Ceftazidime-Avibactam (C/A) Resistant, Meropenem Sensitive KPC-Producing Klebsiella pneumoniae in ICU Setting: We Are What We Are Treated with?
Abstract
1. Introductions
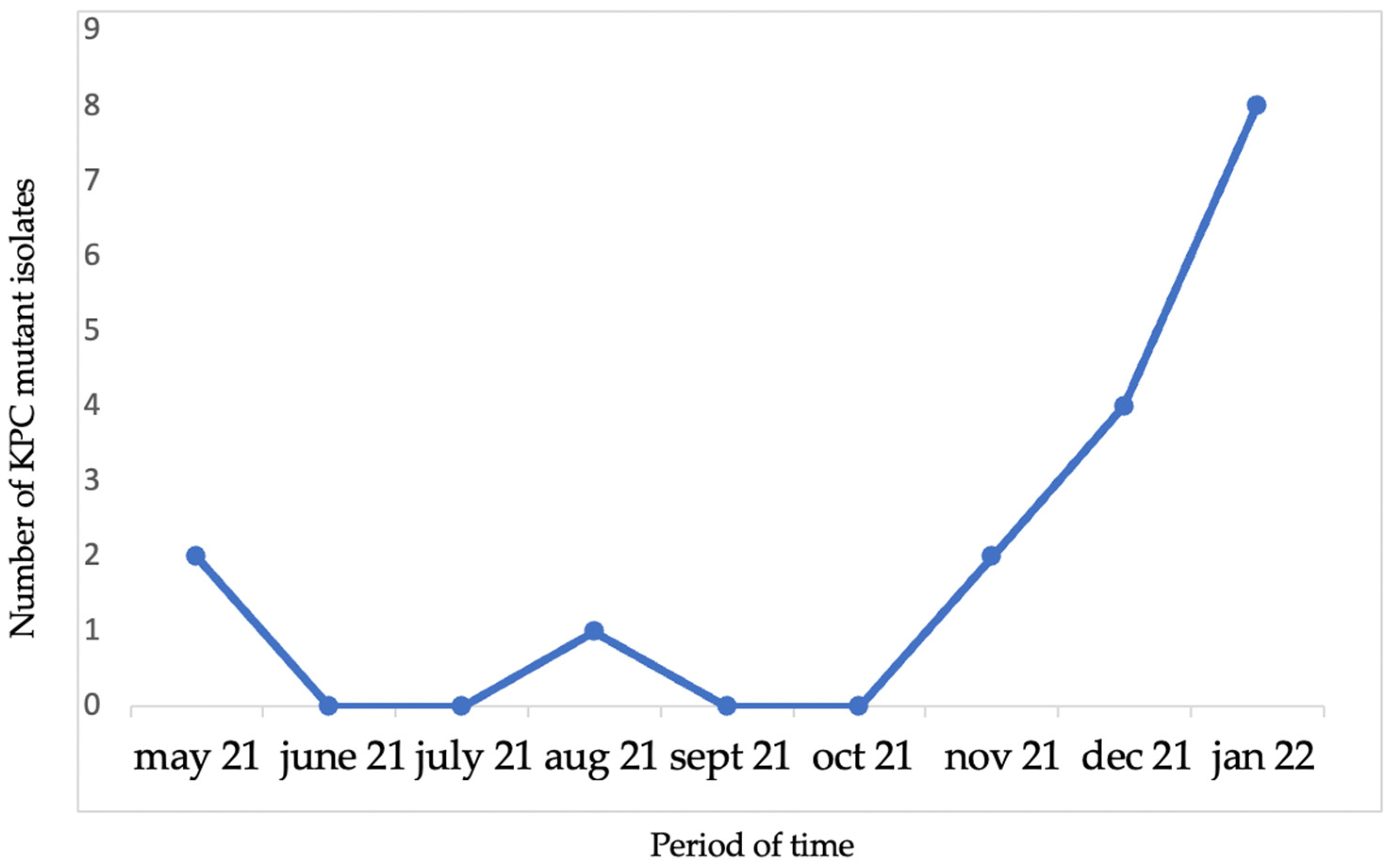
2. Results
3. Discussion
4. Materials and Methods
4.1. Statistical Analysis
4.2. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Logan, L.K.; Weinstein, R.A. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Brink, A.J. Epidemiology of carbapenem-resistant Gram-negative infections globally. Curr. Opin. Infect. Dis. 2019, 32, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Brolund, A.; Lagerqvist, N.; Byfors, S.; Struelens, M.J.; Monnet, D.L.; Albiger, B.; Kohlenberg, A.; European Antimicrobial Resistance Genes Surveillance Network (EURGen-Net) capacity survey group. Worsening epidemiological situation of carbapenemase-producing Enterobacteriaceae in Europe, assessment by national experts from 37 countries, July 2018. Eurosurveillance 2019, 24, 1900123. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022.
- Rossi, M.; Chatenoud, L.; Gona, F.; Sala, I.; Nattino, G.; D’Antonio, A.; Castelli, D.; Itri, T.; Morelli, P.; Bigoni, S.; et al. Characteristics and Clinical Implications of Carbapenemase-Producing Klebsiella pneumoniae Colonization and Infection, Italy. Emerg. Infect. Dis. 2021, 27, 1416–1426. [Google Scholar] [CrossRef]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasević, A.T.; Cantón, R.; Carmeli, Y.; Friedrich, A.W.; Giske, C.G.; et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Raffaelli, F.; Giannella, M.; Mantengoli, E.; Mularoni, A.; Venditti, M.; De Rosa, F.G.; Sarmati, L.; Bassetti, M.; Brindicci, G.; et al. Ceftazidime-Avibactam Use for Klebsiella pneumoniae Carbapenemase–Producing K. pneumoniae Infections: A Retrospective Observational Multicenter Study. Clin. Infect. Dis. 2021, 73, 1664–1676. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; De Benedetto, I.; Shbaklo, N.; Ranzani, F.; Pinna, S.M.; Castiglione, A.; Scabini, S.; Bianco, G.; Cavallo, R.; Mirabella, S.; et al. Ten Years of KPC-Kp Bloodstream Infections Experience: Impact of Early Appropriate Empirical Therapy on Mortality. Biomedicines 2022, 10, 3268. [Google Scholar] [CrossRef]
- Cano, A.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Gracia-Ahufinger, I.; Pérez-Nadales, E.; Causse, M.; Castón, J.J.; Guzman-Puche, J.; Torre-Giménez, J.; Kindelán, L.; et al. Risks of Infection and Mortality Among Patients Colonized With Klebsiella pneumoniae Carbapenemase–Producing K. pneumoniae: Validation of Scores and Proposal for Management. Clin. Infect. Dis. 2018, 66, 1204–1210. [Google Scholar] [CrossRef]
- Falcone, M.; Daikos, G.L.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients With Bloodstream Infections Caused by Metallo-β-lactamase–Producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef]
- Boattini, M.; Comini, S.; Bianco, G.; Iannaccone, M.; Casale, R.; Cavallo, R.; Costa, C. Activity of cefiderocol and synergy of novel β-lactam-β-lactamase inhibitor-based combinations against metallo-β-lactamase-producing gram-negative bacilli: Insights from a two-year study (2019–2020). J. Chemother. 2022, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.A.; Melano, R.; Cárdenas, P.A.; Trueba, G. Mobile genetic elements associated with carbapenemase genes in South American Enterobacterales. Braz. J. Infect. Dis. 2020, 24, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Kitchel, B.; Rasheed, J.K.; Patel, J.B.; Srinivasan, A.; Navon-Venezia, S.; Carmeli, Y.; Brolund, A.; Giske, C.G. Molecular Epidemiology of KPC-Producing Klebsiella pneumoniae Isolates in the United States: Clonal Expansion of Multilocus Sequence Type 258. Antimicrob. Agents Chemother. 2009, 53, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mathema, B.; Chavda, K.D.; DeLeo, F.R.; Bonomo, R.A.; Kreiswirth, B.N. Carbapenemase-producing Klebsiella pneumoniae: Molecular and genetic decoding. Trends Microbiol. 2014, 22, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Principe, L.; Lupia, T.; Andriani, L.; Campanile, F.; Carcione, D.; Corcione, S.; De Rosa, F.G.; Luzzati, R.; Stroffolini, G.; Steyde, M.; et al. Microbiological, Clinical, and PK/PD Features of the New Anti-Gram-Negative Antibiotics: β-Lactam/β-Lactamase Inhibitors in Combination and Cefiderocol—An All-Inclusive Guide for Clinicians. Pharmaceuticals 2022, 15, 463. [Google Scholar] [CrossRef]
- Di Bella, S.; Giacobbe, D.R.; Maraolo, A.E.; Viaggi, V.; Luzzati, R.; Bassetti, M.; Luzzaro, F.; Principe, L. Resistance to ceftazidime/avibactam in infections and colonisations by KPC-producing Enterobacterales: A systematic review of observational clinical studies. J. Glob. Antimicrob. Resist. 2021, 25, 268–281. [Google Scholar] [CrossRef]
- Gaibani, P.; Campoli, C.; Lewis, R.E.; Volpe, S.L.; Scaltriti, E.; Giannella, M.; Pongolini, S.; Berlingeri, A.; Cristini, F.; Bartoletti, M.; et al. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J. Antimicrob. Chemother. 2018, 73, 1525–1529. [Google Scholar] [CrossRef]
- Tiseo, G.; Falcone, M.; Leonildi, A.; Giordano, C.; Barnini, S.; Arcari, G.; Carattoli, A.; Menichetti, F. Meropenem-Vaborbactam as Salvage Therapy for Ceftazidime-Avibactam-, Cefiderocol-Resistant ST-512 Klebsiella pneumoniae–Producing KPC-31, a D179Y Variant of KPC-3. Open Forum Infect. Dis. 2021, 8, ofab141. [Google Scholar] [CrossRef]
- Athans, V.; Neuner, E.A.; Hassouna, H.; Richter, S.S.; Keller, G.; Castanheira, M.; Brizendine, K.D.; Mathers, A.J. Meropenem-Vaborbactam as Salvage Therapy for Ceftazidime-Avibactam-Resistant Klebsiella pneumoniae Bacteremia and Abscess in a Liver Transplant Recipient. Antimicrob. Agents Chemother. 2018, 63, e01551-18. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Yao, X.; Xian, H.; Liu, Y.; Li, H.; Chen, H.; Wang, X.; Wang, R.; Zhao, C.; et al. Risk factors and clinical outcomes for carbapenem-resistant Enterobacteriaceae nosocomial infections. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1679–1689. [Google Scholar] [CrossRef]
- Nicolas-Chanoine, M.-H.; Vigan, M.; Laouénan, C.; Robert, J.; E-carb Study Group. Risk factors for carbapenem-resistant Enterobacteriaceae infections: A French case-control-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Adembri, C.; Novelli, A.; Nobili, S. Some Suggestions from PK/PD Principles to Contain Resistance in the Clinical Setting—Focus on ICU Patients and Gram-Negative Strains. Antibiotics 2020, 9, 676. [Google Scholar] [CrossRef] [PubMed]
- Hites, M. Minireview on Novel Anti-infectious Treatment Options and Optimized Drug Regimens for Sepsis. Front. Med. 2021, 8, 640740. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Gatti, M.; Rinaldi, M.; Pesce, C.C.; Lazzarotto, T.; Giannella, M.; Lombardo, D.; Amadesi, S.; Viale, P.; Pea, F.; et al. Suboptimal drug exposure leads to selection of different subpopulations of ceftazidime-avibactam-resistant Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae in a critically ill patient. Int. J. Infect. Dis. 2021, 113, 213–217. [Google Scholar] [CrossRef]
- Rakovitsky, N.; Frenk, S.; Kon, H.; Schwartz, D.; Temkin, E.; Solter, E.; Paikin, S.; Cohen, R.; Schwaber, M.J.; Carmeli, Y.; et al. Fourier Transform Infrared Spectroscopy Is a New Option for Outbreak Investigation: A Retrospective Analysis of an Extended-Spectrum-Beta-Lactamase-Producing Klebsiella pneumoniae Outbreak in a Neonatal Intensive Care Unit. J. Clin. Microbiol. 2020, 58, e00098-20. [Google Scholar] [CrossRef]
- Rapid Risk Assessment: Emergence of Resistance to Ceftazidime-Avibactam in Carbapenem-Resistant Enterobacteriaceae. European Centre for Disease Prevention and Control. 13 June 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-emergence-resistance-ceftazidime-avibactam-carbapenem (accessed on 31 December 2022).
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. In Vitro Selection of Meropenem Resistance among Ceftazidime-Avibactam-Resistant, Meropenem-Susceptible Klebsiella pneumoniae Isolates with Variant KPC-3 Carbapenemases. Antimicrob. Agents Chemother. 2017, 61, e00079-17. [Google Scholar] [CrossRef]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne bla KPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar] [CrossRef]
- Corcione, S.; Lupia, T.; Maraolo, A.E.; Pinna, S.M.; Gentile, I.; De Rosa, F.G. Carbapenem-sparing strategy: Carbapenemase, treatment, and stewardship. Curr. Opin. Infect. Dis. 2019, 32, 663–673. [Google Scholar] [CrossRef]
- Winkler, M.L.; Papp-Wallace, K.M.; Bonomo, R.A. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J. Antimicrob. Chemother. 2015, 70, 2279–2286. [Google Scholar] [CrossRef]
- Zhang, P.; Shi, Q.; Hu, H.; Hong, B.; Wu, X.; Du, X.; Akova, M.; Yu, Y. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin. Microbiol. Infect. 2020, 26, 124.e1–124.e4. [Google Scholar] [CrossRef]
- Woodford, N.; Tierno, P.M., Jr.; Young, K.; Tysall, L.; Palepou, M.-F.I.; Ward, E.; Painter, R.E.; Suber, D.F.; Shungu, D.; Silver, L.L.; et al. Outbreak of Klebsiella pneumoniae Producing a New Carbapenem-Hydrolyzing Class A β-Lactamase, KPC-3, in a New York Medical Center. Antimicrob. Agents Chemother. 2004, 48, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Kline, E.G.; Jones, C.E.; Morder, K.T.; Mettus, R.T.; Doi, Y.; Nguyen, M.H.; Clancy, C.J.; Shields, R.K. Effects of KPC Variant and Porin Genotype on the In Vitro Activity of Meropenem-Vaborbactam against Carbapenem-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e02048-18. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Doyle, T.B.; Deshpande, L.M.; Mendes, R.E.; Sader, H.S. Activity of ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam against carbapenemase-negative carbapenem-resistant Enterobacterales isolates from US hospitals. Int. J. Antimicrob. Agents 2021, 58, 106439. [Google Scholar] [CrossRef]
- Antonelli, A.; Giani, T.; Di Pilato, V.; Riccobono, E.; Perriello, G.; Mencacci, A.; Rossolini, G.M. KPC-31 expressed in a ceftazidime/avibactam-resistant Klebsiella pneumoniae is associated with relevant detection issues. J. Antimicrob. Chemother. 2019, 74, 2464–2466. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Boattini, M.; Iannaccone, M.; Bondi, A.; Ghibaudo, D.; Zanotto, E.; Peradotto, M.; Cavallo, R.; Costa, C. Carbapenemase detection testing in the era of ceftazidime/avibactam-resistant KPC-producing Enterobacterales: A 2-year experience. J. Glob. Antimicrob. Resist. 2021, 24, 411–414. [Google Scholar] [CrossRef]
- Mueller, L.; Masseron, A.; Prod’Hom, G.; Galperine, T.; Greub, G.; Poirel, L.; Nordmann, P. Phenotypic, Biochemical, and Genetic Analysis of KPC-41, a KPC-3 Variant Conferring Resistance to Ceftazidime-Avibactam and Exhibiting Reduced Carbapenemase Activity. Antimicrob. Agents Chemother. 2019, 63, e01111-19. [Google Scholar] [CrossRef]
- Gaibani, P.; Re, M.C.; Campoli, C.; Viale, P.; Ambretti, S. Bloodstream infection caused by KPC-producing Klebsiella pneumoniae resistant to ceftazidime/avibactam: Epidemiology and genomic characterization. Clin. Microbiol. Infect. 2020, 26, 516.e1–516.e4. [Google Scholar] [CrossRef]
- Iannaccone, M.; Boattini, M.; Bianco, G.; Corcione, S.; Cavallo, R.; Costa, C. Ceftazidime-avibactam susceptible to resistant KPC-producing Enterobacterales bloodstream infections: An observational study. J. Chemother. 2020, 32, 160–162. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Iannaccone, M.; Cavallo, R.; Costa, C. Bloodstream infection by two subpopulations of Klebsiella pneumoniae ST1685 carrying KPC-33 or KPC-14 following ceftazidime/avibactam treatment: Considerations regarding acquired heteroresistance and choice of carbapenemase detection assay. J. Antimicrob. Chemother. 2020, 75, 3075–3076. [Google Scholar] [CrossRef]
- Bianco, G.; Boattini, M.; Comini, S.; Leone, A.; Bondi, A.; Zaccaria, T.; Cavallo, R.; Costa, C. Implementation of Chromatic Super CAZ/AVI® medium for active surveillance of ceftazidime-avibactam resistance: Preventing the loop from becoming a spiral. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1165–1171. [Google Scholar] [CrossRef]
- Shbaklo, N.; Corcione, S.; Vicentini, C.; Giordano, S.; Fiorentino, D.; Bianco, G.; Cattel, F.; Cavallo, R.; Zotti, C.M.; De Rosa, F.G. An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 695. [Google Scholar] [CrossRef] [PubMed]
| Antibiotic | Sensitivity | MIC (µg/L) | MIC Breakpoint | |
|---|---|---|---|---|
| S≤ | R> | |||
| Ampicillin | R | >8 | 8 | 8 |
| Amoxicillin/clavulanic acid | >32 | |||
| Piperacillin | R | >16 | 8 | 8 |
| Pip/Tazobactam | R | >16 | 8 | 8 |
| Ticarcillin | R | >16 | 8 | 16 |
| Cefazolin | >16 | 0.001 | 4 | |
| Cefuroxime OV | R | >8 | 0.001 | 8 |
| Ceftazidime | R | >32 | 1 | 4 |
| Cefotaxime | R | 16 | 1 | 2 |
| Cefepime | R | >8 | 1 | 4 |
| Cefixime | R | >1 | 1 | 1 |
| Ertapenem | R | >1 | 0.5 | 0.5 |
| Imipenem | S | ≤1 | 2 | 4 |
| Meropenem | S | 2 | 2 | 8 |
| Aztreonam | I | 2 | 1 | 4 |
| Ciprofloxacin | R | >1 | 0.25 | 0.5 |
| Levofloxacin | R | >1 | 0.5 | 1 |
| Amikacin | R | >16 | 8 | 8 |
| Gentamicin | S | ≤2 | 2 | 2 |
| Tobramicin | R | >4 | 2 | 2 |
| Colistin | R | >4 | 2 | 2 |
| Fosfomicin IV | R | >64 | 32 | 32 |
| Ceftazidime/avibactam | R | >8 | 8 | 8 |
| Ceftolozane/tazobactam | R | >4 | 2 | 2 |
| Trimetoprim-sulfametoxazole | R | >4/76 | 2 | 4 |
| Carbapenemase | + | |||
| Carbapenemase KPC | + | |||
| Sex/Age | Principle Diagnosis | Comorbidities | COVID-19 | Time from ICU Admission | KPC Mutant Specimen | KPC Sensitive Isolation | Previous Infections | A. baumannii Intestinal Colonization | Previous Broad-Spectrum ATB | Previous Antifungal Therapy | CZA Treatment before Isolation | In-Hospital Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F/72 | ARDS COVID-19 | AH, NIDDM, Obesity, malignancy | Yes | 3 | RS | Yes | no | no | yes | no | no | no |
| M/52 | HAP in COVID-19 | Yes | 64 | Blood, UC | Yes | VAP E. coli, MRSA, A. baumannii, KPC + BSI MRSE, KPC, E. faecium. | yes | yes | yes | yes | yes | |
| M/56 | Difficult weaning | Smoker, COPD | No | 51 | BAL | Yes | Serratia marcescens + Pseudomonas in BAL, CMV, BSI MRSE. | yes | yes | yes | yes | Yes |
| M/35 | Pulmonary embolism in trauma (vv-ECMO) | Obesity | No | 72 | BAL | Yes | BSI A. baumannii, SSTI VRE | yes | yes | no | yes | no |
| F/61 | Difficult weaning in H. influenzae SCAP | No | 14 | RS | Yes | BSI H. influenzae, MRSE, S. marcescens, P. aeruginosa. | no | yes | yes | no | yes | |
| M/49 | ARDS COVID-19 | Obesity | Yes | 15 | RS | Yes | VAP H. influenzae | no | yes | no | no | no |
| F/72 | ARDS COVID-19 | AH, autoimmune disease | Yes | 17 | RS | Yes | VAP H. influenzae, CAPA | no | yes | Yes | no | yes |
| M/46 | ARDS COVID-19 | Previous smoker, haematological disease | Yes | 10 | RS | No | VAP A. baumannii, KPC, MSSA | yes | no | no | no | yes |
| M/71 | Difficult weaning | AH, COPD, smoker | No | 87 | RS | Yes | VAP A. baumannii, KPC, K. oxytoca, P. aeruginosa; C. difficile, BSI MRSE + Candida | yes | yes | yes | no | yes |
| F/35 | ARDS COVID-19, pulomonary embolism in ECMO VV | AH, NIDDM, obesity | Yes | 8 | RS | Yes | VAP MRSE + K. aerogenes | yes | yes | no | no | no |
| F/34 | ARDS COVID-19 | obesity | Yes | 5 | RS | Yes | VAP E. coli + S. marcescens, CAPA | no | yes | yes | no | no |
| F/55 | ARDS COVID-19 | AH, haematological disease | Yes | 13 | RS, blood | Yes | UTI E. faecalis + E. coli, BSI S. capitis, UTI E. coli | no | yes | no | no | no |
| F/69 | ARDS COVID-19 | AH | Yes | 13 | RS | No | VAP P. aeruginosa, CAPA | no | yes | yes | yes | yes |
| F/58 | ARDS COVID-19 | AH, obesity, NIDDM, respiratory and autoimmune disease | Yes | 12 | RS | Yes | VAP MRSA + Proteus | no | yes | yes | no | no |
| M/57 | ARDS COVID-19 | autoimmune disease | Yes | 15 | RS | Yes | VAP P. aeruginosa, BSI C. albicans, C. auris colonization. | no | yes | yes | no | yes |
| M/69 | ARDS COVID-19 | autoimmune disease, respiratory disease, CKD | Yes | 5 | RS | No | VAP P. aeruginosa | no | yes | no | no | yes |
| M/71 | ARDS COVID-19 | Previous smoker, NIDDM, CRF, Obesity, Malignancy, CKD | Yes | 6 | RS | Yes | no | yes | yes | no | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corcione, S.; De Benedetto, I.; Shbaklo, N.; Torsello, G.; Lupia, T.; Bianco, G.; Cavallo, R.; Brazzi, L.; Montrucchio, G.; De Rosa, F.G. Ceftazidime-Avibactam (C/A) Resistant, Meropenem Sensitive KPC-Producing Klebsiella pneumoniae in ICU Setting: We Are What We Are Treated with? Int. J. Mol. Sci. 2023, 24, 4767. https://doi.org/10.3390/ijms24054767
Corcione S, De Benedetto I, Shbaklo N, Torsello G, Lupia T, Bianco G, Cavallo R, Brazzi L, Montrucchio G, De Rosa FG. Ceftazidime-Avibactam (C/A) Resistant, Meropenem Sensitive KPC-Producing Klebsiella pneumoniae in ICU Setting: We Are What We Are Treated with? International Journal of Molecular Sciences. 2023; 24(5):4767. https://doi.org/10.3390/ijms24054767
Chicago/Turabian StyleCorcione, Silvia, Ilaria De Benedetto, Nour Shbaklo, Giulia Torsello, Tommaso Lupia, Gabriele Bianco, Rossana Cavallo, Luca Brazzi, Giorgia Montrucchio, and Francesco Giuseppe De Rosa. 2023. "Ceftazidime-Avibactam (C/A) Resistant, Meropenem Sensitive KPC-Producing Klebsiella pneumoniae in ICU Setting: We Are What We Are Treated with?" International Journal of Molecular Sciences 24, no. 5: 4767. https://doi.org/10.3390/ijms24054767
APA StyleCorcione, S., De Benedetto, I., Shbaklo, N., Torsello, G., Lupia, T., Bianco, G., Cavallo, R., Brazzi, L., Montrucchio, G., & De Rosa, F. G. (2023). Ceftazidime-Avibactam (C/A) Resistant, Meropenem Sensitive KPC-Producing Klebsiella pneumoniae in ICU Setting: We Are What We Are Treated with? International Journal of Molecular Sciences, 24(5), 4767. https://doi.org/10.3390/ijms24054767









