of grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2020, 97, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Li, M.; He, J.; Chen, H.; Zheng, P.; Yu, J.; Luo, Y.; et al. Effects of dietary grape seed proanthocyanidin extract supplementation on meat quality, muscle fiber characteristics and antioxidant capacity of finishing pigs. Food Chem. 2022, 367, 130781. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Semba, S.; Khan, R.I.; Bochimoto, H.; Watanabe, T.; Fujiya, M.; Kohgo, Y.; Liu, Y.; Taniguchi, T. Focal adhesion kinase regulates intestinal epithelial barrier function via redistribution of tight junction. Biochim. Biophys. Acta 2013, 1832, 151–159. [Google Scholar] [CrossRef]
- Deng, Y.P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Wu, P.; Zhang, Y.A.; Feng, L.; Zhou, X.Q. Differential growth performance, intestinal antioxidant status and relative expression of Nrf2 and its target genes in young grass carp (Ctenopharyngodon idella) fed with graded levels of leucine. Aquaculture 2014, 434, 66–73. [Google Scholar] [CrossRef]
- Piche, T.; Barbara, G.; Aubert, P.; Bruley, D.V.S.; Dainese, R.; Nano, J.L.; Cremon, C.; Stanghellini, V.; De Giorgio, R.; Galmiche, J.P.; et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: Involvement of soluble mediators. Gut 2009, 58, 196–201. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Jiang, W.; Deng, Y.; Liu, Y.; Qu, B.; Jiang, J.; Kuang, S.; Tang, L.; Tang, W.; Wu, P.; Zhang, Y.; et al. Dietary leucine regulates the intestinal immune status, immune-related signalling molecules and tight junction transcript abundance in grass carp (Ctenopharyngodon idella). Aquaculture 2015, 444, 134–142. [Google Scholar] [CrossRef]
- Mao, X.; Hu, H.; Tang, J.; Chen, D.; Yu, B. Leucine increases mucin 2 and occludin production in LS174T cells partially via PI3K-Akt-mTOR pathway. Anim. Nutr. 2016, 2, 218–224. [Google Scholar] [CrossRef]
- Gaffney-Stomberg, E.; Marszewski, P.; MacArthur, M.; McClung, J.P.; Matheny, R.W. Paracellular calcium flux across Caco-2 cell monolayers: Effects of individual amino acids. J. Nutr. Biochem. 2018, 59, 114–122. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, D.; Kim, J.H.; Shin, Y.J.; Kim, E.S.; Akram, M.; Kim, E.H.; Majid, A.; Baek, S.H.; Bae, O.N. Autophagy-mediated occludin degradation contributes to blood-brain barrier disruption during ischemia in bEnd.3 brain endothelial cells and rat ischemic stroke models. Fluids Barriers CNS 2020, 17, 21. [Google Scholar] [CrossRef]
- Zhang, S.; An, Q.; Wang, T.; Gao, S.; Zhou, G. Autophagy- and MMP-2/9-mediated reduction and redistribution of ZO-1 contribute to hyperglycemia-increased blood-brain barrier permeability during early reperfusion in stroke. Neuroscience 2018, 377, 126–137. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Liu, Y.; Su, Z.; Dawar, F.U.; Dan, H.; He, Y.; Gui, J.F.; Mei, J. Leucine mediates autophagosome-lysosome fusion and improves sperm motility by activating the PI3K/Akt pathway. Oncotarget 2017, 8, 111807–111818. [Google Scholar] [CrossRef]
- Li, S.A.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary myo-inositol deficiency decreased intestinal immune function related to NFκB and TOR signaling in the intestine of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2018, 76, 333–346. [Google Scholar] [CrossRef]
- Liang, D.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S. Dietary vitamin A deficiency reduces growth per-formance, immune function of intestine, and alters tight junction proteins of intestine for juvenile hybrid grouper (Epinephelus fuscoguttatus female symbol x Epinephelus lanceolatus male symbol). Fish Shellfish Immunol. 2020, 107, 346–356. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac Res 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enriquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Veterinární Med. 2011, 56, 486–503. [Google Scholar] [CrossRef]
- Ramasamy Harikrishnansupa Sup, C.B.S.M.; Heosupc Sup, M.S. Effect of a mixed herb-enriched diet on the innate immune response and disease resistance of Paralichthys olivaceus against Philasterides dicentrarchi infection. J. Aquat. Anim. Health 2010, 22, 235–243. [Google Scholar]
- Harikrishnan, R.; Balasundaram, C.; Heo, M.S. Lactobacillus sakei BK19 enriched diet enhances the immunity status and disease resistance to streptococcosis infection in kelp grouper, Epinephelus bruneus. Fish Shellfish Immunol. 2010, 29, 1037–1043. [Google Scholar] [CrossRef]
- Rajalakshmi, S.; Mohandas, A. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicol. Environ. Saf. 2005, 62, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Boshra, H.; Li, J.; Sunyer, J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006, 20, 239–262. [Google Scholar] [CrossRef] [PubMed]
- Pilstr, M.L.; Bengtén, E. Immunoglobulin in fish-genes, expression and structure. Fish Shellfish Immunol. 1996, 6, 243–262. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Chi, C.; Kim, H.J.; Yun, S.; Park, S.C.; Sukumaran, V. Effect of dietary leucine on the growth parameters and expression of antioxidant, immune, and inflammatory genes in the head kidney of Labeo rohita fingerlings. Vet. Immunol. Immunop. 2015, 167, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhao, X.; Wang, J.; Zhang, F.; Hong, W.; Liu, H. Intelectin mediated phagocytosis and killing activity of macrophages in blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2019, 87, 129–135. [Google Scholar] [CrossRef]
- Jiménez-Cantizano, R.M.; Infante, C.; Martin-Antonio, B.; Ponce, M.; Hachero, I.; Navas, J.I.; Manchado, M. Molecular characterization, phylogeny, and expression of c-type and g-type lysozymes in brill (Scophthalmus rhombus). Fish Shellfish Immunol. 2008, 25, 57–65. [Google Scholar] [CrossRef]
- Dong, J.J.; Wu, F.; Ye, X.; Sun, C.F.; Tian, Y.Y.; Lu, M.X.; Zhang, R.; Chen, Z.H. β-Defensin in Nile tilapia (Oreochromis niloticus): Sequence, tissue expression, and anti-bacterial activity of synthetic peptides. Gene 2015, 566, 23–31. [Google Scholar] [CrossRef]
- Zhou, Y.; Lei, Y.; Cao, Z.; Chen, X.; Sun, Y.; Xu, Y.; Guo, W.; Wang, S.; Liu, C. A beta-defensin gene of Trachinotus ovatus might be involved in the antimicrobial and antiviral immune response. Dev. Comp. Immunol. 2019, 92, 105–115. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, S.; Liu, X.; Li, S.; Mao, X.; Zeng, X.; Qiao, S. Different lipopolysaccharide branched-chain amino acids modulate porcine intestinal endogenous beta-defensin expression through the Sirt1/ERK/90RSK pathway. J. Agric. Food Chem. 2016, 64, 3371–3379. [Google Scholar] [CrossRef]
- You, C.; Chen, B.; Wang, M.; Wang, S.; Zhang, M.; Sun, Z.; Juventus, A.J.; Ma, H.; Li, Y. Effects of dietary lipid sources on the intestinal microbiome and health of golden pompano (Trachinotus ovatus). Fish Shellfish Immunol. 2019, 89, 187–197. [Google Scholar] [CrossRef]
- Wang, E.; Yuan, Z.; Wang, K.; Gao, D.; Liu, Z.; Liles, M.R. Consumption of florfenicol-medicated feed alters the composition of the channel catfish intestinal microbiota including enriching the relative abundance of opportunistic pathogens. Aquaculture 2019, 501, 111–118. [Google Scholar] [CrossRef]
- Song, B.; Zheng, C.; Zha, C.; Hu, S.; Yang, X.; Wang, L.; Xiao, H. Dietary leucine supplementation improves intestinal health of mice through intestinal SIgA secretion. J. Appl. Microbiol. 2020, 128, 574–583. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Dignass, A.U. Intestinal barrier function. Curr. Opin. Clin. Nutr. 2002, 5, 685–694. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, X.Y.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhao, Y. Glutamate ameliorates copper-induced oxidative injury by regulating antioxidant defences in fish intestine. Brit. J. Nutr. 2016, 116, 70–79. [Google Scholar] [CrossRef]
- Eldutar, E.; Kandemir, F.M.; Kucukler, S.; Caglayan, C. Restorative effects of Chrysin pretreatment on oxidant-antioxidant status, inflammatory cytokine production, and apoptotic and autophagic markers in acute paracetamol-induced hepatotoxicity in rats an experimental and biochemical study. J. Biochem. Mol. Toxic 2017, 31, e21960. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.B.; Liu, Y.; Kuang, S.Y.; Tang, L.; Jiang, J.; Hu, K.; Jiang, W.-D.; Li, S.-H.; Zhou, X.-Q. Dietary phosphorus prevents oxidative damage and increases antioxidant enzyme activities in intestine and hepatopancreas of juvenile Jian carp. Aquac. Nutr. 2012, 19, 250–257. [Google Scholar] [CrossRef]
- Jiang, W.D.; Deng, Y.P.; Zhou, X.Q.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Wu, P.; Zhang, Y.A.; et al. Towards the modulation of oxidative damage, apoptosis and tight junction protein in response to dietary leucine deficiency: A likely cause of ROS-induced gill structural integrity impairment. Fish Shellfish Immunol. 2017, 70, 609–620. [Google Scholar] [CrossRef]
- Liu, F.; Qu, Y.K.; Geng, C.; Wang, A.M.; Zhang, J.H.; Chen, K.J.; Liu, B.; Tian, H.Y.; Yang, W.P.; Yu, Y.B. Effects of hesperidin on the growth performance, antioxidant capacity, immune responses and disease resistance of red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2020, 99, 154–166. [Google Scholar] [CrossRef]
- Silva, D.C.; Serrano, L.; Oliveira, T.; Mansano, A.S.; Almeida, E.A.; Vieira, E.M. Effects of parabens on antioxidant system and oxidative damages in Nile tilapia (Oreochromis niloticus). Ecotoxicol. Environ. Saf. 2018, 162, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, W.; Liu, Y.; Qu, B.; Jiang, J.; Kuang, S.; Tang, L.; Tang, W.; Wu, P.; Zhang, Y.; et al. Dietary leucine improves flesh quality and alters mRNA expressions of Nrf2-mediated antioxidant enzymes in the muscle of grass carp (Ctenopharyngodon idella). Aquaculture 2016, 452, 380–387. [Google Scholar] [CrossRef]
- Reeds, P.J.; Burrin, D.G.; Stoll, B.; Jahoor, F.; Wykes, L.; Henry, J.; Frazer, M.E. Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am. J. Physiol. 1997, 273, E408–E415. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fontagné-Dicharry, S.; Lataillade, E.; Surget, A.; Larroquet, L.; Cluzeaud, M.; Kaushik, S. Antioxidant defense system is altered by dietary oxidized lipid in first-feeding rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 424–425, 220–227. [Google Scholar] [CrossRef]
- Zhao, J.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Jiang, J.; Zhang, Y.; Zhou, X. Effect of dietary isoleucine on the immuni-ty, antioxidant status, tight junctions and microflora in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2014, 41, 663–673. [Google Scholar] [CrossRef]
- Zeng, L.; Zheng, J.L.; Wang, Y.H.; Xu, M.Y.; Zhu, A.Y.; Wu, C.W. The role of Nrf2/Keap1 signaling in inorganic mercury induced oxidative stress in the liver of large yellow croaker Pseudosciaena crocea. Ecotoxicol. Environ. Saf. 2016, 132, 345–352. [Google Scholar] [CrossRef]
- Dodson, M.; Redmann, M.; Rajasekaran, N.S.; Darley-Usmar, V.; Zhang, J. Keap1-Nrf2 signaling and autophagy in protection against oxidative and reductive proteotoxicity. Biochem. J. 2015, 469, 347–355. [Google Scholar] [CrossRef]
- Chasiotis, H.; Kolosov, D.; Bui, P.; Kelly, S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: A review. Respir. Physiol. Neurobiol. 2012, 184, 269–281. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. J. Virtual Libr. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Gu, W.; Xu, F.; Li, H.; Shan, S.; Sun, X.; Yin, M.; Yang, G.; Chen, L. Characterization of a common carp intelectin gene with bacterial binding and agglutination activity. Fish Shellfish Immunol. 2021, 108, 32–41. [Google Scholar] [CrossRef]
- Beutheu, S.; Ghouzali, I.; Galas, L.; Dechelotte, P.; Coeffier, M. Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin. Nutr. 2013, 32, 863–869. [Google Scholar] [CrossRef]
- Guo, X.; Rao, J.N.; Liu, L.; Zou, T.; Wang, J.Y. Polyamines are necessary for synthesis and stability of occludin protein in intestinal epithelial cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, 1159–1169. [Google Scholar] [CrossRef]
- Liu, W.; Mi, S.; Ruan, Z.; Li, J.; Shu, X.; Yao, K.; Jiang, M.; Deng, Z. Dietary Tryptophan enhanced the expression of tight junction protein ZO-1 in intestine. J. Food Sci. 2017, 82, 562–567. [Google Scholar] [CrossRef]
- Venugopal, S.; Anwer, S.; Szaszi, K. Claudin-2: Roles beyond permeability functions. Int. J. Mol. Sci. 2019, 20, 5655. [Google Scholar] [CrossRef]
- Wada, M.; Tamura, A.; Takahashi, N.; Tsukita, S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 2013, 144, 369–380. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the Inhibition of NLRP3 Inflammasome and autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef]
- Choubey, V.; Cagalinec, M.; Liiv, J.; Safiulina, D.; Hickey, M.A.; Kuum, M.; Liiv, M.; Anwar, T.; Eskelinen, E.L.; Kaasik, A. BECN1 is involved in the initiation of mitophagy: It facilitates PARK2 translocation to mitochondria. Autophagy 2014, 10, 1105–1119. [Google Scholar] [CrossRef]
- Mizushima, N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr. Opin. Cell Biol. 2010, 22, 132–139. [Google Scholar] [CrossRef]
- Guévin, C.; Manna, D.; Bélanger, C.; Konan, K.V.; Mak, P.; Labonté, P. Autophagy protein ATG5 interacts transiently with the hepatitis C virus RNA polymerase (NS5B) early during infection. Virology 2010, 405, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Matoba, K.; Sawada, R.; Fujioka, Y.; Nakatogawa, H.; Yamamoto, H.; Kobashigawa, Y.; Hoshida, H.; Akada, R.; Ohsumi, Y.; et al. Noncanonical recognition and UBL loading of distinct E2s by autophagy-essential Atg7. Nat. Struct. Mol. Biol. 2012, 19, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Tooze, S.A. Autophagy regulation through Atg9 traffic. J. Cell Biol. 2012, 198, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Perez-Perez, M.E.; Lemaire, S.D.; Crespo, J.L. The ATG4 protease integrates redox and stress signals to regulate autophagy. J. Exp. Bot. 2021, 72, 3340–3351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Zhang, H. Autophagy in immunity. Autophagy 2012, 8, 1286–1299. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Kim, J.O.; Kim, K.K. Autophagy-mediated upregulation of cytoplasmic claudin 1 stimulates the degradation of SQSTM1/p62 under starvation. Biochem. Biophys. Res. Commun. 2018, 496, 159–166. [Google Scholar] [CrossRef]
- Son, S.M.; Park, S.J.; Stamatakou, E.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Leucine regulates autophagy via acetylation of the mTORC1 component raptor. Nat. Commun. 2020, 11, 3148. [Google Scholar] [CrossRef]
- Nighot, P.; Ma, T. Role of autophagy in the regulation of epithelial cell junctions. Tissue Barriers 2016, 4, e1171284. [Google Scholar] [CrossRef]
- Ma, Q.; You, X.; Zhu, K.; Zhao, X.; Yuan, D.; Wang, T.; Dun, Y.; Wu, J.; Ren, D.; Zhang, C.; et al. Changes in the tight junctions of the testis during aging: Role of the p38 MAPK/MMP9 pathway and autophagy in Sertoli cells. Exp. Gerontol. 2022, 161, 111729. [Google Scholar] [CrossRef]
- Bi, S.; Li, L.; Gu, H.; Li, M.; Xu, S.; Bu, W.; Zhang, M.; Zhou, Z.; Chen, X. Lycopene upregulates ZO-1 and downregu-lates claudin-1 through autophagy inhibition in the human cutaneous squamous cell carcinoma cell line COLO-16. J. Cancer 2019, 10, 510–521. [Google Scholar] [CrossRef]
- Rosenthal, R.; Günzel, D.; Krug, S.M.; Schulzke, J.D.; Fromm, M.; Yu, A.S. Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 2017, 219, 521–536. [Google Scholar] [CrossRef]
- Hultmann, L.; Phu, T.M.; Tobiassen, T.; Aas-Hansen, O.; Rustad, T. Effects of pre-slaughter stress on proteolytic en-zyme activities and muscle quality of farmed Atlantic cod (Gadus morhua). Food Chem. 2012, 134, 1399–1408. [Google Scholar] [CrossRef]
- MOSS, B.; Allam, B. Fluorometric measurement of oxidative burst in lobster hemocytes and inhibiting effect of pathogenic bacteria and hypoxia. J. Shellfish Res. 2007, 25, 1051–1057. [Google Scholar]
- Chang, C.J.; Robertsen, C.; Sun, B.; Robertsen, B. Protection of Atlantic salmon against virus infection by intramuscular injection of IFNc expression plasmid. Vaccine 2014, 32, 4695–4702. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, X.; Guo, C.; Feng, L.; Liu, Y.; Jiang, W.; Wu, P.; Luo, W.; Huang, X.; Chen, D.; et al. Dietary isoleucine affects muscle fatty acid and amino acid profiles through regulating lipid metabolism and autophagy in hybrid catfish (Pelteobagrus vachelli ♀ × Leiocassis longirostris ♂). Anim. Nutr. 2022, 11, 369–380. [Google Scholar] [CrossRef]
- Yossa, R.; Verdegem, M. Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 2015, 437, 344–350. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, H.; Xu, W.; Pan, Y.; Chen, J.; Zhang, W.; Mai, K. Replacement of dietary fishmeal by Antarctic krill meal on growth performance, intestinal morphology, body composition and organoleptic quality of large yellow croaker Larimichthys crocea. Aquaculture 2019, 512, 734281. [Google Scholar] [CrossRef]

| Items 2 | Dietary Leu Levels, g/kg | Pr > F2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 | ANOVA | Linear | Quadratic | |
| LZM | 73.92 ± 3.06 c | 87.82 ± 3.23b c | 100.07 ± 8.27 abc | 117.71 ± 0.36 a | 104.7 ± 4.14 ab | 85.62 ± 11.13 abc | 83.27 ± 3.31 c | 0.00 | <0.0001 | <0.0001 |
| ACP | 54.23 ± 2.08 e | 77.69 ± 0.63 d | 99.98 ± 2.87 b | 122.71 ± 5.45 a | 100.29 ± 0.50 b | 90.72 ± 0.59 bc | 86.37 ± 5.42 cd | 0.00 | <0.0001 | <0.0001 |
| AKP | 40.75 ± 3.57 d | 64.09 ± 4.78 c | 83.36 ± 3.33 b | 101.82 ± 4.57 a | 82.65 ± 1.88 b | 69.54 ± 3.84 c | 61.94 ± 3.26 c | 0.00 | <0.0001 | <0.0001 |
| C3 | 9.44 ± 0.46 cd | 11.66 ± 0.34 b | 14.36 ± 0.66 a | 16.02 ± 0.24 a | 12.03 ± 0.35 b | 10.83 ± 0.56 bc | 8.05 ± 1.15 d | 0.00 | 0.02 | 0.26 |
| C4 | 0.89 ± 0.04 c | 0.9 ± 0.07 c | 1.07 ± 0.05 bc | 1.38 ± 0.02 a | 1.28 ± 0.04 ab | 1.21 ± 0.04 ab | 0.96 ± 0.14 c | 0.00 | 0.01 | 0.01 |
| IgM | 29.35 ± 0.91 d | 32.64 ± 1.33 bcd | 38.24 ± 0.77 b | 45.05 ± 2.25 a | 35.87 ± 1.32 bc | 34.15 ± 1.78 bcd | 30.21 ± 2.97 cd | 0.00 | 0.74 | <0.0001 |
| Items 2 | Dietary Leu levels, g/kg | Pr > F2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 | ANOVA | Linear | Quadratic | |
| ROS | 100.00 ± 7.11 a | 83.70 ± 6.12 b | 70.10 ± 2.43 cd | 52.30 ± 2.60 e | 66.80 ± 1.90 d | 78.20 ± 1.60 bcd | 81.25 ± 8.81 bc | 0.00 | 0.00 | 0.00 |
| MDA | 0.57 ± 0.08 a | 0.50 ± 0.07 ab | 0.37 ± 0.02 b | 0.38 ± 0.10 b | 0.41 ± 0.06 b | 0.48 ± 0.02 ab | 0.51 ± 0.03 ab | 0.04 | 0.03 | 0.43 |
| PC | 2.25 ± 0.17 a | 1.86 ± 0.05 ab | 1.25 ± 0.26 d | 1.46 ± 0.01 cd | 1.62 ± 0.05 bc | 1.70 ± 0.07 bc | 1.91 ± 0.03 ab | 0.00 | 0.04 | 0.13 |
| GSH | 2.16 ± 0.06 e | 2.47 ± 0.05 cd | 2.64 ± 0.09 bc | 2.87 ± 0.10 a | 2.67 ± 0.01 b | 2.51 ± 0.05 bcd | 2.31 ± 0.11 de | 0.00 | 0.05 | <0.0001 |
| ASA | 184.16 ± 17.73 e | 208.87 ± 9.42 de | 262.87 ± 21.40 b | 293.15 ± 10.29 a | 242.93 ± 4.34 bc | 218.14 ± 10.42 cd | 209.52 ± 5.76 de | 0.00 | 0.13 | 0.00 |
| AHR | 103.79 ± 2.65 e | 113.86 ± 4.30 cde | 126.45 ± 1.81 b | 149.16 ± 8.96 a | 123.88 ± 3.26 bc | 117.19 ± 1.13 bcd | 112.18 ± 2.62 de | 0.00 | 0.08 | 0.00 |
| T-SOD | 26.12 ± 1.30 d | 29.23 ± 0.50 cd | 32.71 ± 1.30 ab | 36.91 ± 1.60 a | 31.42 ± 0.70 bc | 28.54 ± 1.00 c | 27.34 ± 0.90 cd | 0.00 | 0.70 | <0.0001 |
| CAT | 3.05 ± 0.04 d | 3.32 ± 0.08 cd | 3.93 ± 0.14 ab | 4.48 ± 0.43 a | 3.69 ± 0.22 bc | 3.37 ± 0.23 bcd | 3.18 ± 0.110 cd | 0.01 | 0.68 | 0.06 |
| GPX | 8.05 ± 1.22 d | 11.31 ± 0.36 bc | 13.08 ± 1.43 ab | 15.50 ± 0.90 a | 13.31 ± 1.42 ab | 12.57 ± 0.62 ab | 8.88 ± 0.42 cd | 0.00 | 0.14 | 0.85 |
| GST | 42.68 ± 2.57 e | 54.26 ± 4.51 cd | 60.65 ± 1.11 ab | 63.44 ± 0.10 a | 57.76 ± 1.70 abc | 55.49 ± 1.07 bcd | 50.85 ± 0.13 d | 0.00 | 0.01 | 0.67 |
| GR | 16.79 ± 0.11 c | 19.49 ± 1.22 b | 21.22 ± 0.22 b | 24.22 ± 0.38 a | 21.36 ± 0.52 b | 19.88 ± 0.56 b | 17.47 ± 1.16 c | 0.00 | 0.29 | <0.0001 |
| Independent Parameters | Dependent Parameters | Correlation Coefficients | p |
|---|---|---|---|
| ROS | MDA | 0.912 | 0.004 |
| PC | 0.793 | 0.033 | |
| ASA | −0.956 | 0.001 | |
| AHR | −0.954 | 0.001 | |
| T-SOD | −0.933 | 0.002 | |
| CAT | −0.928 | 0.003 | |
| GPX | −0.920 | 0.003 | |
| GST | −0.943 | 0.001 | |
| GR | −0.942 | 0.001 | |
| GSH | −0.965 | 0.000 | |
| CuZnSOD mRNA | T-SOD | 0.981 | 0.000 |
| CAT mRNA | CAT | 0.861 | 0.013 |
| GPX1α mRNA | GPX | 0.643 | 0.119 |
| GST mRNA | GST | −0.792 | 0.034 |
| Nrf2 mRNA | CuZnSOD mRNA | 0.892 | 0.007 |
| CAT mRNA | 0.794 | 0.033 | |
| GPX1α mRNA | 0.849 | 0.016 | |
| GCLC mRNA | 0.818 | 0.025 | |
| Keap1 mRNA | CuZnSOD mRNA | −0.975 | 0.000 |
| CAT mRNA | −0.906 | 0.005 | |
| GPX1α mRNA | −0.794 | 0.033 | |
| GCLC mRNA | −0.919 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhao, Y.; Liu, H.; Cao, Q.; Feng, L.; Zhang, Z.; Jiang, W.; Wu, P.; Liu, Y.; Luo, W.; et al. Dietary Leucine Improves Fish Intestinal Barrier Function by Increasing Humoral Immunity, Antioxidant Capacity, and Tight Junction. Int. J. Mol. Sci. 2023, 24, 4716. https://doi.org/10.3390/ijms24054716
Zhao J, Zhao Y, Liu H, Cao Q, Feng L, Zhang Z, Jiang W, Wu P, Liu Y, Luo W, et al. Dietary Leucine Improves Fish Intestinal Barrier Function by Increasing Humoral Immunity, Antioxidant Capacity, and Tight Junction. International Journal of Molecular Sciences. 2023; 24(5):4716. https://doi.org/10.3390/ijms24054716
Chicago/Turabian StyleZhao, Ju, Ye Zhao, Haifeng Liu, Quanquan Cao, Lin Feng, Zhihao Zhang, Weidan Jiang, Pei Wu, Yang Liu, Wei Luo, and et al. 2023. "Dietary Leucine Improves Fish Intestinal Barrier Function by Increasing Humoral Immunity, Antioxidant Capacity, and Tight Junction" International Journal of Molecular Sciences 24, no. 5: 4716. https://doi.org/10.3390/ijms24054716
APA StyleZhao, J., Zhao, Y., Liu, H., Cao, Q., Feng, L., Zhang, Z., Jiang, W., Wu, P., Liu, Y., Luo, W., Huang, X., & Jiang, J. (2023). Dietary Leucine Improves Fish Intestinal Barrier Function by Increasing Humoral Immunity, Antioxidant Capacity, and Tight Junction. International Journal of Molecular Sciences, 24(5), 4716. https://doi.org/10.3390/ijms24054716









