Depolarization and Hyperexcitability of Cortical Motor Neurons after Spinal Cord Injury Associates with Reduced HCN Channel Activity
Abstract
1. Introduction
2. Results
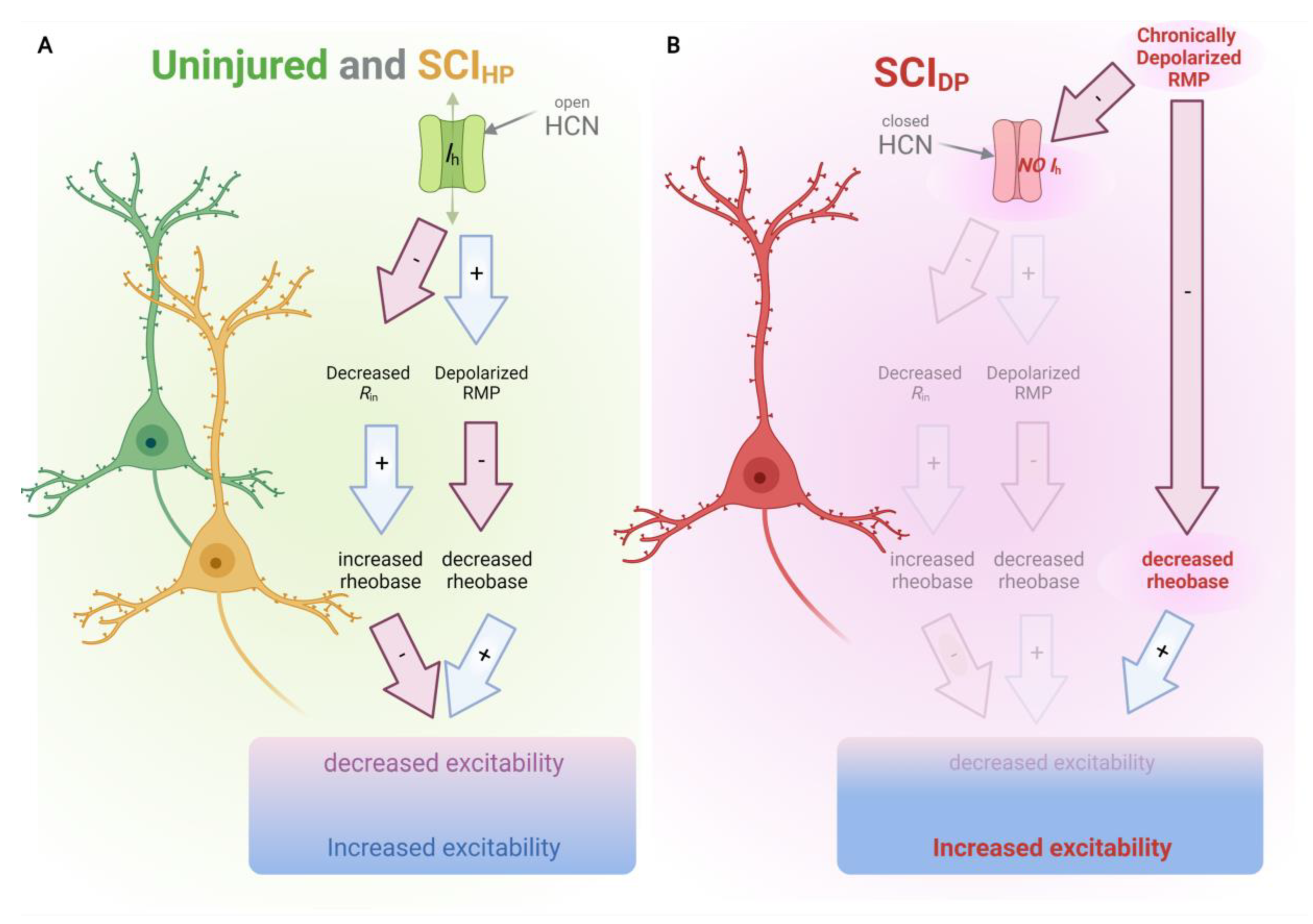
3. Discussion
4. Materials and Methods
4.1. Surgery
4.2. Electrophysiology
4.3. Histology
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagendran, T.; Larsen, R.S.; Bigler, R.L.; Frost, S.B.; Philpot, B.D.; Nudo, R.J.; Taylor, A.M. Distal axotomy enhances retrograde presynaptic excitability onto injured pyramidal neurons via trans-synaptic signaling. Nat. Commun. 2017, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Jure, I.; Labombarda, F. Spinal cord injury drives chronic brain changes. Neural Regen. Res. 2017, 12, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Höller, Y.; Brigo, F.; Höller, P.; Christova, M.; Tezzon, F.; Golaszewski, S.; Trinka, E. Fatigue-induced motor cortex excitability changes in subjects with spinal cord injury. Brain Res. Bull. 2013, 99, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Saturno, E.; Bonato, C.; Miniussi, C.; Lazzaro, V.; Callea, L. Motor cortex changes in spinal cord injury: A TMS study. Neurol. Res. 2008, 30, 1084–1085. [Google Scholar] [CrossRef]
- Benedetti, B.; Weidenhammer, A.; Reisinger, M.; Couillard-despres, S. Spinal Cord Injury and Loss of Cortical Inhibition. Int. J. Mol. Sci. 2022, 23, 5622. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Dai, H.-N.; McAtee, M.; Vicini, S.; Bregman, B.S. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp. Neurol. 2006, 198, 401–415. [Google Scholar] [CrossRef]
- Kim, B.G.; Dai, H.-N.; McAtee, M.; Bregman, B.S. Modulation of dendritic spine remodeling in the motor cortex following spinal cord injury: Effects of environmental enrichment and combinatorial treatment with transplants and neurotrophin-3. J. Comp. Neurol. 2008, 508, 473–486. [Google Scholar] [CrossRef]
- Tan, A.M.; Waxman, S.G. Spinal cord injury, dendritic spine remodeling, and spinal memory mechanisms. Exp. Neurol. 2012, 235, 142–151. [Google Scholar] [CrossRef]
- Beaud, M.; Schmidlin, E.; Wannier, T.; Freund, P.; Bloch, J.; Mir, A.; Schwab, M.E.; Rouiller, E.M. Anti-Nogo-A antibody treatment does not prevent cell body shrinkage in the motor cortex in adult monkeys subjected to unilateral cervical cord lesion. BMC Neurosci. 2008, 9, 5. [Google Scholar] [CrossRef]
- Hains, B.C.; Black, J.A.; Waxman, S.G. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J. Comp. Neurol. 2003, 462, 328–341. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, K.H.; Kim, U.J.; Yoon, D.H.; Sohn, J.H.; Choi, S.S.; Yi, I.G.; Park, Y.G. Injury in the spinal cord may produce cell death in the brain. Brain Res. 2004, 1020, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Bareyre, F.M.; Kerschensteiner, M.; Raineteau, O.; Mettenleiter, T.C.; Weinmann, O.; Schwab, M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004, 7, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Bieler, L.; Grassner, L.; Zaunmair, P.; Kreutzer, C.; Lampe, L.; Trinka, E.; Marschallinger, J.; Aigner, L.; Couillard-despres, S. Motor deficits following dorsal corticospinal tract transection in rats: Voluntary versus skilled locomotion readouts. Heliyon 2018, 4, e00540. [Google Scholar] [CrossRef]
- Siegenthaler, M.M.; Tu, M.K.; Keirstead, H.S. The extent of myelin pathology differs following contusion and transection spinal cord injury. J. Neurotrauma 2007, 24, 1631–1646. [Google Scholar] [CrossRef]
- Greer, J.E.; Hånell, A.; McGinn, M.J.; Povlishock, J.T. Mild traumatic brain injury in the mouse induces axotomy primarily within the axon initial segment. Acta Neuropathol. 2013, 126, 59–74. [Google Scholar] [CrossRef]
- Zhang, J.M.; Donnelly, D.F.; Song, X.J.; LaMotte, R.H. Axotomy increases the excitability of dorsal root ganglion cells with unmyelinated axons. J. Neurophysiol. 1997, 78, 2790–2794. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, Z.; Sabirzhanov, B.; Stoica, B.A.; Kumar, A.; Luo, T.; Skovira, J.; Faden, A.I. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: Role of cell cycle pathways. J. Neurosci. 2014, 34, 10989–11006. [Google Scholar] [CrossRef]
- Kase, D.; Imoto, K. The Role of HCN Channels on Membrane Excitability in the Nervous System. J. Signal Transduct. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Lewis, A.S.; Chetkovich, D.M. HCN channels in behavior and neurological disease: Too hyper or not active enough? Mol. Cell. Neurosci. 2011, 46, 357–367. [Google Scholar] [CrossRef]
- Sheets, P.L.; Suter, B.A.; Kiritani, T.; Savio Chan, C.S.; James Surmeier, D.; Shepherd, G.M.G. Corticospinal-specific HCN expression in mouse motor cortex: I h)-dependent synaptic integration as a candidate microcircuit mechanism involved in motor control. J. Neurophysiol. 2011, 106, 2216–2231. [Google Scholar] [CrossRef]
- Yuemin, D.; Abba, K.J.; Weihong, P. Neural plasticity after spinal cord injury. Neural Regen. Res. 2005, 7, 386–391. [Google Scholar] [CrossRef]
- Xerri, C. Plasticity of cortical maps: Multiple triggers for adaptive reorganization following brain damage and spinal cord injury. Neuroscientist 2012, 18, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Isa, T. Compensatory changes at the cerebral cortical level after spinal cord injury. Neuroscientist 2009, 15, 436–444. [Google Scholar] [CrossRef]
- Orlando, C.; Raineteau, O. Integrity of cortical perineuronal nets influences corticospinal tract plasticity after spinal cord injury. Brain Struct. Funct. 2014, 220, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Nardone, R.; Höller, Y.; Brigo, F.; Seidl, M.; Christova, M.; Bergmann, J.; Golaszewski, S.; Trinka, E. Functional brain reorganization after spinal cord injury: Systematic review of animal and human studies. Brain Res. 2013, 1504, 58–73. [Google Scholar] [CrossRef]
- Jain, N.; Florence, S.L.; Kaas, J.H. Reorganization of somatosensory cortex after nerve and spinal cord injury. News Physiol. Sci. 1998, 13, 143–149. [Google Scholar] [CrossRef]
- Manganotti, P.; Acler, M.; Zanette, G.P.; Smania, N.; Fiaschi, A. Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil. Neural Repair 2008, 22, 396–403. [Google Scholar] [CrossRef]
- Miyake, S.; Higuchi, H.; Honda-Wakasugi, Y.; Fujimoto, M.; Kawai, H.; Nagatsuka, H.; Maeda, S.; Miyawaki, T. Locally injected ivabradine inhibits carrageenan-induced pain and inflammatory responses via hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. PLoS ONE 2019, 14, e0217209. [Google Scholar] [CrossRef]
- Djouhri, L.; Smith, T.; Ahmeda, A.; Alotaibi, M.; Weng, X. Hyperpolarization-activated cyclic nucleotide-gated channels contribute to spontaneous activity in L4 C-fiber nociceptors, but not Aβ-non-nociceptors, after axotomy of L5-spinal nerve in the rat in vivo. Pain 2018, 159, 1392–1402. [Google Scholar] [CrossRef]
- Smith, T.; Al Otaibi, M.; Sathish, J.; Djouhri, L. Increased expression of HCN2 channel protein in L4 dorsal root ganglion neurons following axotomy of L5- and inflammation of L4-spinal nerves in rats. Neuroscience 2015, 295, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Schiemann, J.; Puggioni, P.; Dacre, J.; Pelko, M.; Domanski, A.; van Rossum, M.C.W.; Duguid, I. Cellular Mechanisms Underlying Behavioral State-Dependent Bidirectional Modulation of Motor Cortex Output. Cell Rep. 2015, 11, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, J.C.; Barbuti, A.; Milanesi, R.; Coco, S.; Bucchi, A.; Bottelli, G.; Ferrarese, C.; Franceschetti, S.; Terragni, B.; Baruscotti, M.; et al. Recessive loss-of-function mutation in the pacemaker HCN2 Channel causing increased neuronal excitability in a patient with idiopathic generalized epilepsy. J. Neurosci. 2011, 31, 17327–17337. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Jones, T.D.; Lugo, J.N.; Sheerin, A.H.; Miller, J.W.; D’Ambrosio, R.; Anderson, A.E.; Poolos, N.P. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J. Neurosci. 2007, 27, 13012–13021. [Google Scholar] [CrossRef]
- Jung, S.; Warner, L.N.; Pitsch, J.; Becker, A.J.; Poolos, N.P. Rapid loss of dendritic HCN channel expression in hippocampal pyramidal neurons following status epilepticus. J. Neurosci. 2011, 31, 14291–14295. [Google Scholar] [CrossRef] [PubMed]
- Powell, K.L.; Ng, C.; O’Brien, T.; Xu, S.H.; Williams, D.A.; Foote, S.; Reid, C.A. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia 2008, 49, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Dibbens, L.M.; Reid, C.A.; Hodgson, B.; Thomas, E.A.; Phillips, A.M.; Gazina, E.; Cromer, B.A.; Clarke, A.L.; Baram, T.Z.; Scheffer, I.E.; et al. Augmented currents of an HCN2 variant in patients with febrile seizure syndromes. Ann. Neurol. 2010, 67, 542–546. [Google Scholar] [CrossRef]
- Benedetti, B.; Dannehl, D.; Janssen, J.M.; Corcelli, C.; Couillard-Després, S.; Engelhardt, M. Structural and Functional Maturation of Rat Primary Motor Cortex Layer V Neurons. Int. J. Mol. Sci. 2020, 21, 6101. [Google Scholar] [CrossRef]
- Albertson, A.J.; Williams, S.B.; Hablitz, J.J. Regulation of epileptiform discharges in rat neocortex by HCN channels. J. Neurophysiol. 2013, 110, 1733–1743. [Google Scholar] [CrossRef]
- Kriz, J.; Kozak, J.; Zedka, M. Primary motor cortex inhibition in spinal cord injuries. Neuroendocrinol. Lett. 2012, 33, 431–441. [Google Scholar]
- Ellaway, P.H.; Catley, M.; Davey, N.J.; Kuppuswamy, A.; Strutton, P.; Frankel, H.L.; Jamous, A.; Savic, G. Review of physiological motor outcome measures in spinal cord injury using transcranial magnetic stimulation and spinal reflexes. J. Rehabil. Res. Dev. 2007, 44, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Belci, M.; Catley, M.; Husain, M.; Frankel, H.L.; Davey, N.J. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004, 42, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Nelson, R.; Nissim, N.; Parmer, R.; Thompson, F.J.; Bose, P. Effect of combined treadmill training and magnetic stimulation on spasticity and gait impairments after cervical spinal cord injury. J. Neurotrauma 2014, 31, 1088–1106. [Google Scholar] [CrossRef]
- Moxon, K.A.; Oliviero, A.; Aguilar, J.; Foffani, G. Cortical reorganization after spinal cord injury: Always for good? Neuroscience 2014, 283, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.N.; Michaelis, M.; Amir, R.; Devor, M. Spinal nerve injury enhances subthreshold membrane potential oscillations in DRG neurons: Relation to neuropathic pain. J. Neurophysiol. 2000, 84, 205–215. [Google Scholar] [CrossRef]
- Mandolesi, G.; Madeddu, F.; Bozzi, Y.; Maffei, L.; Ratto, G.M. Acute physiological response of mammalian central neurons to axotomy: Ionic regulation and electrical activity. FASEB J. 2004, 18, 1934–1936. [Google Scholar] [CrossRef] [PubMed]
- Hogan, Q.H. Role of decreased sensory neuron membrane calcium currents in the genesis of neuropathic pain. Croat. Med. J. 2007, 48, 9–21. [Google Scholar]
- Hogan, Q.H.; Sprick, C.; Guo, Y.; Mueller, S.; Bienengraeber, M.; Pan, B.; Wu, H.E. Divergent effects of painful nerve injury on mitochondrial Ca2+ buffering in axotomized and adjacent sensory neurons. Brain Res. 2014, 1589, 112–125. [Google Scholar] [CrossRef]
- Muramoto, A. Ionic dependence of the axotomy-induced long-lasting firing in an identified crayfish motoneuron. Zoolog. Sci. 1998, 15, 11–18. [Google Scholar] [CrossRef]
- Petrosyan, H.A.; Alessi, V.; Sisto, S.A.; Kaufman, M.; Arvanian, V.L. Transcranial magnetic stimulation (TMS) responses elicited in hindlimb muscles as an assessment of synaptic plasticity in spino-muscular circuitry after chronic spinal cord injury. Neurosci. Lett. 2017, 642, 37–42. [Google Scholar] [CrossRef]
- Ichiyama, R.M.; Gerasimenko, Y.P.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci. Lett. 2005, 383, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Weise, D.; Mann, J.; Ridding, M.; Eskandar, K.; Huss, M.; Rumpf, J.J.; Di Lazzaro, V.; Mazzone, P.; Ranieri, F.; Classen, J. Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability. J. Physiol. 2013, 591, 4903–4920. [Google Scholar] [CrossRef]
- Bunday, K.L.; Urbin, M.A.; Perez, M.A. Potentiating paired corticospinal-motoneuronal plasticity after spinal cord injury. Brain Stimul. 2018, 11, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Van Aerde, K.I.; Feldmeyer, D. Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb. Cortex 2015, 25, 788–805. [Google Scholar] [CrossRef] [PubMed]




| Uninjured (n = 11) | SCI (n = 21) | p-Value | |
|---|---|---|---|
| RMP (mV) | −72.9 ± 5.0 | −68.4 ± 5.1 | 0.0108 1 |
| Rheobase (pA) | 59.3 ± 21.2 | 102.3 ± 43.7 | <0.001 1 |
| Rin (GΩ) | 0.22 ± 0.14 | 0.26 ± 0.12 | 0.1167 2 |
| AP threshold (mV) | −40.2 ± 4.1 | −43.4 ± 5.3 | 0.1100 1 |
| AP half-width (ms) | 1.37 ± 0.24 | 1.47 ± 0.21 | 0.1000 2 |
| Ih (pA/pF) | 0.6 ± 0.3 | 0.6 ± 0.4 | 0.4736 2 |
| CM (pF) | 258.5 ± 130.5 | 191.9 ± 71.3 | 0.1390 1 |
| Ih Vhalf | −86.9 ± 4.6 | −85.3 ± 9.6 | 0.1506 2 |
| RMP/Ih correlation (R) | 0.76 | 0.36 | 0.0067 (uninjured) 0.1429 (SCI) |
| Uninjured (n = 11) | Uninjured ZD7288 (n = 11) | Adjusted p-Value | |
|---|---|---|---|
| RMP (mV) | −72.9 ± 5.0 | −80.1 ± 4.9 | <0.001 2 |
| Rin (GΩ) | 0.22 ± 0.14 | 0.30 ± 0.17 | <0.001 2 |
| Rheobase (pA) | 102.3 ± 43.7 | 117.7 ± 61.5 | 0.0300 1 |
| SCIHP (n = 12) | SCIHP ZD7288 | SCIDP (n = 6) | SCIDP ZD7288 | Adjusted p-Value | |
|---|---|---|---|---|---|
| RMP (mV) | −71.2 ± 4.5 | −80.7 ± 3.7 | −63.1 ± 1.9 | −57.7 ± 7.3 | <0.0001 1a 0.0051 1b |
| Rin (GΩ) | 0.24 ± 0.08 | 0.35 ± 0.11 | 0.23 ± 0.07 | 0.25 ± 0.11 | <0.0001 1a 0.3868 1b |
| Rheobase (pA) | 72.9 ± 15.9 | 88.7 ± 31.4 | 33.3 ± 11.5 | 20.0 ± 34.6 | 0.0007 1a 0.3237 1b |
| Uninjured (n = 8) | SCIHP (n = 12) | SCIDP (n = 3) | p-Value | |
|---|---|---|---|---|
| Rheobase-70 (pA) | 74.04 ± 48.5 | 47.6 ± 21.3 | 30.0 ± 34.6 | 0.0300 1 n.a. 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, B.; Bieler, L.; Erhardt-Kreutzer, C.; Jakubecova, D.; Benedetti, A.; Reisinger, M.; Dannehl, D.; Thome, C.; Engelhardt, M.; Couillard-Despres, S. Depolarization and Hyperexcitability of Cortical Motor Neurons after Spinal Cord Injury Associates with Reduced HCN Channel Activity. Int. J. Mol. Sci. 2023, 24, 4715. https://doi.org/10.3390/ijms24054715
Benedetti B, Bieler L, Erhardt-Kreutzer C, Jakubecova D, Benedetti A, Reisinger M, Dannehl D, Thome C, Engelhardt M, Couillard-Despres S. Depolarization and Hyperexcitability of Cortical Motor Neurons after Spinal Cord Injury Associates with Reduced HCN Channel Activity. International Journal of Molecular Sciences. 2023; 24(5):4715. https://doi.org/10.3390/ijms24054715
Chicago/Turabian StyleBenedetti, Bruno, Lara Bieler, Christina Erhardt-Kreutzer, Dominika Jakubecova, Ariane Benedetti, Maximilian Reisinger, Dominik Dannehl, Christian Thome, Maren Engelhardt, and Sebastien Couillard-Despres. 2023. "Depolarization and Hyperexcitability of Cortical Motor Neurons after Spinal Cord Injury Associates with Reduced HCN Channel Activity" International Journal of Molecular Sciences 24, no. 5: 4715. https://doi.org/10.3390/ijms24054715
APA StyleBenedetti, B., Bieler, L., Erhardt-Kreutzer, C., Jakubecova, D., Benedetti, A., Reisinger, M., Dannehl, D., Thome, C., Engelhardt, M., & Couillard-Despres, S. (2023). Depolarization and Hyperexcitability of Cortical Motor Neurons after Spinal Cord Injury Associates with Reduced HCN Channel Activity. International Journal of Molecular Sciences, 24(5), 4715. https://doi.org/10.3390/ijms24054715








