An Early and Sustained Inflammatory State Induces Muscle Changes and Establishes Obesogenic Characteristics in Wistar Rats Exposed to the MSG-Induced Obesity Model
Abstract
1. Introduction
2. Results
2.1. Murinometric Profile
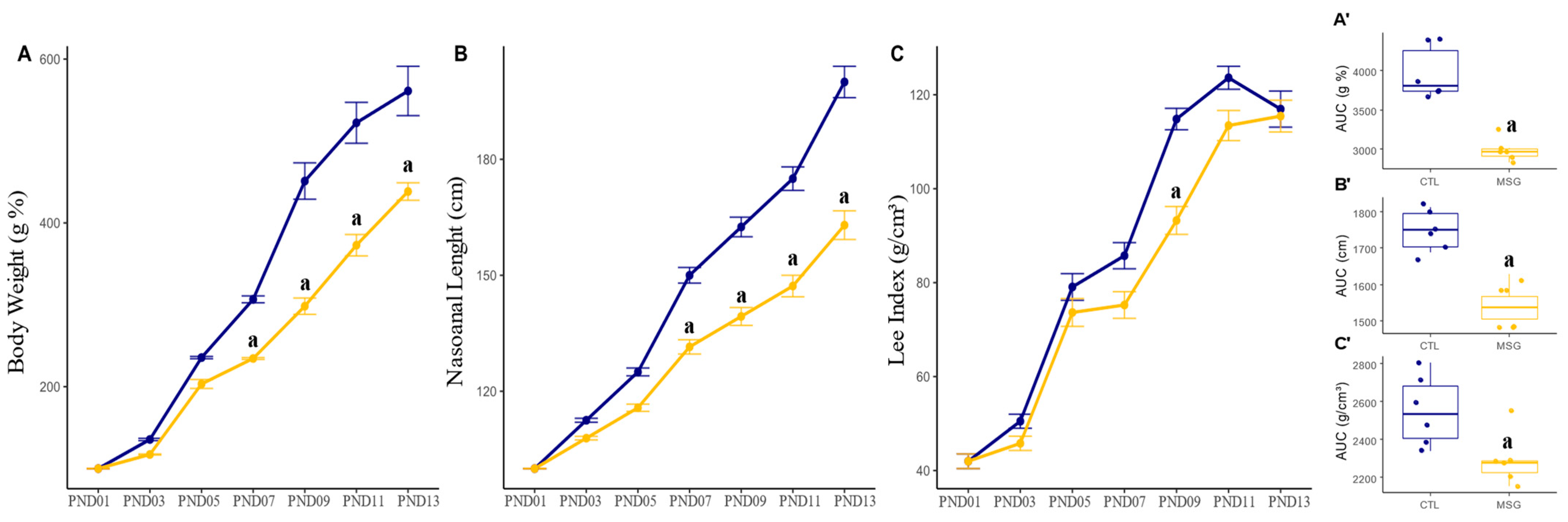
2.1.1. Lactation Period
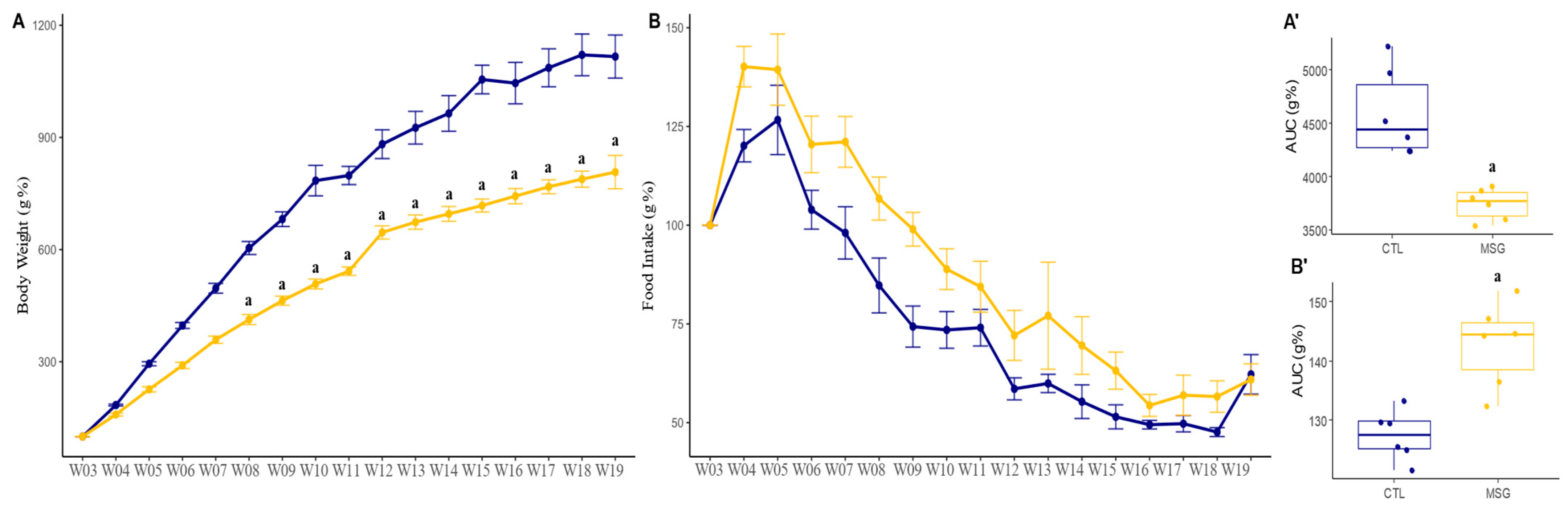
2.1.2. Post-Weaning Period
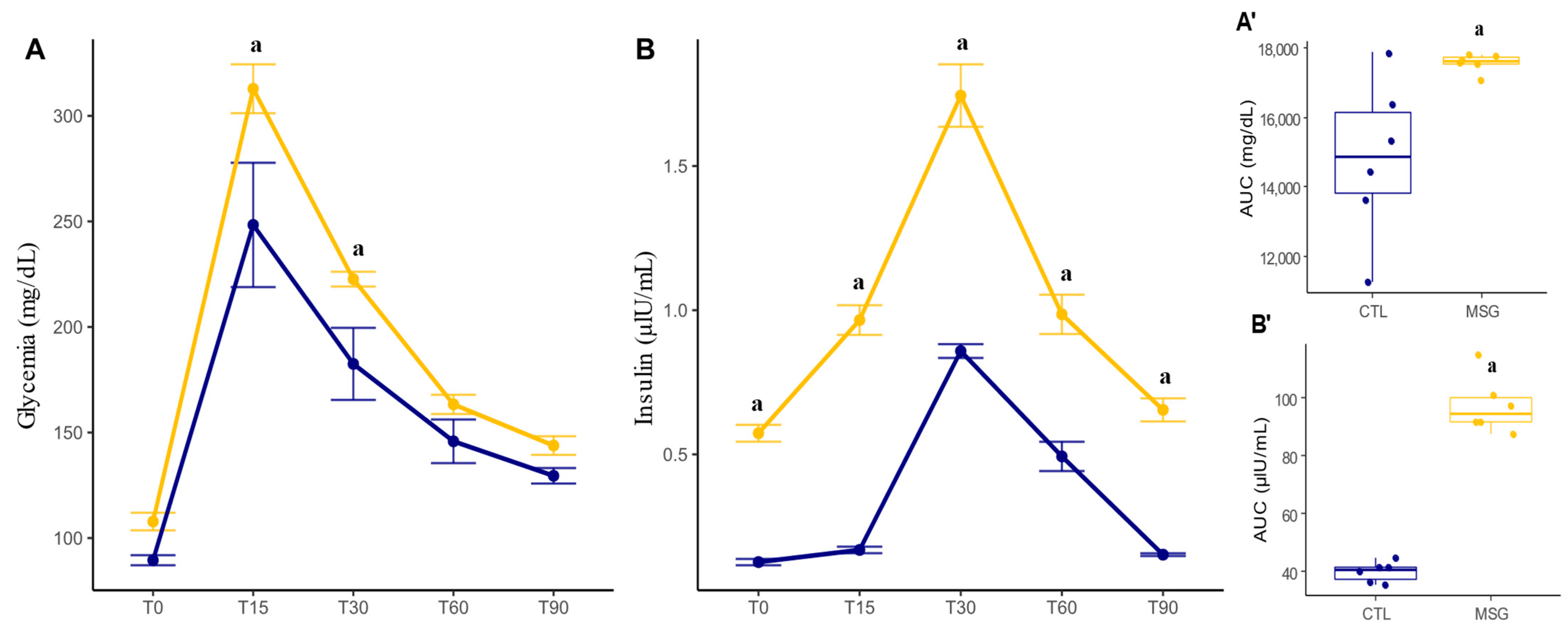
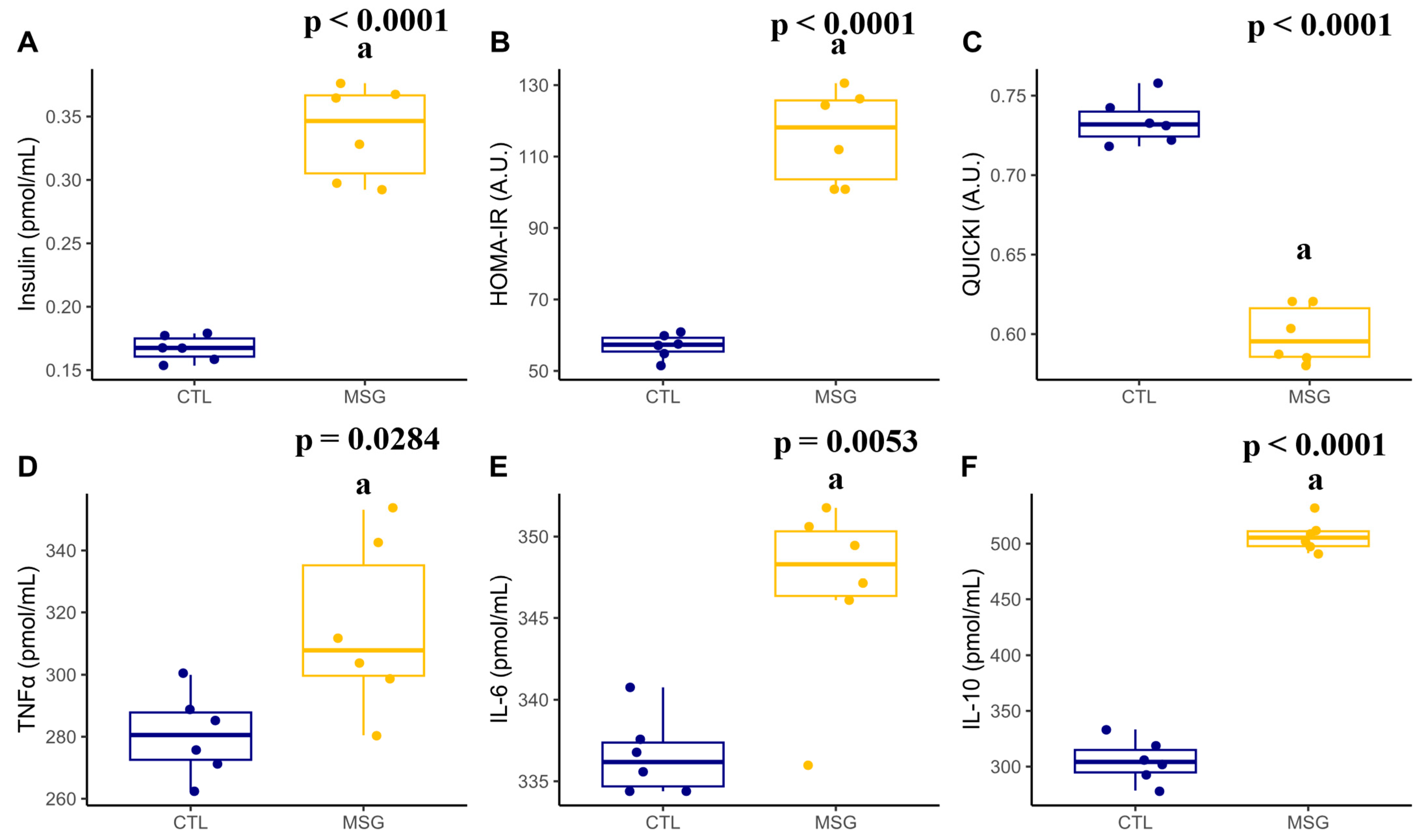
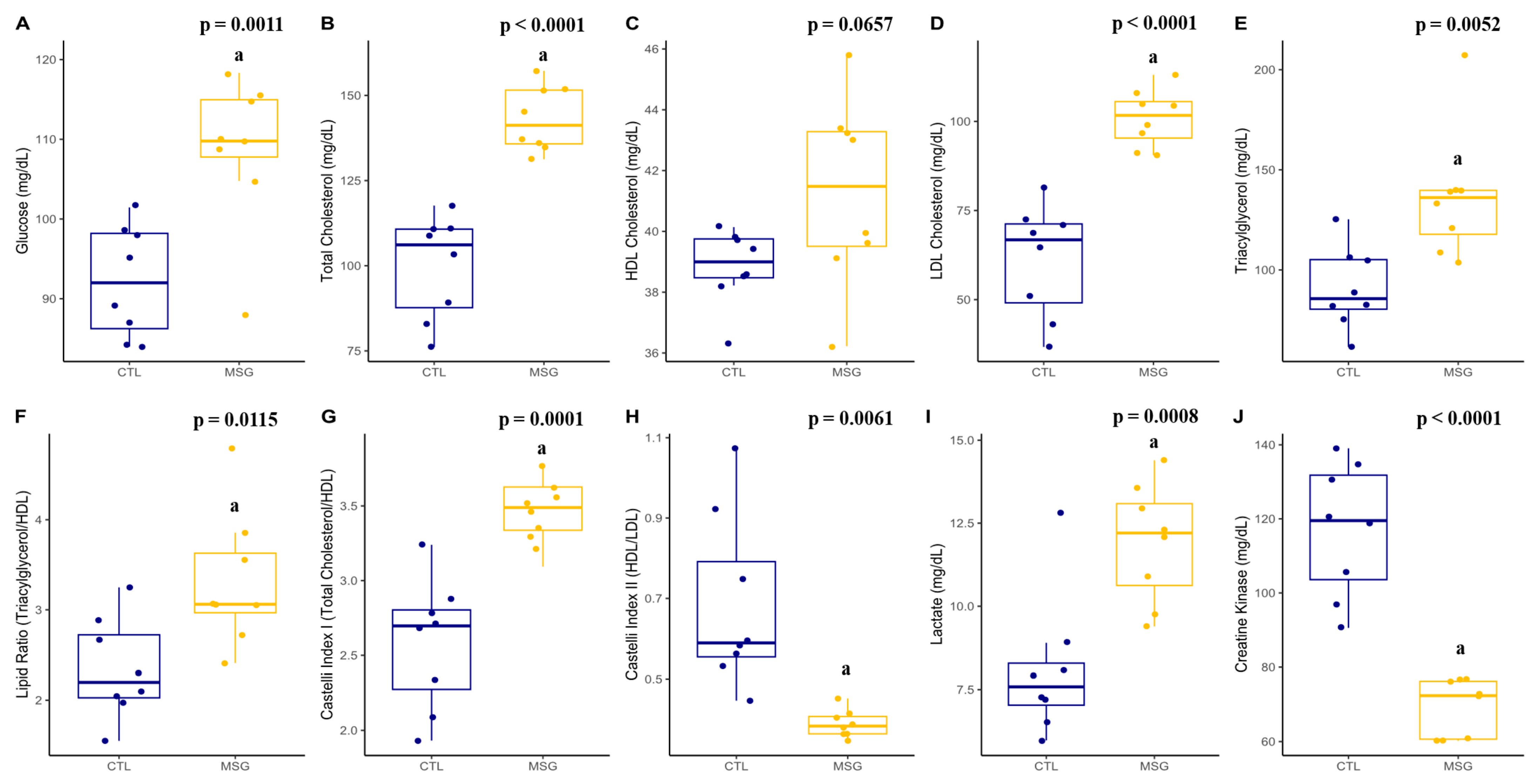
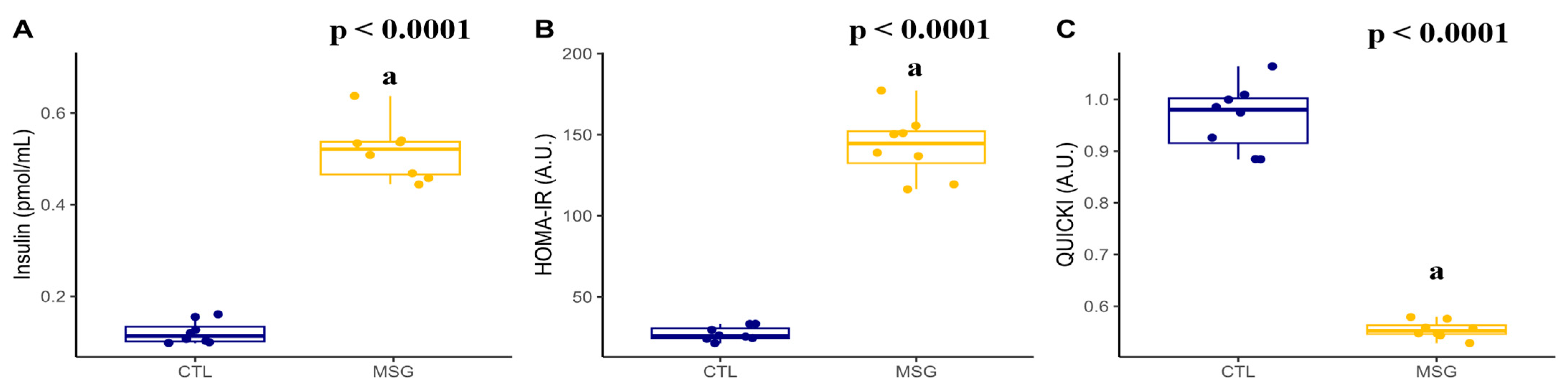
2.2. Intraperitoneal Glucose Tolerance Test (ipGTT) and Insulin Measurement
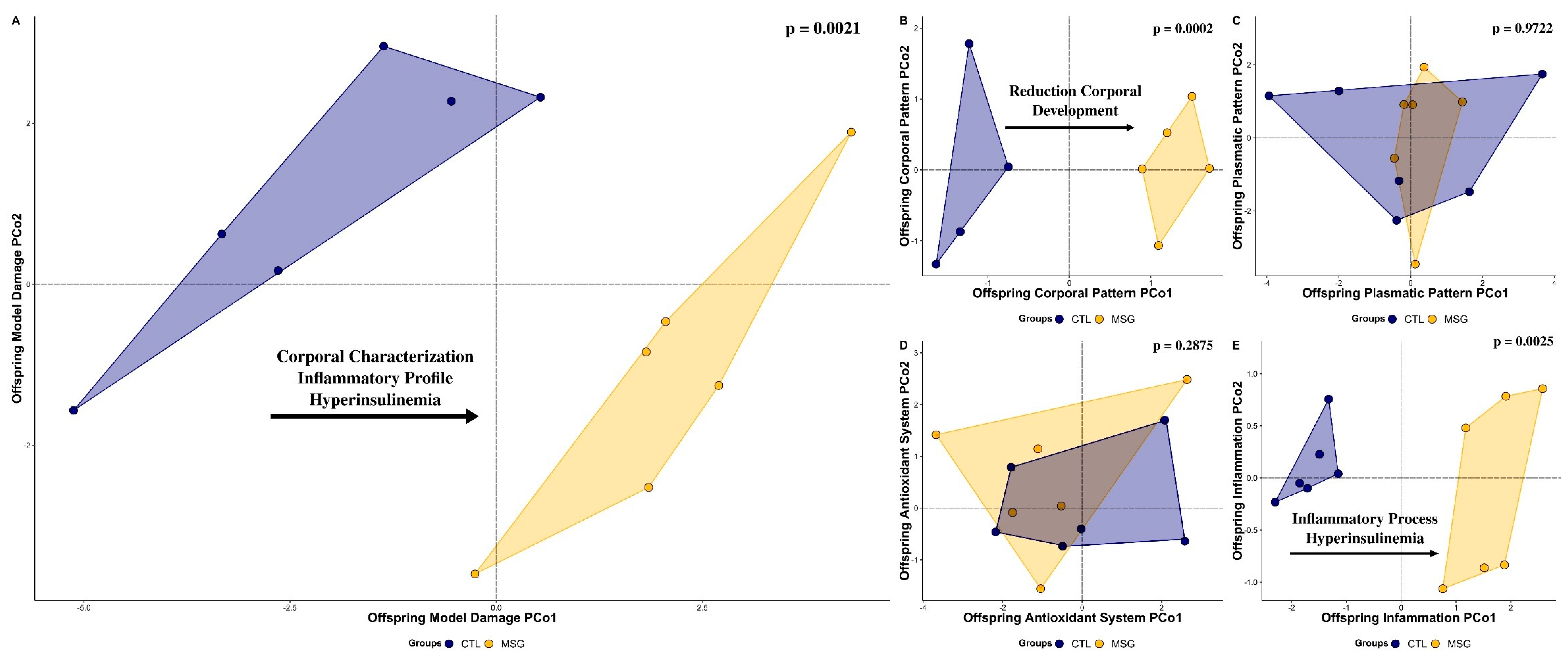
2.3. Inflammatory and Obesogenic Scenario at 15 Days
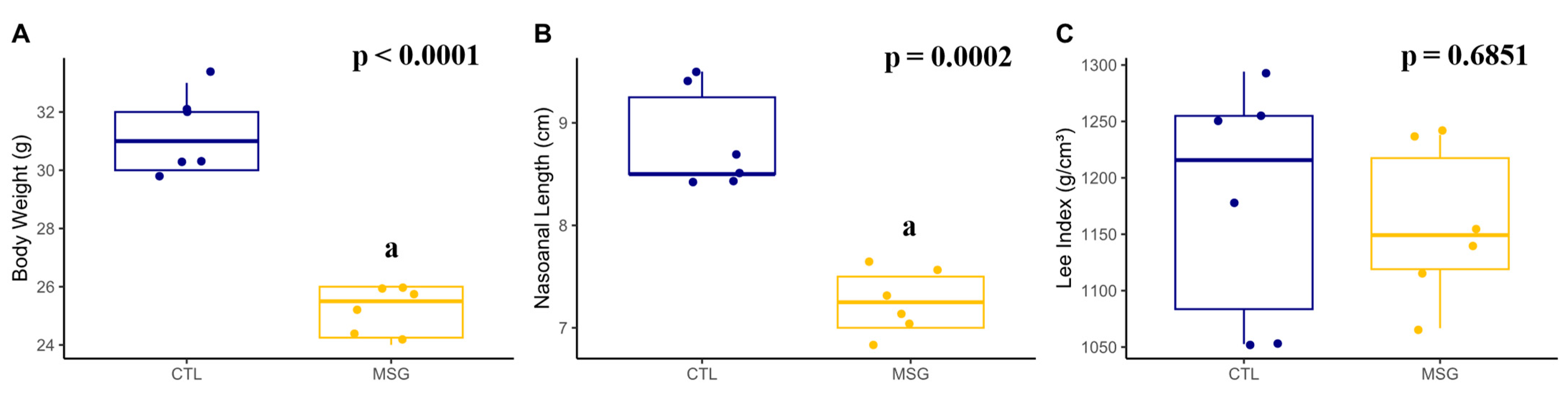
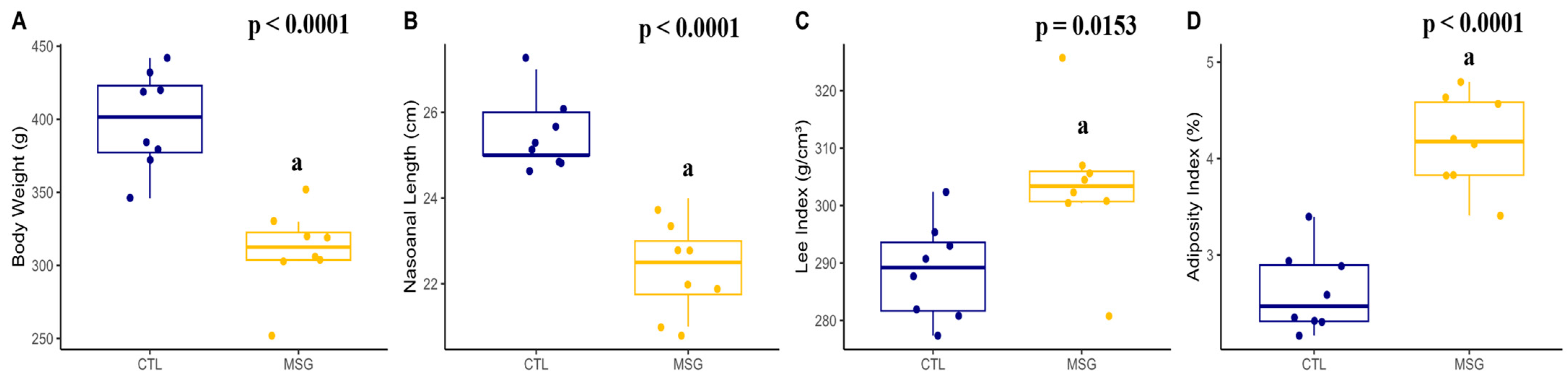
2.3.1. Corporal Characterization
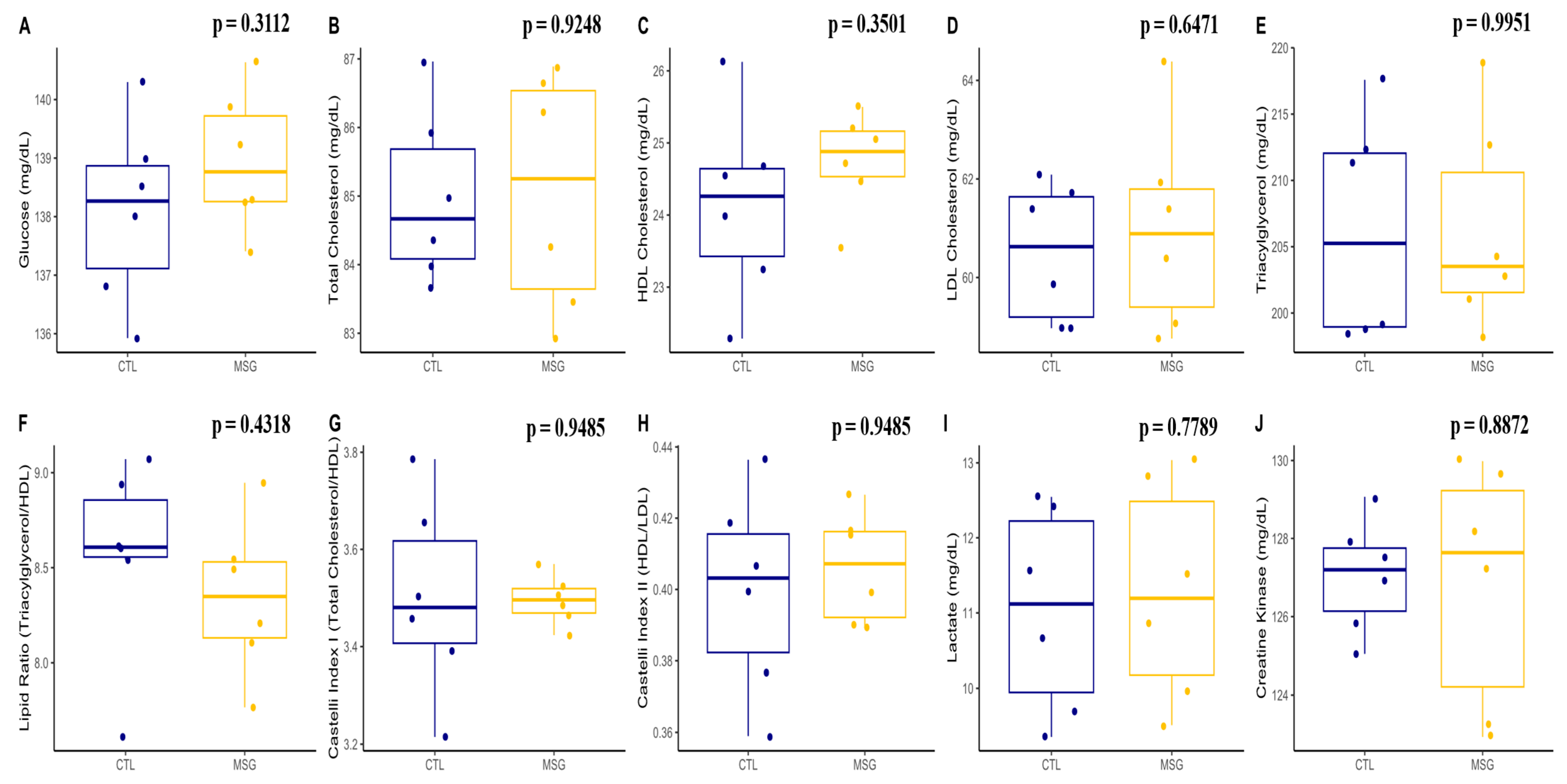
2.3.2. Plasmatic Profile
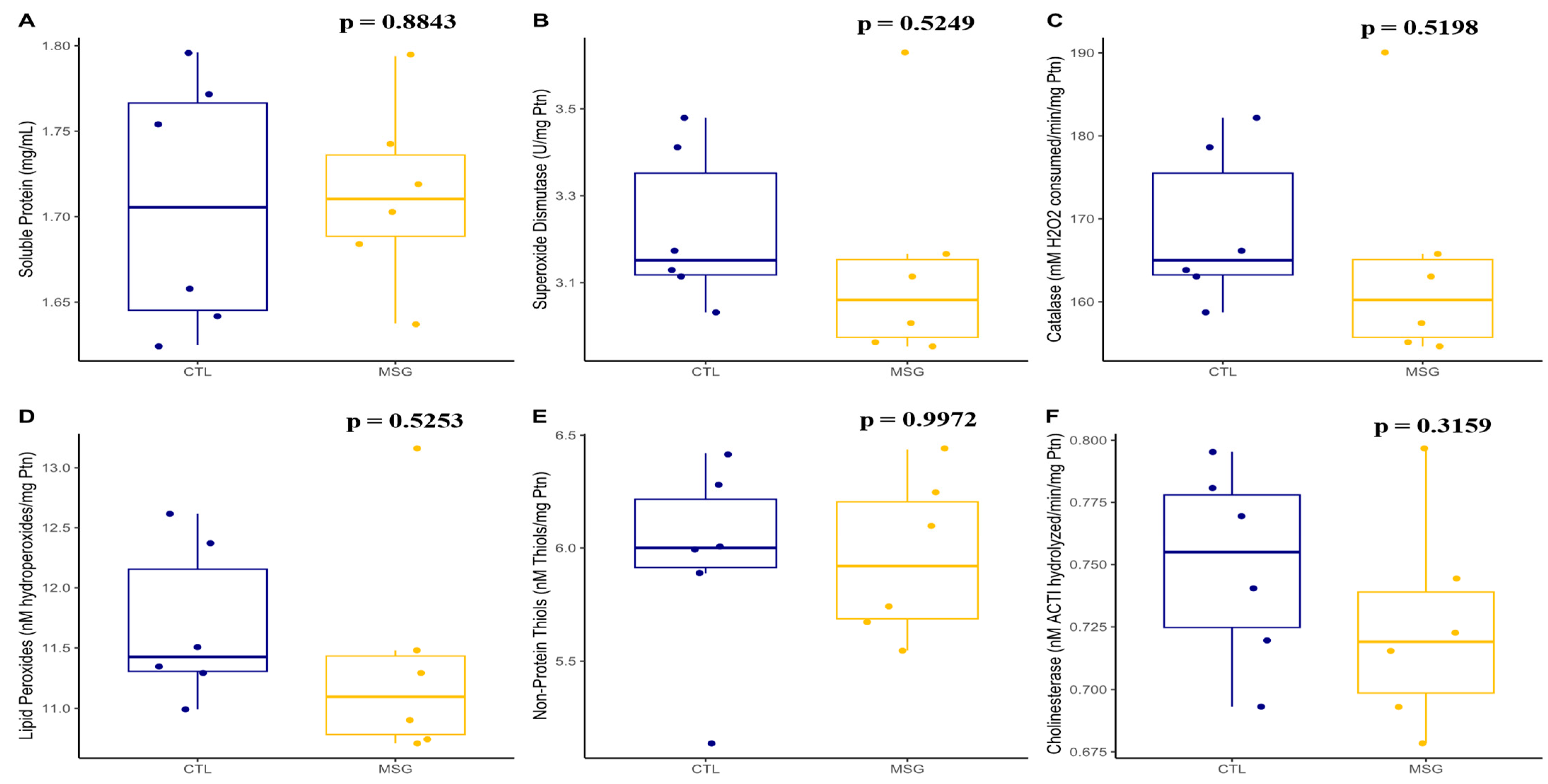
2.3.3. Skeletal Muscle Antioxidant System and Oxidative Damage
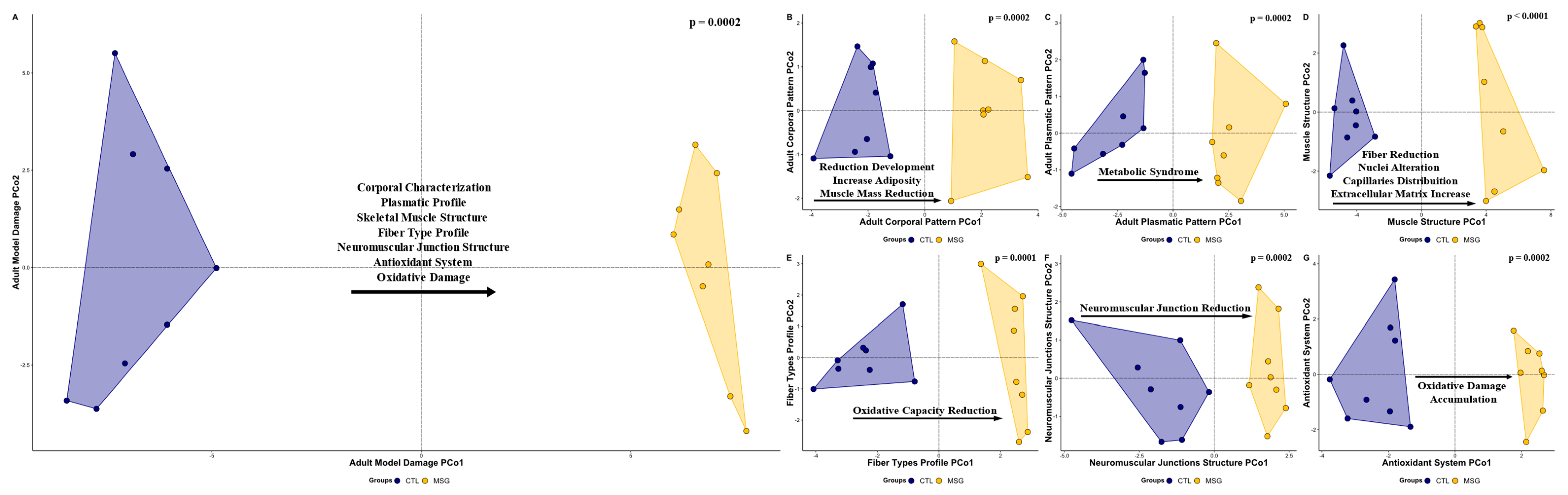
2.4. Obesity and Muscle Damage at 142 Days
2.4.1. Corporal Characterization
2.4.2. Plasmatic Profile
2.4.3. Skeletal Muscle Structure
2.4.4. Fiber Types Profile and Neuromuscular Junction Structure
2.5. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Animals and Experimental Design
4.3. Intraperitoneal Glucose Tolerance Test (ipGTT) and Insulin Dosage
4.4. Euthanasia and Material Collection
4.5. Skeletal Muscle Structure Analysis
4.6. Antioxidant System and Oxidative Damages Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernández Bautista, R.J.; Mahmoud, A.M.; Königsberg, M.; López Díaz Guerrero, N.E. Obesity: Pathophysiology, Monosodium Glutamate-Induced Model and Anti-Obesity Medicinal Plants. Biomed. Pharmacother. 2019, 111, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Borck, P.C.; de C Leite, N.; Valcanaia, A.C.; Rickli, S.; de L Alípio, J.C.; Machado, M.; Vellosa, J.C.; de F Mathias, P.C.; Boschero, A.C.; Grassiolli, S. Swimming Training Reduces Glucose-amplifying Pathway and Cholinergic Responses in Islets from Lean- and MSG-obese Rats. Clin. Exp. Pharmacol. Physiol. 2020, 47, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W. Brain Lesions, Obesity, and Other Disturbances in Mice Treated with Monosodium Glutamate. Science 1969, 164, 719–721. [Google Scholar] [CrossRef]
- Torii, K.; Takasaki, Y.; Iwata, S.; Wurtman, R.J. Changes in Blood Osmolarity, Electrolytes, and Metabolites among Adult Rats Treated with a Neurotoxic Dose of MSG1. Life Sci. 1981, 28, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Lemkey-Johnston, N.; Butler, V.; Reynolds, W.A. Brain Damage in Neonatal Mice Following Monosodium Glutamate Administration: Possible Involvement of Hypernatremia and Hyperosmolality. Exp. Neurol. 1975, 48, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.; Mostafa, E.M.; Toama, F.N. Histological Effects of Monosodium Glutamate on Brain of Infant Albino Swiss Mice Mus Musculus. J. Med. Chem. Sci. 2021, 4, 564–570. [Google Scholar] [CrossRef]
- de Souza, T.A.; de Souza, D.W.; Siqueira, B.S.; Rentz, T.; de Oliveria Emílio, H.R.; Grassiolli, S. Splenic Participation in Glycemic Homeostasis in Obese and Non-Obese Male Rats. Obes. Res. Clin. Pract. 2020, 14, 479–486. [Google Scholar] [CrossRef]
- Quines, C.B.; Rosa, S.G.; Velasquez, D.; Prado, V.C.; Neto, J.S.S.; Nogueira, C.W. (P-ClPhSe)2 Stabilizes Metabolic Function in a Rat Model of Neuroendocrine Obesity Induced by Monosodium Glutamate. Food Chem. Toxicol. 2018, 118, 168–180. [Google Scholar] [CrossRef]
- de Andrade, B.Z.; Zazula, M.F.; Bittencourt Guimarães, A.T.; Sagae, S.C.; Boaretto, M.L.; Felicio Poncio, A.C.; Hoff Nunes Maciel, J.I.; de Oliveira, C.M.T.; Costa, R.M.; Flor Bertolini, G.R.; et al. Whole-Body Vibration Promotes Lipid Mobilization in Hypothalamic Obesity Rat. Tissue Cell 2021, 68, 101456. [Google Scholar] [CrossRef]
- Hazzaa, S.M.; Abdelaziz, S.A.M.; Eldaim, M.A.A.; Abdel-Daim, M.M.; Elgarawany, G.E. Neuroprotective Potential of Allium Sativum against Monosodium Glutamate-Induced Excitotoxicity: Impact on Short-Term Memory, Gliosis, and Oxidative Stress. Nutrients 2020, 12, 1028. [Google Scholar] [CrossRef]
- Guareschi, Z.M.; Valcanaia, A.C.; Ceglarek, V.M.; Hotz, P.; Amaral, B.K.; De Souza, D.W.; De Souza, T.A.; Nardelli, T.; Ferreira, T.R.; Leite, N.C.; et al. The Effect of Chronic Oral Vitamin D Supplementation on Adiposity and Insulin Secretion in Hypothalamic Obese Rats. Br. J. Nutr. 2019, 121, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bautista, R.; Alarcón-Aguilar, F.; Escobar-Villanueva, M.D.C.; Almanza-Pérez, J.; Merino-Aguilar, H.; Fainstein, M.; López-Diazguerrero, N. Biochemical Alterations during the Obese-Aging Process in Female and Male Monosodium Glutamate (MSG)-Treated Mice. Int. J. Mol. Sci. 2014, 15, 11473–11494. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Araujo, T.R.; Freitas, I.N.; Vettorazzi, J.F.; Batista, T.M.; Santos-Silva, J.C.; Bonfleur, M.L.; Balbo, S.L.; Boschero, A.C.; Carneiro, E.M.; Ribeiro, R.A. Benefits of L-Alanine or l-Arginine Supplementation against Adiposity and Glucose Intolerance in Monosodium Glutamate-Induced Obesity. Eur. J. Nutr. 2017, 56, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Quines, C.B.; Jardim, N.S.; Araujo, P.C.O.; Cechella, J.L.; Prado, V.C.; Nogueira, C.W. Resistance Training Restores Metabolic Alterations Induced by Monosodium Glutamate in a Sex-Dependent Manner in Male and Female Rats. J. Cell. Biochem. 2019, 120, 13426–13440. [Google Scholar] [CrossRef] [PubMed]
- Hoppeler, H. Molecular Networks in Skeletal Muscle Plasticity. J. Exp. Biol. 2016, 219, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kido, K.; Sase, K.; Yokokawa, T.; Fujita, S. Enhanced Skeletal Muscle Insulin Sensitivity after Acute Resistance-Type Exercise Is Upregulated by Rapamycin-Sensitive MTOR Complex 1 Inhibition. Sci. Rep. 2020, 10, 8509. [Google Scholar] [CrossRef] [PubMed]
- Pomytkin, I.; Krasil’nikova, I.; Bakaeva, Z.; Surin, A.; Pinelis, V. Excitotoxic Glutamate Causes Neuronal Insulin Resistance by Inhibiting Insulin Receptor/Akt/MTOR Pathway. Mol. Brain 2019, 12, 112. [Google Scholar] [CrossRef]
- Hung IKKβ Suppression of TSC1 Function Links the MTOR Pathway with Insulin Resistance. Int. J. Mol. Med. 1998, 22, 633–638. [CrossRef]
- Shan, T.; Zhang, P.; Jiang, Q.; Xiong, Y.; Wang, Y.; Kuang, S. Adipocyte-Specific Deletion of MTOR Inhibits Adipose Tissue Development and Causes Insulin Resistance in Mice. Diabetologia 2016, 59, 1995–2004. [Google Scholar] [CrossRef]
- Zazula, M.F.; Bergmann Kirsch, C.; Theodoro, J.L.; de Toni Boaro, C.; Saraiva, D.F.; de Oliveira Gonçalves, S.; Zanardini de Andrade, B.; Peretti, A.L.; Naliwaiko, K.; Flor Bertolini, G.R.; et al. Whole-Body Vibration Promotes Beneficial Changes on the Anterior Tibial Muscle Histomorphometry of Hypothalamic Obese Rats. Muscles. Ligaments Tendons J. 2021, 11, 657. [Google Scholar] [CrossRef]
- de Paula, D.G.; Bohlen, T.M.; Zampieri, T.T.; Mansano, N.S.; Vieira, H.R.; Gusmao, D.O.; Wasinski, F.; Donato, J.; Frazao, R. Distinct Effects of Growth Hormone Deficiency and Disruption of Hypothalamic Kisspeptin System on Reproduction of Male Mice. Life Sci. 2021, 285, 119970. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I.; Hatano, H.; Minami, S.; Tonegawa, Y.; Akira, S.; Sugihara, H.; Ling, N.C. Effects of Neonatal Administration of Monosodium Glutamate on Plasma Growth Hormone (GH) Response to GH-Releasing Factor in Adult Male and Female Rats. Brain Res. 1986, 372, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Maiter, D.; Underwood, L.E.; Martin, J.B.; Koenig, J.I. Neonatal Treatment with Monosodium Glutamate: Effects of Prolonged Growth Hormone (GH)-Releasing Hormone Deficiency on Pulsatile GH Secretion and Growth in Female Rats. Endocrinology 1991, 128, 1100–1106. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, M.; Dodson, M.V.; Du, M. Developmental Programming of Fetal Skeletal Muscle and Adipose Tissue Development. J. Genomics 2013, 1, 29–38. [Google Scholar] [CrossRef]
- Tarry-adkins, J.L.; Fernandez-twinn, D.S.; Chen, J.H.; Hargreaves, I.P.; Neergheen, V.; Aiken, C.E.; Ozanne, S.E. Poor Maternal Nutrition and Accelerated Postnatal Growth Induces an Accelerated Aging Phenotype and Oxidative Stress in Skeletal Muscle of Male Rats. Dis. Model. Mech. 2016, 9, 1221–1229. [Google Scholar] [CrossRef]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.M.; Holland, O.J.; Perkins, A.V.; Yau, S.Y.; McAinch, A.J.; Hryciw, D.H. Role of Omega-6 and Omega-3 Fatty Acids in Fetal Programming. Clin. Exp. Pharmacol. Physiol. 2020, 47, 907–915. [Google Scholar] [CrossRef]
- Davies, J.S.; Gevers, E.F.; Stevenson, A.E.; Coschigano, K.T.; El-Kasti, M.M.; Bull, M.J.; Elford, C.; Evans, B.A.J.; Kopchick, J.J.; Wells, T. Adiposity Profile in the Dwarf Rat: An Unusually Lean Model of Profound Growth Hormone Deficiency. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1483–E1494. [Google Scholar] [CrossRef]
- Boaretto, M.L.; de Andrade, B.Z.; Maciel, J.I.H.N.; Moha, I.; Schneider, S.C.S.; Torrejais, M.M.; de Fátima Chasko Ribeiro, L.; Bertolini, G.R.F. Effects of Vibratory Platform Training on the Histomorphometric Parameters of the Soleus Muscle in Obese Wistar Rats. Sport Sci. Health 2020, 16, 501–510. [Google Scholar] [CrossRef]
- Maciel, J.I.H.N.; Zazula, M.F.; Rodrigues, D.F.S.; De Toni Boaro, C.; Boaretto, M.L.; de Andrade, B.Z.; Schneider, S.C.S.; Naliwaiko, K.; Torrejais, M.M.; Costa, R.M.; et al. Whole-Body Vibration Promotes Skeletal Muscle Restructuring and Reduced Obesogenic Effect of MSG in Wistar Rats. Appl. Biochem. Biotechnol. 2022, 194, 3594–3608. [Google Scholar] [CrossRef]
- Hirata, A.E.; Andrade, I.S.; Vaskevicius, P.; Dolnikoff, M.S. Monosodium Glutamate (MSG)-Obese Rats Develop Glucose Intolerance and Insulin Resistance to Peripheral Glucose Uptake. Brazilian J. Med. Biol. Res. 1997, 30, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Marcell, T.J.; Harman, S.M.; Urban, R.J.; Metz, D.D.; Rodgers, B.D.; Blackman, M.R. Comparison of GH, IGF-I, and Testosterone with MRNA of Receptors and Myostatin in Skeletal Muscle in Older Men. Am. J. Physiol. Metab. 2001, 281, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Grade, C.V.C.; Mantovani, C.S.; Alvares, L.E. Myostatin Gene Promoter: Structure, Conservation and Importance as a Target for Muscle Modulation. J. Anim. Sci. Biotechnol. 2019, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yan, X.; Tong, J.F.; Zhao, J.; Zhu, M.J. Maternal Obesity, Inflammation, and Fetal Skeletal Muscle Development. Biol. Reprod. 2010, 82, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant Repair, and Fibrosis Development in Skeletal Muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Lima, C.B.; de Sousa Ferreira Soares, G.; Vitor, S.M.; Andrade-da-Costa, B.L.d.S.; Castellano, B.; Guedes, R.C.A. Spreading Depression Features and Iba1 Immunoreactivity in the Cerebral Cortex of Developing Rats Submitted to Treadmill Exercise after Treatment with Monosodium Glutamate. Int. J. Dev. Neurosci. 2014, 33, 98–105. [Google Scholar] [CrossRef]
- Jung, U.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central Factors in the Altered Cytokine Network of Uremia—The Good, the Bad, and the Ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef]
- Sharma, B.; Dabur, R. Role of Pro-Inflammatory Cytokines in Regulation of Skeletal Muscle Metabolism: A Systematic Review. Curr. Med. Chem. 2018, 27, 2161–2188. [Google Scholar] [CrossRef]
- Bian, A.L.; Hu, H.Y.; Rong, Y.D.; Wang, J.; Wang, J.X.; Zhou, X.Z. A Study on Relationship between Elderly Sarcopenia and Inflammatory Factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef]
- Kim, H.J.; Higashimori, T.; Park, S.Y.; Choi, H.; Dong, J.; Kim, Y.J.; Noh, H.L.; Cho, Y.R.; Cline, G.; Kim, Y.B.; et al. Differential Effects of Interleukin-6 and -10 on Skeletal Muscle and Liver Insulin Action In Vivo. Diabetes 2004, 53, 1060–1067. [Google Scholar] [CrossRef]
- Von Dentz, K.E.; Silva, B.S.; Queiroz, E.A.I.F.; Bomfim, G.F.; Nascimento, A.F.; Sugizaki, M.M.; Luvizotto, R.A.M. Hibiscus Sabdariffa Ethanolic Extract Modulates Adipokine Levels, Decreases Visceral Fat and Improves Glycemic Profile in High-Fat/Sugar Diet-Induced Obese Rats. Nutr. Food Sci. 2020, 51, 222–233. [Google Scholar] [CrossRef]
- Yonamine, C.Y.; Pinheiro-Machado, E.I.; Michalani, M.L.; Alves-Wagner, A.B.; Esteves ID, J.V.; Freitas, H.S.; Machado, U.F. Molecules Resveratrol Improves Glycemic Control in Type 2 Diabetic Obese Mice by Regulating Glucose Transporter Expression in Skeletal Muscle and Liver. Molecules 2017, 22, 1180. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilar, F.J.; Almanza-Perez, J.; Blancas, G.; Angeles, S.; Garcia-Macedo, R.; Roman, R.; Cruz, M. Glycine Regulates the Production of Pro-Inflammatory Cytokines in Lean and Monosodium Glutamate-Obese Mice. Eur. J. Pharmacol. 2008, 599, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Cutler, A.A.; Jackson, J.B.; Corbett, A.H.; Pavlath, G.K. Non-Equivalence of Nuclear Import among Nuclei in Multinucleated Skeletal Muscle Cells. J. Cell Sci. 2018, 131, jcs207670. [Google Scholar] [CrossRef]
- Dungan, C.M.; Peck, B.D.; Walton, R.G.; Huang, Z.; Bamman, M.M.; Kern, P.A.; Peterson, C.A. In Vivo Analysis of ΓH2AX+ Cells in Skeletal Muscle from Aged and Obese Humans. FASEB J. 2020, 34, 7018–7035. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.; Schiaffino, S.; Reggiani, C. Mechanisms Modulating Skeletal Muscle Phenotype. Compr. Physiol. 2013, 3, 1645–1687. [Google Scholar] [CrossRef]
- Hoppeler, H.; Flück, M. Normal Mammalian Skeletal Muscle and Its Phenotypic Plasticity. J. Exp. Biol. 2002, 205, 2143–2152. [Google Scholar] [CrossRef]
- Hu, C.; Yang, Y.; Chen, M.; Hao, X.; Wang, S.; Yang, L.; Yin, Y.; Tan, C. A Maternal High-Fat/Low-Fiber Diet Impairs Glucose Tolerance and Induces the Formation of Glycolytic Muscle Fibers in Neonatal Offspring. Eur. J. Nutr. 2021, 60, 2709–2718. [Google Scholar] [CrossRef]
- Kong, X.F.; Zhou, X.L.; Feng, Z.M.; Li, F.N.; Ji, Y.J.; Tan, B.E.; Liu, Y.Y.; Geng, M.M.; Wu, G.Y.; Blachier, F.; et al. Dietary Supplementation with Monosodium L-Glutamate Modifies Lipid Composition and Gene Expression Related to Lipid Metabolism in Growing Pigs Fed a Normal- or High-Fat Diet. Livest. Sci. 2015, 180, 247–252. [Google Scholar] [CrossRef]
- Jana, B.A.; Chintamaneni, P.K.; Krishnamurthy, P.T.; Wadhwani, A.; Mohankumar, S.K. Cytosolic Lipid Excess-Induced Mitochondrial Dysfunction Is the Cause or Effect of High Fat Diet-Induced Skeletal Muscle Insulin Resistance: A Molecular Insight. Mol. Biol. Rep. 2019, 46, 957–963. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Papa, P.; Vargas, A.M.; Tavares Da Silva, J.L.; Nunes, M.T.; Machado, U.F. GLUT4 Protein Is Differently Modulated during Development of Obesity in Monosodium Glutamate-Treated Mice. Life Sci. 2002, 71, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A. Monosodium Glutamate (MSG)-Induced Animal Model of Type 2 Diabetes. Methods Mol. Biol. 2019, 1916, 49–65. [Google Scholar] [PubMed]
- Reijrink, M.; De Boer, S.A.; Antunes, I.F.; Spoor, D.S.; Heerspink, H.J.L.; Lodewijk, M.E.; Mastik, M.F.; Boellaard, R.; Greuter, M.J.W.; Benjamens, S.; et al. [18 F]FDG Uptake in Adipose Tissue Is Not Related to Inflammation in Type 2 Diabetes Mellitus. Mol. Imaging Biol. 2021, 23, 117–126. [Google Scholar] [CrossRef]
- Kazmi, Z.; Fatima, I.; Perveen, S.; Malik, S.S. Monosodium Glutamate: Review on Clinical Reports. Int. J. Food Prop. 2017, 20, 1807–1815. [Google Scholar] [CrossRef]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Tsoukalas, D.; Engin, A.B.; Aschner, M.; Margină, D. A Review of the Alleged Health Hazards of Monosodium Glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef]
- Dolnikoff, M.; Martín-Hidalgo, A.; Machado, U.F.; Lima, F.B.; Herrera, E. Decreased Lipolysis and Enhanced Glycerol and Glucose Utilization by Adipose Tissue Prior to Development of Obesity in Monosodium Glutamate (MSG) Treated-Rats. Int. J. Obes. 2001, 25, 426–433. [Google Scholar] [CrossRef]
- Svidnicki, P.V.; de Carvalho Leite, N.; Venturelli, A.C.; Camargo, R.L.; Vicari, M.R.; de Almeida, M.C.; Artoni, R.F.; Nogaroto, V.; Grassiolli, S. Swim Training Restores Glucagon-like Peptide-1 Insulinotropic Action in Pancreatic Islets from Monosodium Glutamate-Obese Rats. Acta Physiol. 2013, 209, 34–44. [Google Scholar] [CrossRef]
- Boaretto, M.; de Andrade, B.Z.; Maciel, J.I.H.N.; de Campos Oliveira, M.; de Oliveira, C.M.T.; Guimarães, A.T.B.; Torrejais, M.M.; Schneider, S.C.S.; Ribeiro, L.d.F.C.; Bertolini, G.R.F. Alterations in Neuromuscular Junctions and Oxidative Stress of the Soleus Muscle of Obese Wistar Rats Caused by Vibratory Platform Training. J. Musculoskelet. Neuronal Interact. 2020, 20, 570–578. [Google Scholar]
- de Campos Oliveira, M.; Laís Boaretto, M.; Barbosa, A.; Bittencourt Guimarães, A.T.; Flor Bertolini, G.R.; Torrejais, M.M.; Costa, R.M. Whole-Body Vibration, Morphological and Antioxidant Effects on the Diaphragm Muscle of Obese Rats. Muscles. Ligaments Tendons J. 2021, 11, 648. [Google Scholar] [CrossRef]
- Ceglarek, V.M.; Coelho, M.L.; Coelho, R.L.; Almeida, D.L.; de Souza Rodrigues, W.; Camargo, R.L.; Barella, L.F.; de Freitas Mathias, P.C.; Grassiolli, S. Chronic Leucine Supplementation Does Not Prevent the Obesity and Metabolic Abnormalities Induced by Monosodium Glutamate. Clin. Nutr. Exp. 2020, 29, 62–75. [Google Scholar] [CrossRef]
- Wen, C.; Li, F.; Zhang, L.; Duan, Y.; Guo, Q.; Wang, W.; He, S.; Li, J.; Yin, Y. Taurine Is Involved in Energy Metabolism in Muscles, Adipose Tissue, and the Liver. Mol. Nutr. Food Res. 2019, 63, e1800536. [Google Scholar] [CrossRef] [PubMed]
- Fouda, Y.B.; Ngo Lemba Tom, E.; Atsamo, A.D.; Bonabe, C.; Dimo, T. Effects of Stem Bark Aqueous Extract of Fagara Tessmannii Engl (Rutaceae) on Cardiovascular Risks Related to Monosodium Glutamate-Induced Obesity in Rat: In Vivo and in Vitro Assessments. J. Ethnopharmacol. 2020, 260, 112972. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The Arrive Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Crouch, R.K.; Gandy, S.E.; Kimsey, G.; Galbraith, R.A.; Galbraith, G.M.; Buse, M.G. The Inhibition of Islet Superoxide Dismutase by Diabetogenic Drugs. Diabetes 1981, 30, 235–241. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzimol. 1985, 105, 121–126. [Google Scholar]
- Jiang, Z.-Y.; Woollard, A.C.S.; Wolff, S.P. Lipid Hydroperoxides Measurement by Oxidation of Fe2+ in the Presence of Xylenol Orange. Comparison with the TBA Assay and an Iodometric Method. Lipids 1991, 26, 853–856. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
| Grouping Category | Variable | EDL | SOL | ||||
|---|---|---|---|---|---|---|---|
| CTL | MSG | p-Value | CTL | MSG | p-Value | ||
| Macroscopical Structure | Muscle Weight | 0.16 ± 0.01 | 0.12 ± 0.01 a | <0.0001 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.1382 |
| Muscle Length | 31.60 ± 1.18 | 27.55 ± 0.92 a | <0.0001 | 23.64 ± 1.80 | 20.38 ± 0.64 a | 0.0011 | |
| Skeletal Muscle Structure | Fiber Density | 574.82 ± 24.01 | 629.13 ± 32.43 a | 0.0022 | 318.60 ± 27.67 | 466.44 ± 15.27 a | <0.0001 |
| Cross-Sectional Area | 1743.2 ± 73.4 | 1595.3 ± 82.2 a | 0.0020 | 3165.9 ± 284.4 | 2146.2 ± 70.7 a | <0.0001 | |
| Larger Diameter | 58.86 ± 7.14 | 43.37 ± 2.22 a | 0.0003 | 78.51 ± 5.68 | 57.00 ± 5.59 a | <0.0001 | |
| Smaller Diameter | 42.37 ± 5.76 | 31.31 ± 2.60 a | 0.0006 | 46.77 ± 2.98 | 36.99 ± 3.83 a | <0.0001 | |
| Diameter Ratio | 1.39 ± 0.03 | 1.39 ± 0.05 | 0.9332 | 1.68 ± 0.12 | 1.54 ± 0.05 a | 0.0178 | |
| Capillaries/Fibers | 1.50 ± 0.14 | 3.03 ± 0.41 a | <0.0001 | 3.28 ± 0.24 | 2.05 ± 0.02 a | <0.0001 | |
| Nuclei/Fibers | 2.12 ± 0.37 | 1.88 ± 0.19 | 0.1891 | 2.72 ± 0.09 | 1.85 ± 0.08 a | <0.0001 | |
| Central Nuclei | 1.63 ± 0.41 | 4.87 ± 1.17 a | <0.0001 | 1.63 ± 0.24 | 2.96 ± 0.49 a | <0.0001 | |
| Myonuclear Domain | 1195.1 ± 208.3 | 909.6 ± 152.3 a | 0.0081 | 1160.6 ± 97.1 | 1019.1 ± 198.5 | 0.0998 | |
| Nuclei/Sarcoplasm Area | 0.010 ± 0.001 | 0.017 ± 0.001 a | <0.0001 | 0.013 ± 0.001 | 0.012 ± 0.000 | 0.1082 | |
| Total Connective Tissue | 3.58 ± 0.56 | 6.83 ± 1.10 a | <0.0001 | 4.31 ± 0.41 | 6.91 ± 1.38 a | 0.0008 | |
| Epimysium | 1.92 ± 0.30 | 3.35 ± 0.54 a | <0.0001 | 2.32 ± 0.21 | 3.39 ± 0.68 a | <0.0001 | |
| Perimysium | 0.64 ± 0.10 | 1.96 ± 0.31 a | <0.0001 | 0.77 ± 0.07 | 1.98 ± 0.39 a | <0.0001 | |
| Endomysium | 1.01 ± 0.15 | 1.51 ± 0.24 a | 0.0004 | 1.22 ± 0.11 | 1.52 ± 0.31 | 0.2689 | |
| Collagen Type I | 75.27 ± 3.49 | 66.32 ± 5.44 a | 0.0014 | 75.27 ± 6.36 | 78.52 ± 8.56 | 0.4048 | |
| Collagen Type III | 24.23 ± 3.49 | 33.67 ± 5.44 a | 0.0014 | 33.09 ± 5.86 | 21.47 ± 8.56 a | 0.0078 | |
| Fiber Types Profile | Proportion Fiber Type I | 9.31 ± 1.49 | 5.40 ± 1.18 a | <0.0001 | 86.05 ± 1.89 | 79.29 ± 1.35 a | <0.0001 |
| Proportion Fiber Type IIA | 41.68 ± 2.59 | 43.61 ± 3.08 | 0.1968 | 13.94 ± 1.89 | 20.71 ± 1.35 | <0.0001 | |
| Proportion Fiber Type IIB | 49.00 ± 2.28 | 50.98 ± 2.16 | 0.0973 | NA | NA | NA | |
| Cross-Sectional Area Type I | 833.87 ± 26.92 | 779.70 ± 22.64 a | 0.0007 | 2923.4 ± 843.8 | 1669.2 ± 273.5 a | <0.0001 | |
| Cross-Sectional Area Type IIA | 1158.1 ± 87.3 | 985.7 ± 131.6 a | 0.0093 | 4233.6 ± 685.6 | 3867.7 ± 463.9 a | <0.0001 | |
| Cross-Sectional Area Type IIB | 2514.3 ± 149.2 | 2357.4 ± 292.4 | 0.2048 | NA | NA | NA | |
| Neuromuscular Junctions Structure | NMJ Cross-Sectional Area | 151.38 ± 15.10 | 134.31 ± 7.75 a | 0.0167 | 161.64 ± 10.84 | 96.03 ± 6.55 a | <0.0001 |
| NMJ Larger Diameter | 25.71 ± 4.19 | 22.90 ± 1.53 | 0.1089 | 23.69 ± 0.94 | 16.17 ± 1.21 a | <0.0001 | |
| NMJ Smaller Diameter | 10.03 ± 0.88 | 10.10 ± 1.23 | 0.8939 | 7.11 ± 0.40 | 5.58 ± 0.42 a | <0.0001 | |
| NMJ Diameter Ratio | 2.56 ± 0.29 | 2.29 ± 0.22 | 0.0621 | 3.34 ± 0.30 | 2.90 ± 0.28 a | 0.0102 | |
| Antioxidant System | Superoxide Dismutase | 6.61 ± 0.69 | 6.95 ± 0.70 | 0.3439 | 5.65 ± 0.25 | 5.64 ± 0.43 | 0.9409 |
| Catalase | 403.37 ± 107.70 | 258.43 ± 84.11 a | 0.0101 | 204.45 ± 37.45 | 435.22 ± 28.13 a | <0.0001 | |
| Soluble Proteins | 1.74 ± 0.06 | 1.81 ± 0.03 a | 0.0268 | 1.83 ± 0.07 | 1.91 ± 0.03 a | 0.0268 | |
| Lipid Peroxides | 27.60 ± 3.85 | 21.51 ± 2.95 a | 0.0405 | 13.44 ± 1.50 | 17.58 ± 1.37 a | <0.0001 | |
| Non-Protein Thiols | 6.73 ± 0.44 | 5.42 ± 0.44 a | <0.0001 | 7.37 ± 0.70 | 5.89 ± 0.45 a | 0.0003 | |
| Total Cholinesterase’s | 0.99 ± 0.06 | 0.85 ± 0.04 a | 0.0001 | 1.10 ± 0.12 | 0.84 ± 0.03 a | 0.0003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zazula, M.F.; Saraiva, D.F.; Theodoro, J.L.; Maciel, M.; Sepulveda, E.V.d.S.; Zanardini de Andrade, B.; Boaretto, M.L.; Maciel, J.I.H.N.; Bronczek, G.A.; Soares, G.M.; et al. An Early and Sustained Inflammatory State Induces Muscle Changes and Establishes Obesogenic Characteristics in Wistar Rats Exposed to the MSG-Induced Obesity Model. Int. J. Mol. Sci. 2023, 24, 4730. https://doi.org/10.3390/ijms24054730
Zazula MF, Saraiva DF, Theodoro JL, Maciel M, Sepulveda EVdS, Zanardini de Andrade B, Boaretto ML, Maciel JIHN, Bronczek GA, Soares GM, et al. An Early and Sustained Inflammatory State Induces Muscle Changes and Establishes Obesogenic Characteristics in Wistar Rats Exposed to the MSG-Induced Obesity Model. International Journal of Molecular Sciences. 2023; 24(5):4730. https://doi.org/10.3390/ijms24054730
Chicago/Turabian StyleZazula, Matheus Felipe, Diego Francis Saraiva, João Lucas Theodoro, Mônica Maciel, Eliel Vieira dos Santos Sepulveda, Bárbara Zanardini de Andrade, Mariana Laís Boaretto, Jhyslayne Ignácia Hoff Nunes Maciel, Gabriela Alves Bronczek, Gabriela Moreira Soares, and et al. 2023. "An Early and Sustained Inflammatory State Induces Muscle Changes and Establishes Obesogenic Characteristics in Wistar Rats Exposed to the MSG-Induced Obesity Model" International Journal of Molecular Sciences 24, no. 5: 4730. https://doi.org/10.3390/ijms24054730
APA StyleZazula, M. F., Saraiva, D. F., Theodoro, J. L., Maciel, M., Sepulveda, E. V. d. S., Zanardini de Andrade, B., Boaretto, M. L., Maciel, J. I. H. N., Bronczek, G. A., Soares, G. M., Sagae Schneider, S. C., Bertolini, G. R. F., Torrejais, M. M., Ribeiro, L. F. C., Fernandes, L. C., & Naliwaiko, K. (2023). An Early and Sustained Inflammatory State Induces Muscle Changes and Establishes Obesogenic Characteristics in Wistar Rats Exposed to the MSG-Induced Obesity Model. International Journal of Molecular Sciences, 24(5), 4730. https://doi.org/10.3390/ijms24054730









