Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Phosphate Buffer Release Test
2.2. Cytotoxicity Results
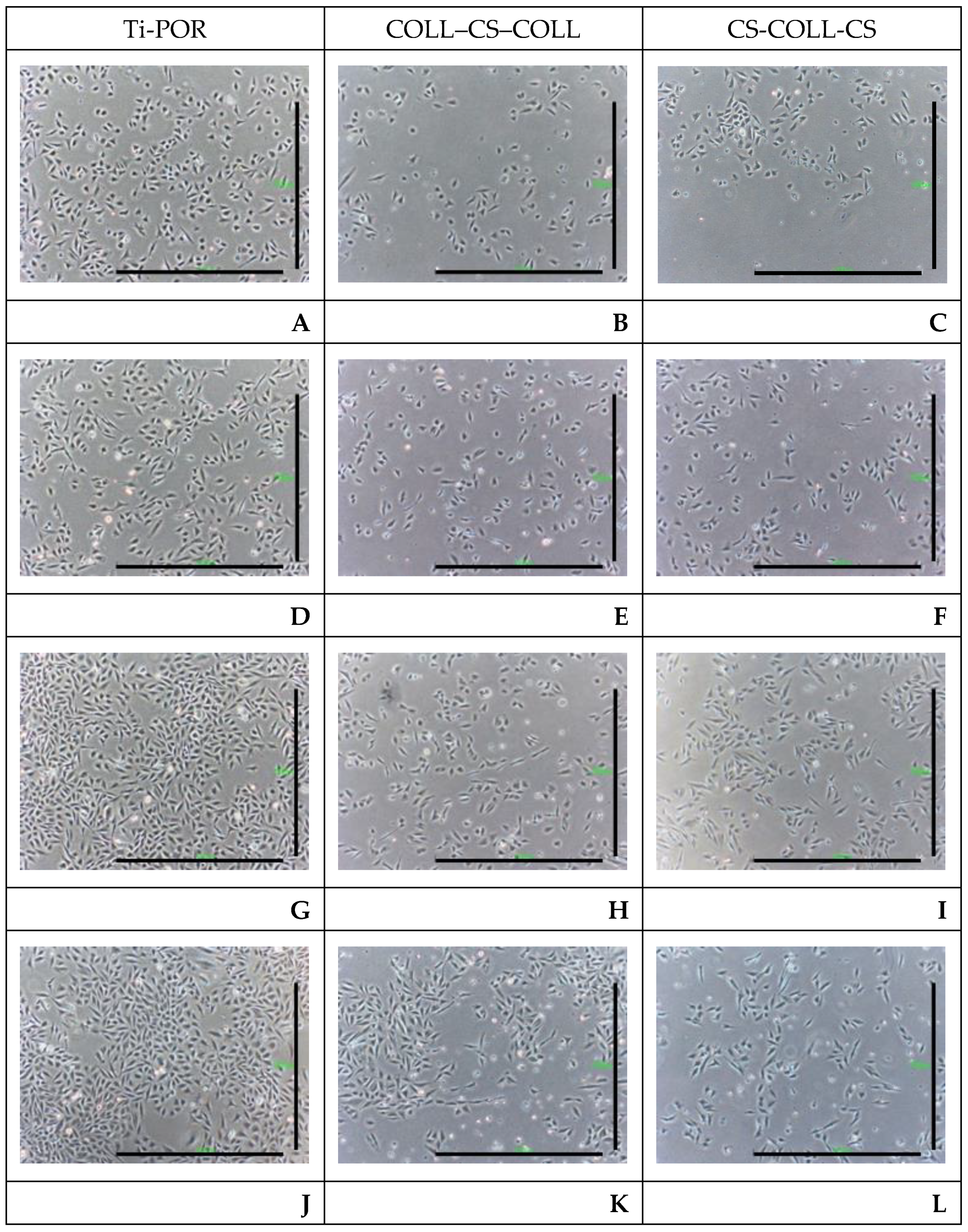
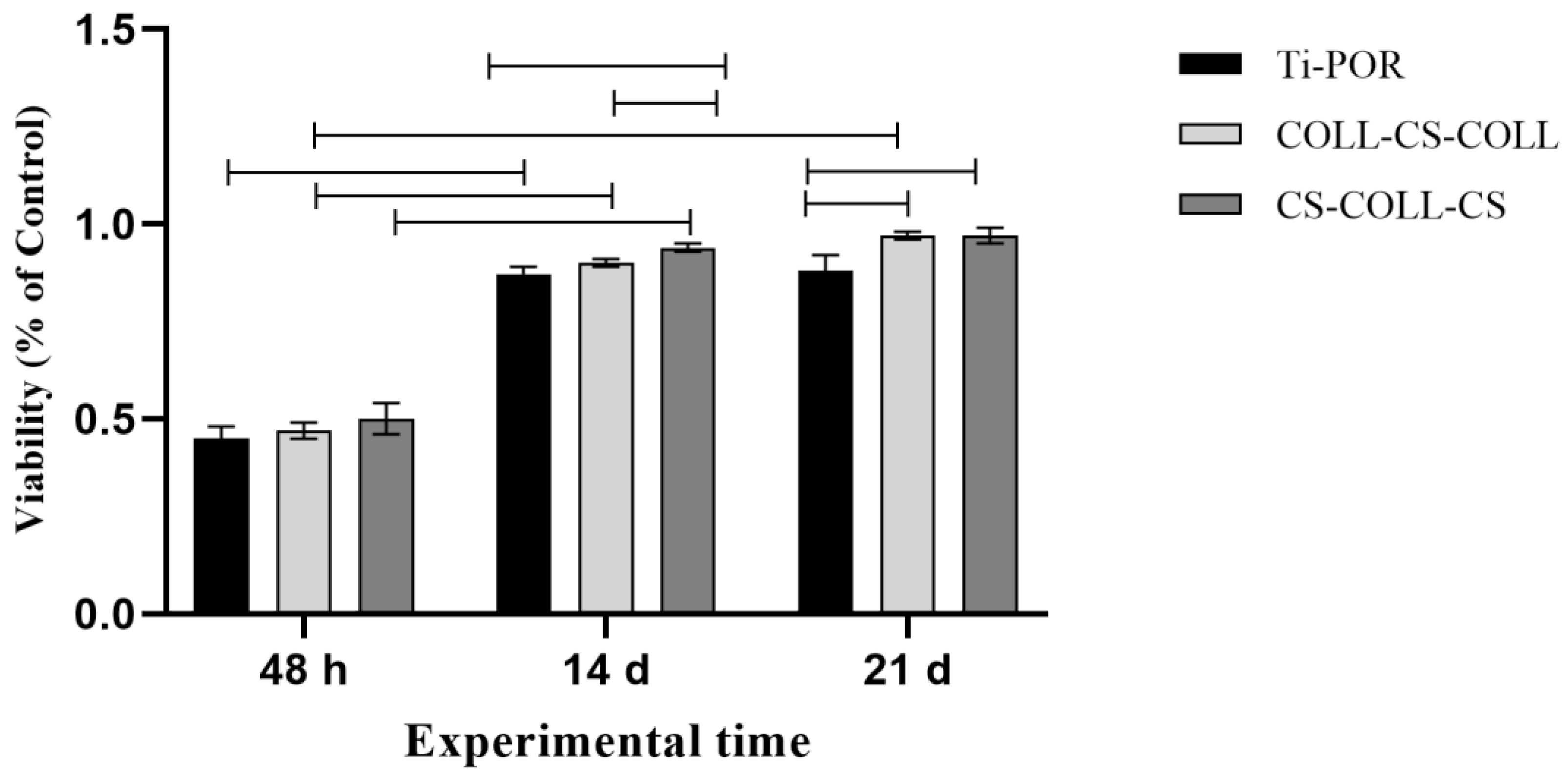
2.3. hBMSC Viability and Gene Expression Results
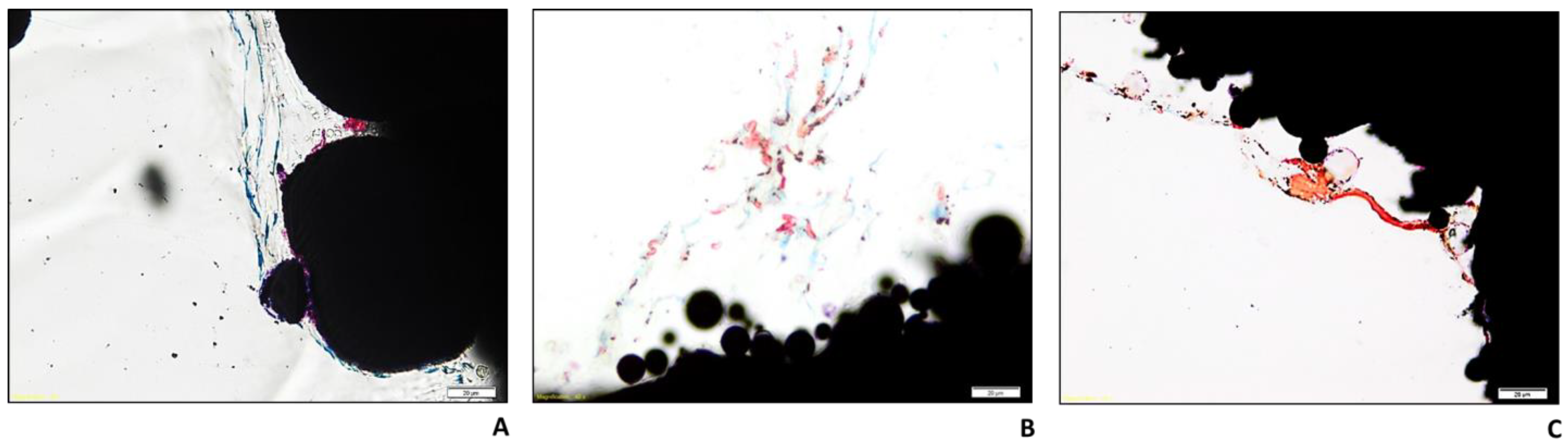
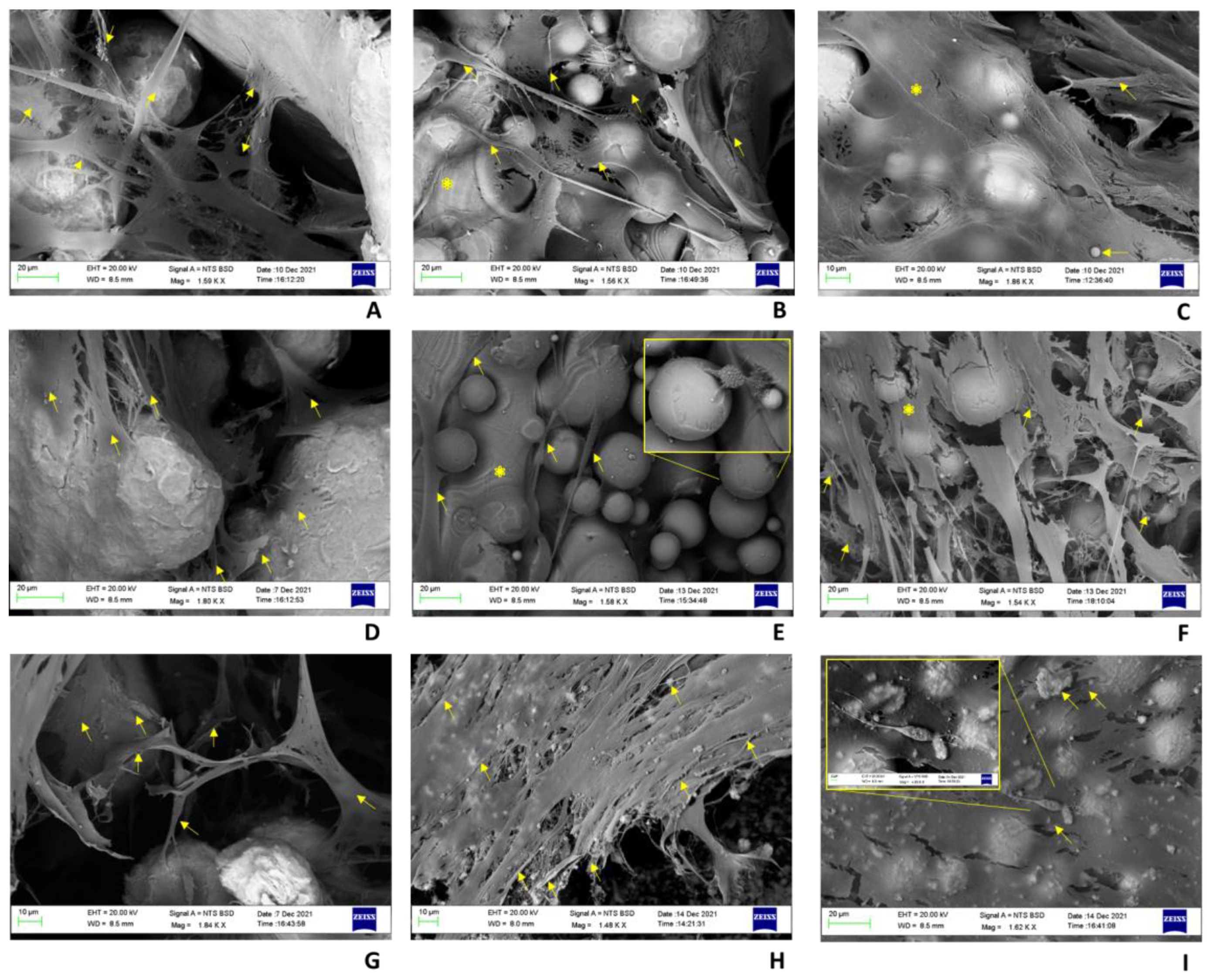
2.4. Histology and BS-SEM Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Titanium Specimens (Ti-POR)
4.2. Multilayer Coating of Ti-POR (COLL–CS–COLL and CS-COLL-CS)
- The spraying of fluidized COLL or CS on the surface of the Ti-POR implants;
- Rapid freezing at −40 °C;
- Freeze-drying.
4.3. Phosphate Buffer Release Test
4.4. In Vitro Cytotoxicity Evaluation
4.5. hBMSC Viability
4.6. hBMSC Gene Expression
4.7. Histology
4.8. BS-SEM
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Dindelegan, G.C.; Caziuc, A.; Brie, I.; Soritau, O.; Dindelegan, M.G.; Bintintan, V.; Pascalau, V.; Mihu, C.; Popa, C. Multilayered Porous Titanium-Based 3rd Generation Biomaterial Designed for Endosseous Implants. Materials 2021, 14, 1727. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Hung, C.H.; Kuo, C.N.; Chen, C.Y.; Peng, Y.N.; Shie, M.Y. Improved Bioactivity of 3D Printed Porous Titanium Alloy Scaffold with Chitosan/Magnesium-Calcium Silicate Composite for Orthopaedic Applications. Materials 2019, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Mao, M.; Li, J.; Wei, T.; Sun, H. Chitosan/hydroxyapatite composite coatings on porous Ti6Al4V titanium implants: In vitro and in vivo studies. J. Periodontal Implant. Sci. 2020, 50, 392–405. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Medina, F.; Lopez, H.; Martinez, E.; Machado, B.I.; Hernandez, D.H.; Martinez, L.; Lopez, M.I.; Wicker, R.B.; et al. Next-generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1999–2032. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Ueno, T.; Takeuchi, M.; Hori, N.; Iwasa, F.; Minamikawa, H.; Igarashi, Y.; Anpo, M.; Ogawa, T. Gamma ray treatment enhances bioactivity and osseointegration capability of titanium. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2279–2287. [Google Scholar] [CrossRef]
- Chen, W.C.; Chen, Y.S.; Chang, K.C.; Chen, C.H.; Lin, D.J. An in vitro assessment and comparative effectiveness of silanized-glutaraldehyde functionalized titanium surfaces with phosphatidylcholine and type I collagen grafts. Dent. Mater. 2020, 36, 320–328. [Google Scholar] [CrossRef]
- Liu, C.F.; Chang, K.C.; Sun, Y.S.; Nguyen, D.T.; Huang, H.H. Combining Sandblasting, Alkaline Etching, and Collagen Immobilization to Promote Cell Growth on Biomedical Titanium Implants. Polymers 2021, 13, 2550. [Google Scholar] [CrossRef]
- Brogini, S.; Sartori, M.; Giavaresi, G.; Cremascoli, P.; Alemani, F.; Bellini, D.; Martini, L.; Maglio, M.; Pagani, S.; Fini, M. Osseointegration of additive manufacturing Ti-6Al-4V and Co-Cr-Mo alloys, with and without surface functionalization with hydroxyapatite and type I collagen. J. Mech. Behav. Biomed. Mater. 2021, 115, 104262. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.T.; Kim, S.Y.; Jung, S.I.; Kang, S.S.; Kim, S.E.; Hwang, S.H.; Jeong, C.M.; Huh, J.B. Effects of Gamma Radiation-Induced Crosslinking of Collagen Type I Coated Dental Titanium Implants on Osseointegration and Bone Regeneration. Materials 2021, 14, 3268. [Google Scholar] [CrossRef] [PubMed]
- Türk, S.; Altınsoy, I.; Efe, G.Ç.; Ipek, M.; Özacar, M.; Bindal, C. 3D porous collagen/functionalized multiwalled carbon nanotube/chitosan/hydroxyapatite composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Nie, B.; Zhang, S.; Long, T.; Yue, B. Immobilization of type I collagen/hyaluronic acid multilayer coating on enoxacin loaded titania nanotubes for improved osteogenesis and osseointegration in ovariectomized rats. Colloids Surf. B Biointerfaces 2019, 175, 409–420. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, L.; Huang, X.; Wei, S.; Zhai, M. Structural study and preliminary biological evaluation on the collagen hydrogel crosslinked by γirradiation. J. Biomed. Mater. Res. A 2012, 100, 2960–2969. [Google Scholar] [CrossRef]
- Bae, E.B.; Yoo, J.H.; Jeong, S.I.; Kim, M.S.; Lim, Y.M.; Ahn, J.J.; Lee, J.J.; Lee, S.H.; Kim, H.J.; Huh, J.B. Effect of Titanium Implants Coated with Radiation-Crosslinked Collagen on Stability and Osseointegration in Rat Tibia. Materials 2018, 11, 2520. [Google Scholar] [CrossRef]
- Sun, Y.K.; Cha, J.K.; Thoma, D.S.; Yoon, S.R.; Lee, J.S.; Choi, S.H.; Jung, U.W. Bone Regeneration of Peri-Implant Defects Using a Collagen Membrane as a Carrier for Recombinant Human Bone Morphogenetic Protein-2. BioMed Res. Int. 2018, 2018, 5437361. [Google Scholar] [CrossRef]
- Benea, L.; Celis, J.P. Reactivity of porous titanium oxide film and chitosan layer electrochemically formed on Ti-6Al-4V alloy in biological solution. Surf. Coat. Technol. 2018, 354, 145–152. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Chesnutt, B.M.; Yuan, Y.; Yang, Y.; Appleford, M.; Oh, S.; McLaughlin, R.; Elder, S.H.; Ong, J.L. The Integration of Chitosan-Coated Titanium in Bone: An In Vivo Study in Rabbits. Implant Dent. 2007, 16, 66–79. [Google Scholar] [CrossRef]
- Farghali, R.A.; Fekry, A.M.; Ahmed, R.A.; Elhakim, H.K. Corrosion resistance of Ti modified by chitosan-gold nanoparticles for orthopedic implantation. Int. J. Biol. Macromol. 2015, 79, 787–799. [Google Scholar] [CrossRef]
- Thulasi, G.; Pragathiswaran, C.; Anusuya, N. Experimental investigation and analysis of corrosion inhibition of titanium modified by chitosan silver nanomaterials and its applications. Mater. Today Proc. 2021, 37, 2780–2785. [Google Scholar] [CrossRef]
- Jabłonski, P.; Hebda, M.; Pytlak, P.; Kyzioł, A.; Krawiec, H.; Grzesik, Z.; Kyzioł, K. Impact of chitosan/noble metals-based coatings on the plasmochemically activated surface of NiTi alloy. Mater. Chem. Phys. 2020, 248, 122931. [Google Scholar] [CrossRef]
- Kleszcz, K.; Hebda, M.; Kyzioł, A.; Krawiec, H.; Kyzioł, K. Towards prevention of biofilm formation: Ti6Al7Nb modified with nanocomposite layers of chitosan and Ag/Au nanoparticles. Appl. Surf. Sci. 2021, 557, 149795. [Google Scholar] [CrossRef]
- Tozar, A.; Karahan, İ.H. A comparative study on the effect of collagen and h-BN reinforcement of hydroxyapatite/chitosan biocomposite coatings electrophoretically deposited on Ti-6Al-4V biomedical implants. Surf. Coat. Technol. 2018, 340, 167–176. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, H.; Wei, Q.; Tang, X.; Zhuang, W. Biocompatible chitosan-collagen-hydroxyapatite nanofibers coated with platelet-rich plasma for regenerative engineering of the rotator cuff of the shoulder. RSC Adv. 2019, 9, 27013–27020. [Google Scholar] [CrossRef] [PubMed]
- Abinaya, B.; Prasith, T.P.; Ashwin, B.; Viji Chandran, S.; Selvamurugan, N. Chitosan in Surface Modification for Bone Tissue Engineering Applications. Biotechnol. J. 2019, 14, e1900171. [Google Scholar] [CrossRef]
- Zanca, C.; Patella, B.; Capuana, E.; Lopresti, F.; Brucato, V.; Carfì Pavia, F.; La Carrubba, V.; Inguanta, R. Behavior of Calcium Phosphate-Chitosan-Collagen Composite Coating on AISI 304 for Orthopedic Applications. Polymers 2022, 14, 5108. [Google Scholar] [CrossRef]
- Veronesi, F.; Torricelli, P.; Martini, L.; Tschon, M.; Giavaresi, G.; Bellini, D.; Casagranda, V.; Alemani, F.; Fini, M. An alternative ex vivo method to evaluate the osseointegration of Ti-6Al-4V alloy also combined with collagen. Biomed. Mater. 2021, 16, 025007. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 690701. [Google Scholar] [CrossRef]
- Alnufaiy, B.A.; Lambarte, R.N.A.; Al-Hamdan, K.S. The Osteogenetic Potential of Chitosan Coated Implant: An In Vitro Study. J. Stem Cells Regen. Med. 2020, 16, 44–49. [Google Scholar]
- Haktaniyan, M.; Bradley, M. Polymers showing intrinsic antimicrobial activity. Chem. Soc. Rev. 2022, 51, 8584–8611. [Google Scholar] [CrossRef] [PubMed]
- Szulc, M.; Lewandowska, K. Biomaterials Based on Chitosan and Its Derivatives and Their Potential in Tissue Engineering and Other Biomedical Applications-A Review. Molecules 2022, 28, 247. [Google Scholar] [CrossRef] [PubMed]
- Govindharajulu, J.P.; Chen, X.; Li, Y.; Rodriguez-Cabello, J.C.; Battacharya, M.; Aparicio, C. Chitosan-Recombinamer Layer-by-Layer Coatings for Multifunctional Implants. Int. J. Mol. Sci. 2017, 18, 369. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Sadraei, F.; Ghollasi, M.; Khakpai, F.; Halabian, R.; Tehrani, H.J. Osteogenic differentiation of pre-conditioned bone marrow mesenchymal stem cells with Nisin on modified poly-L-lactic-acid nanofibers. Regen. Ther. 2022, 21, 263–270. [Google Scholar] [CrossRef]
- Rashid, U.; Yousaf, A.; Yaqoob, M.; Saba, E.; Moaeen-Ud-Din, M.; Waseem, S.; Becker, S.K.; Sponder, G.; Aschenbach, J.R.; Sandhu, M.A. Characterization and differentiation potential of mesenchymal stem cells isolated from multiple canine adipose tissue sources. BMC Vet. Res. 2021, 17, 388. [Google Scholar] [CrossRef]
- Gousopoulou, E.; Bakopoulou, A.; Apatzidou, D.A.; Leyhausen, G.; Volk, J.; Staufenbiel, I.; Geurtsen, W.; Adam, K. Evaluation of stemness properties of cells derived from granulation tissue of peri-implantitis lesions. Clin. Exp. Dent. Res. 2021, 7, 739–753. [Google Scholar] [CrossRef]
- Fukuda, C.; Goto, K.; Imamura, M.; Nakamura, T. Bioactive bone cement with a low content of titania particles without postsilanization: Effect of filler content on osteoconductivity, mechanical properties, and handling characteristics. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 95, 407–413. [Google Scholar] [CrossRef]
- Sieberath, A.; Della Bella, E.; Ferreira, A.M.; Gentile, P.; Eglin, D.; Dalgarno, K. A Comparison of Osteoblast and Osteoclast In Vitro Co-Culture Models and Their Translation for Preclinical Drug Testing Applications. Int. J. Mol. Sci. 2020, 21, 912. [Google Scholar] [CrossRef]
- Cope, P.J.; Ourradi, K.; Li, Y.; Sharif, M. Models of osteoarthritis: The good, the bad and the promising. Osteoarthr. Cartil. 2019, 27, 230–239. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 January 2023).




| Gene | Primer Forward | Primer Reverse | Amplicon Length | Annealing Temperature |
|---|---|---|---|---|
| GAPDH | QuantiTect Primer Assay (Qiagen) Hs_GAPDH_1_SG | 95 bp | 55 °C | |
| RUNX2 | CTTCACAAATCCTCCCCAAGT | AGGCGGTCAGAGAACAAAC | 212 bp | 60 °C |
| COL1A1 | QuantiTect Primer Assay (Qiagen) Hs_COL1A1_1_SG | 118 bp | 55 °C | |
| ALPL | QuantiTect Primer Assay (Qiagen) Hs_ALPL_1_SG | 110 bp | 55 °C | |
| SP7 | QuantiTect Primer Assay (Qiagen) Hs_SP7_1_SG | 120 bp | 55 °C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veronesi, F.; Brogini, S.; De Luca, A.; Bellini, D.; Casagranda, V.; Fini, M.; Giavaresi, G. Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 4810. https://doi.org/10.3390/ijms24054810
Veronesi F, Brogini S, De Luca A, Bellini D, Casagranda V, Fini M, Giavaresi G. Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study. International Journal of Molecular Sciences. 2023; 24(5):4810. https://doi.org/10.3390/ijms24054810
Chicago/Turabian StyleVeronesi, Francesca, Silvia Brogini, Angela De Luca, Davide Bellini, Veronica Casagranda, Milena Fini, and Gianluca Giavaresi. 2023. "Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study" International Journal of Molecular Sciences 24, no. 5: 4810. https://doi.org/10.3390/ijms24054810
APA StyleVeronesi, F., Brogini, S., De Luca, A., Bellini, D., Casagranda, V., Fini, M., & Giavaresi, G. (2023). Cell Adhesion and Initial Bone Matrix Deposition on Titanium-Based Implants with Chitosan–Collagen Coatings: An In Vitro Study. International Journal of Molecular Sciences, 24(5), 4810. https://doi.org/10.3390/ijms24054810









