The Role of Microbiota in Liver Transplantation and Liver Transplantation-Related Biliary Complications
Abstract
:1. Introduction
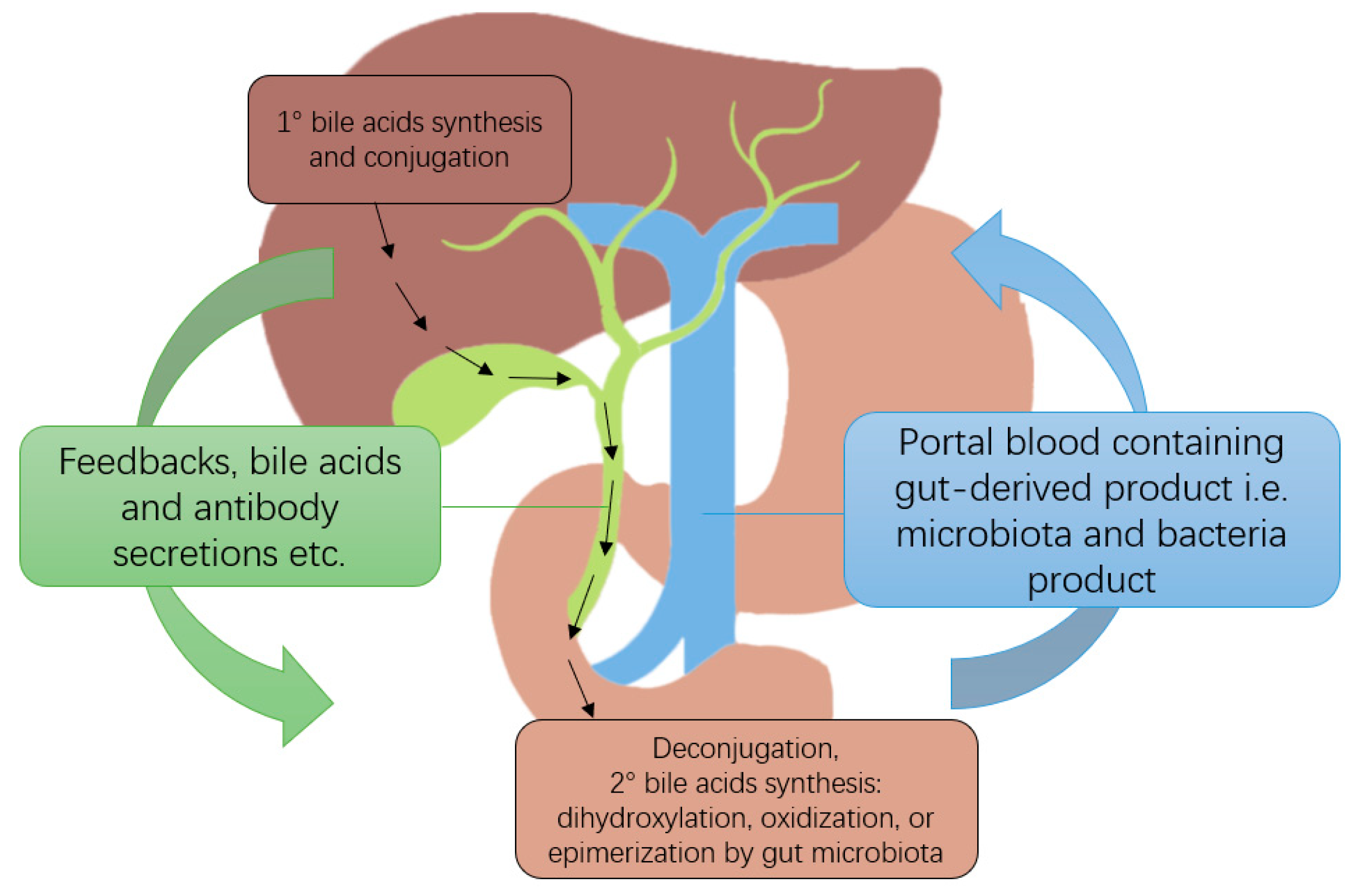
2. Gut-Liver Axis
3. Gut Microbiota in Chronic Liver Disease and Liver Transplantation
4. Infections and Colonization, Especially with Multi-Drug Resistant Microbiota
5. Biliary Complications of Liver Transplantation
5.1. Biliary Reconstruction
5.2. Types of Biliary Complications
6. Microbiota in Liver Transplantation and Associated Biliary Complications
6.1. Gut Microbiota
6.2. Biliary Microbiota
7. Microbiota as a Predictive Tool and Therapeutic Target
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samstein, B.; Smith, A.R.; Freise, C.E.; Zimmerman, M.A.; Baker, T.; Olthoff, K.M.; Fisher, R.A.; Merion, R.M. Complications and Their Resolution in Recipients of Deceased and Living Donor Liver Transplants: Findings from the A2ALL Cohort Study. Am. J. Transplant. 2016, 16, 594–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farkas, S.; Hackl, C.; Schlitt, H.J. Overview of the Indications and Contraindications for Liver Transplantation. Cold Spring Harb. Perspect. Med. 2014, 4, a015602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemes, B.; Gámán, G.; Doros, A. Biliary Complications after Liver Transplantation. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Dubbeld, J.; van Hoek, B.; Ringers, J.; Metselaar, H.; Kazemier, G.; van den Berg, A.; Porte, R.J. Biliary Complications after Liver Transplantation from Donation after Cardiac Death Donors. Ann. Surg. 2015, 261, e64. [Google Scholar] [CrossRef]
- Azzam, A.Z.; Tanaka, K. Biliary Complications after Living Donor Liver Transplantation: A Retrospective Analysis of the Kyoto Experience 1999–2004. Indian J. Gastroenterol. 2017, 36, 296–304. [Google Scholar] [CrossRef]
- Hong, S.Y.; Hu, X.; Lee, H.Y.; Won, J.H.; Kim, J.W.; Shen, X.; Wang, H.; Kim, B. Longterm Analysis of Biliary Complications after Duct-to-Duct Biliary Reconstruction in Living Donor Liver Transplantations. Liver Transplant. 2018, 24, 1050–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vij, V.; Makki, K.; Chorasiya, V.K.; Sood, G.; Singhal, A.; Dargan, P. Targeting the Achilles’ Heel of Adult Living Donor Liver Transplant: Corner-Sparing Sutures with Mucosal Eversion Technique of Biliary Anastomosis. Liver Transplant. 2016, 22, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.K.; Mathew, J.S.; Balakrishnan, D.; Bharathan, V.K.; Amma, B.S.P.T.; Gopalakrishnan, U.; Menon, R.N.; Dhar, P.; Vayoth, S.O.; Sudhindran, S. Intraductal Transanastomotic Stenting in Duct-to-Duct Biliary Reconstruction after Living-Donor Liver Transplantation: A Randomized Trial. J. Am. Coll. Surg. 2017, 225, 747–754. [Google Scholar] [CrossRef]
- Schielke, A.; Scatton, O.; Boelle, P.-Y.; Perdigao, F.; Bernard, D.; Soubrane, O.; Conti, F. Ischemic-Type Biliary Lesions: A Leading Indication of Liver Retransplantation with Excellent Results. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 131–139. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, L.-Y.; Zhu, Z.-J.; Wei, L.; Qu, W.; Zeng, Z.-G. Bile Microbiota: New Insights into Biliary Complications in Liver Transplant Recipients. Ann. Transl. Med. 2020, 8, 354. [Google Scholar] [CrossRef]
- Özdirik, B.; Müller, T.; Wree, A.; Tacke, F.; Sigal, M. The Role of Microbiota in Primary Sclerosing Cholangitis and Related Biliary Malignancies. Int. J. Mol. Sci. 2021, 22, 6975. [Google Scholar] [CrossRef]
- Zigmond, E.; Zecher, B.F.; Bartels, A.-L.; Ziv-Baran, T.; Rösch, T.; Schachschal, G.; Lohse, A.W.; Ehlken, H.; Schramm, C. Bile Duct Colonization with Enterococcus sp. Associates with Disease Progression in Primary Sclerosing Cholangitis. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Wellhöner, F.; Plumeier, I.; Kahl, S.; Chhatwal, P.; Vital, M.; Voigtländer, T.; Pieper, D.H.; Manns, M.P.; Lenzen, H.; et al. The Biliary Microbiome in Ischaemic-Type Biliary Lesions Can Be Shaped by Stenting but Is Resilient to Antibiotic Treatment. Liver Int. 2022, 42, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Contreras, F.; Guevara, F. Incidence and Risk Factors for Infections after Liver Transplant: Single-Center Experience at the University Hospital Fundación Santa Fe de Bogotá, Colombia. Transplant. Infect. Dis. 2011, 13, 608–615. [Google Scholar] [CrossRef]
- Kim, H.K.; Park, Y.K.; Wang, H.J.; Kim, B.W.; Shin, S.Y.; Lim, S.K.; Choi, Y.H. Epidemiology and Clinical Features of Post-Transplant Bloodstream Infection: An Analysis of 222 Consecutive Liver Transplant Recipients. Infect. Chemother. 2013, 45, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Macesic, N.; Sullivan, S.B.; Kress, A.; Khan, S.D.; Giddins, M.J.; Stump, S.; Kim, G.I.; Narain, R.; et al. Colonizing Multidrug-Resistant Bacteria and the Longitudinal Evolution of the Intestinal Microbiome after Liver Transplantation. Nat. Commun. 2019, 10, 4715. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, S.; Roebuck, A.; Khoo, E.; Wigmore, S.J.; Harrison, E.M. A Meta-Analysis and Meta-Regression of Outcomes Including Biliary Complications in Donation after Cardiac Death Liver Transplantation. Transplant. Int. 2014, 27, 1159–1174. [Google Scholar] [CrossRef]
- Foley, D.P.; Fernandez, L.A.; Leverson, G.; Anderson, M.; Mezrich, J.; Sollinger, H.W.; D’Alessandro, A. Biliary Complications after Liver Transplantation from Donation after Cardiac Death Donors. Ann. Surg. 2011, 253, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, V.; di Gregorio, V.; Iebba, V.; Giusto, M.; Schippa, S.; Merli, M.; Thalheimer, U. Microbiota and the Gut-Liver Axis: Bacterial Translocation, Inflammation and Infection in Cirrhosis. World J. Gastroenterol. WJG 2014, 20, 16795. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Valenza, V.; Nure, E.; Bianco, G.; Marrone, G.; Grieco, A.; Pompili, M.; Gasbarrini, A.; Agnes, S.; Sganga, G. Effect of Liver Transplantation on Intestinal Permeability and Correlation with Infection Episodes. PLoS ONE 2020, 15, e0235359. [Google Scholar] [CrossRef]
- Kriss, M.; Verna, E.C.; Rosen, H.R.; Lozupone, C.A. Functional Microbiomics in Liver Transplantation: Identifying Novel Targets for Improving Allograft Outcomes. Transplantation 2019, 103, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kakiyama, G.; Cox, I.J.; Nittono, H.; Takei, H.; White, M.; Fagan, A.; Gavis, E.A.; Heuman, D.M.; Gilles, H.C.; et al. Alterations in Gut Microbial Function Following Liver Transplant. Liver Transplant. 2018, 24, 752–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, Q.; Xu, X.; Wang, B.; Li, L.; Zheng, S. The Origin of New-Onset Diabetes after Liver Transplantation. Transplantation 2016, 100, 808–813. [Google Scholar] [CrossRef]
- Jiménez, E.; Sánchez, B.; Farina, A.; Margolles, A.; Rodríguez, J.M. Characterization of the Bile and Gall Bladder Microbiota of Healthy Pigs. Microbiologyopen 2014, 3, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Begley, M.; Gahan, C.G.M.; Hill, C. The Interaction between Bacteria and Bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.; Aho, V.; Arola, J.; Boyd, S.; Jokelainen, K.; Paulin, L.; Auvinen, P.; Färkkilä, M. Bile Microbiota in Primary Sclerosing Cholangitis: Impact on Disease Progression and Development of Biliary Dysplasia. PLoS ONE 2017, 12, e0182924. [Google Scholar] [CrossRef] [Green Version]
- Verdier, J.; Luedde, T.; Sellge, G. Biliary Mucoosal Barrier and Microbiome. Visc. Med. 2015, 31, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 32 Cancer Groups, 1990 to 2015. JAMA Oncol. 2017, 3, 524. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Z.; Liu, B.; Hou, D.; Liang, Y.; Zhang, J.; Shi, P. Gut Microbiota Dysbiosis and Bacterial Community Assembly Associated with Cholesterol Gallstones in Large-Scale Study. BMC Genom. 2013, 14, 669. [Google Scholar] [CrossRef] [Green Version]
- Moy, B.T.; Birk, J.W. A Review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J. Clin. Transl. Hepatol. 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Yoshizumi, T.; Shirabe, K.; Ikegami, T.; Yamashita, N.; Mano, Y.; Yoshiya, S.; Matono, R.; Harimoto, N.; Uchiyama, H.; Toshima, T.; et al. Decreased Immunoglobulin G Levels after Living-Donor Liver Transplantation Is a Risk Factor for Bacterial Infection and Sepsis. Transplant. Infect. Dis. 2014, 16, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.N.; Weiss, K.H.; Rupp, C.; Bode, K.; Eckerle, I.; Rudolph, G.; Bergemann, J.; Kloeters-Plachky, P.; Chahoud, F.; Büchler, M.W.; et al. Bacteriobilia and Fungibilia Are Associated with Outcome in Patients with Endoscopic Treatment of Biliary Complications after Liver Transplantation. Endoscopy 2013, 45, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the Fecal Bile Acid Profile by Gut Microbiota in Cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, J.S.; Betrapally, N.S.; Gillevet, P.M. Decompensated Cirrhosis and Microbiome Interpretation. Nature 2015, 525, E1–E2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision Microbiome Reconstitution Restores Bile Acid Mediated Resistance to Clostridium Difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Tilg, H.; Cani, P.D.; Mayer, E.A. Gut Microbiome and Liver Diseases. Gut 2016, 65, 2035–2044. [Google Scholar] [CrossRef]
- Giordano, D.M.; Pinto, C.; Maroni, L.; Benedetti, A.; Marzioni, M. Inflammation and the Gut-Liver Axis in the Pathophysiology of Cholangiopathies. Int. J. Mol. Sci. 2018, 19, 3003. [Google Scholar] [CrossRef] [Green Version]
- Tabibian, J.H.; Talwalkar, J.A.; Lindor, K.D. Role of the Microbiota and Antibiotics in Primary Sclerosing Cholangitis. Biomed. Res. Int. 2013, 2013, 389537. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, S.P.; Tabibian, J.H.; Splinter, P.L.; LaRusso, N.F. The Dynamic Biliary Epithelia: Molecules, Pathways, and Disease. J. Hepatol. 2013, 58, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Vera, A.; Moledina, S.; Gunson, B.; Hubscher, S.; Mirza, D.; Olliff, S.; Neuberger, J. Risk Factors for Recurrence of Primary Sclerosing Cholangitis of Liver Allograft. Lancet 2002, 360, 1943–1944. [Google Scholar] [CrossRef]
- Alabraba, E.; Nightingale, P.; Gunson, B.; Hubscher, S.; Olliff, S.; Mirza, D.; Neuberger, J. A Re-Evaluation of the Risk Factors for the Recurrence of Primary Sclerosing Cholangitis in Liver Allografts. Liver Transplant. 2009, 15, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Nakanuma, Y. Biliary Innate Immunity in the Pathogenesis of Biliary Diseases. Inflamm. Allergy Drug Targets 2010, 9, 83–90. [Google Scholar] [CrossRef]
- Harada, K.; Nakanuma, Y. Biliary Innate Immunity: Function and Modulation. Mediat. Inflamm. 2010, 2010, 373878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.J.; Lim, W.H.; Ng, C.H.; Tan, D.J.H.; Bonney, G.K.; Kow, A.W.C.; Huang, D.Q.; Siddiqui, M.S.; Noureddin, M.; Syn, N.; et al. Predictive and Prognostic Roles of Gut Microbial Variation in Liver Transplant. Front. Med. 2022, 9, 8735233. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Ling, Z.-X.; Lu, H.-F.; Zuo, J.; Sheng, J.-F.; Zheng, S.-S.; Li, L.-J. Changes of Gut Bacteria and Immune Parameters in Liver Transplant Recipients. Hepatobiliary Pancreat. Dis. Int. 2012, 11, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the Human Gut Microbiome in Liver Cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of Fecal Microbial Communities in Patients with Liver Cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Lai, Z.; Chen, Z.; Zhang, A.; Niu, Z.; Cheng, M.; Huo, C.; Xu, J. The Gut Microbiota in Liver Transplantation Recipients during the Perioperative Period. Front. Physiol. 2022, 13, 513. [Google Scholar] [CrossRef]
- Rayes, N.; Seehofer, D.; Theruvath, T.; Schiller, R.A.; Langrehr, J.M.; Jonas, S.; Bengmark, S.; Neuhaus, P. Supply of Pre- and Probiotics Reduces Bacterial Infection Rates After Liver Transplantation—A Randomized, Double-Blind Trial. Am. J. Transplant. 2005, 5, 125–130. [Google Scholar] [CrossRef]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knoll, P.; Schlaak, J.; Uhrig, A.; Kempf, P.; zum Büschenfelde, K.-H.M.; Gerken, G. Human Kupffer Cells Secrete IL-10 in Response to Lipopolysaccharide (LPS) Challenge. J. Hepatol. 1995, 22, 226–229. [Google Scholar] [CrossRef]
- Hackstein, C.-P.; Assmus, L.M.; Welz, M.; Klein, S.; Schwandt, T.; Schultze, J.; Förster, I.; Gondorf, F.; Beyer, M.; Kroy, D.; et al. Gut Microbial Translocation Corrupts Myeloid Cell Function to Control Bacterial Infection during Liver Cirrhosis. Gut 2017, 66, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Taddio, M.F.; Castro Jaramillo, C.A.; Runge, P.; Blanc, A.; Keller, C.; Talip, Z.; Béhé, M.; van der Meulen, N.P.; Halin, C.; Schibli, R.; et al. In Vivo Imaging of Local Inflammation: Monitoring LPS-Induced CD80/CD86 Upregulation by PET. Mol. Imaging Biol. 2021, 23, 196–207. [Google Scholar] [CrossRef]
- Rollins, M.R.; Gibbons Johnson, R.M. CD80 Expressed by CD8 + T Cells Contributes to PD-L1-Induced Apoptosis of Activated CD8+ T Cells. J. Immunol. Res. 2017, 2017, 7659462. [Google Scholar] [CrossRef] [Green Version]
- Wegorzewska, M.M.; Glowacki, R.W.P.; Hsieh, S.A.; Donermeyer, D.L.; Hickey, C.A.; Horvath, S.C.; Martens, E.C.; Stappenbeck, T.S.; Allen, P.M. Diet Modulates Colonic T Cell Responses by Regulating the Expression of a Bacteroides Thetaiotaomicron Antigen. Sci. Immunol. 2019, 4, eaau9079. [Google Scholar] [CrossRef] [PubMed]
- Lathrop, S.K.; Bloom, S.M.; Rao, S.M.; Nutsch, K.; Lio, C.-W.; Santacruz, N.; Peterson, D.A.; Stappenbeck, T.S.; Hsieh, C.-S. Peripheral Education of the Immune System by Colonic Commensal Microbiota. Nature 2011, 478, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Wu, Z.; Chi, Y.; Song, Y.; Xu, J.; Tan, J.; Cong, X.; Liu, Y. Mesenteric Lymph Node CD4+ T Lymphocytes Migrate to Liver and Contribute to Non-Alcoholic Fatty Liver Disease. Cell Immunol 2019, 337, 33–41. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, M.; Brown, K.M.; D’Agati, V.D.; Lee, H.T. Paneth Cell-Derived Interleukin-17A Causes Multiorgan Dysfunction after Hepatic Ischemia and Reperfusion Injury. Hepatology 2011, 53, 1662–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Xie, L.; Li, L.; Chen, X.; Yao, T.; Tian, Y.; Li, Q.; Wang, K.; Huang, C.; Li, C.; et al. The Gut Microbial Metabolite, 3,4-Dihydroxyphenylpropionic Acid, Alleviates Hepatic Ischemia/Reperfusion Injury via Mitigation of Macrophage pro-Inflammatory Activity in Mice. Acta Pharm. Sin. B 2022, 12, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Schurich, A.; Berg, M.; Stabenow, D.; Böttcher, J.; Kern, M.; Schild, H.-J.; Kurts, C.; Schuette, V.; Burgdorf, S.; Diehl, L.; et al. Dynamic Regulation of CD8 T Cell Tolerance Induction by Liver Sinusoidal Endothelial Cells. J. Immunol. 2010, 184, 4107–4114. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; White, M.B.; Monteith, P.; Noble, N.A.; Unser, A.B.; Daita, K.; Fisher, A.R.; et al. Altered Profile of Human Gut Microbiome Is Associated with Cirrhosis and Its Complications. J. Hepatol. 2014, 60, 940–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Guo, J.; Qian, G.; Fang, D.; Shi, D.; Guo, L.; Li, L. Gut Dysbiosis in Acute-on-Chronic Liver Failure and Its Predictive Value for Mortality. J. Gastroenterol. Hepatol. 2015, 30, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Xue, J.; Shan, J.; Hong, Y.; Zhu, W.; Nie, Z.; Zhang, Y.; Ji, N.; Luo, X.; Zhang, T.; et al. Gut-Flora-Dependent Metabolite Trimethylamine-N-Oxide Promotes Atherosclerosis-Associated Inflammation Responses by Indirect ROS Stimulation and Signaling Involving AMPK and SIRT1. Nutrients 2022, 14, 3338. [Google Scholar] [CrossRef]
- Gabarre, P.; Loens, C.; Tamzali, Y.; Barrou, B.; Jaisser, F.; Tourret, J. Immunosuppressive Therapy after Solid Organ Transplantation and the Gut Microbiota: Bidirectional Interactions with Clinical Consequences. Am. J. Transplant. 2022, 22, 1014–1030. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Wu, J.; Chalson, H.; Merigan, L.; Mitchell, A. Probiotic Use in Preventing Postoperative Infection in Liver Transplant Patients. Hepatobiliary Surg. Nutr. 2013, 2, 142. [Google Scholar] [CrossRef]
- Kawecki, D.; Pacholczyk, M.; Lagiewska, B.; Sawicka-Grzelak, A.; Durlik, M.; Mlynarczyk, G.; Chmura, A. Bacterial and Fungal Infections in the Early Post-Transplantation Period after Liver Transplantation: Etiologic Agents and Their Susceptibility. In Proceedings of the Transplantation Proceedings; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 46, pp. 2777–2781. [Google Scholar]
- Piano, S.; Tonon, M.; Angeli, P. Changes in the Epidemiology and Management of Bacterial Infections in Cirrhosis. Clin. Mol. Hepatol. 2021, 27, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Kabar, I.; Hüsing, A.; Cicinnati, V.R.; Heitschmidt, L.; Beckebaum, S.; Thölking, G.; Schmidt, H.H.; Karch, H.; Kipp, F. Analysis of Bile Colonization and Intestinal Flora May Improve Management in Liver Transplant Recipients Undergoing ERCP. Ann. Transplant. 2015, 20, 249–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Men, T.-Y.; Li, H.; Gu, Y.; Ding, X.; Xing, T.-H.; Fan, J.-W.; Peng, Z.-H. Prevalence and Risk Factor for MDR-GNB Infection in Liver Transplantation. Front. Biosci. Landmark 2013, 18, 366–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Kim, S.I.; Wie, S.H.; Kim, Y.R.; Hur, J.A.; Choi, J.Y.; Yoon, S.K.; Moon, I.S.; Kim, D.G.; Lee, M.D.; et al. Infectious Complications in Living-Donor Liver Transplant Recipients: A 9-Year Single-Center Experience. Transplant. Infect. Dis. 2008, 10, 316–324. [Google Scholar] [CrossRef]
- Abad, C.L.R.; Lahr, B.D.; Razonable, R.R. Epidemiology and Risk Factors for Infection after Living Donor Liver Transplantation. Liver Transplant. 2017, 23, 465–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C. Analysis of Infections in the First 3-Month after Living Donor Liver Transplantation. World J. Gastroenterol. 2012, 18, 1975. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.; Kusne, S. Bacterial, Mycobacterial, and Protozoal Infections after Liver Transplantation—Part I. Liver Transplant. 2005, 11, 1452–1459. [Google Scholar] [CrossRef]
- Orloff, S.L.; Busch, A.M.H.; Olyaei, A.J.; Corless, C.L.; Benner, K.G.; Flora, K.D.; Rosen, H.R.; Rabkin, J.M. Vancomycin-Resistant Enterococcus in Liver Transplant Patients. Am. J. Surg. 1999, 177, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Gearhart, M.; Martin, J.E.; Rudich, S.; Thomas, M.; Wetzel, D.; Solomkin, J.; Hanaway, M.J.; Aranda-Michel, J.; Weber, F.; Trumball, L.; et al. Consequences of Vancomycin-Resistant Enterococcus in Liver Transplant Recipients: A Matched Control Study. Clin. Transpl. 2005, 19, 711–716. [Google Scholar] [CrossRef]
- Fishman, J.A.; Rubin, R.H. Infection in Organ-Transplant Recipients. N. Engl. J. Med. 1998, 338, 1741–1751. [Google Scholar] [CrossRef]
- Gurakar, A.; Tasdogan, B.E.; Simsek, C.; Ma, M.; Saberi, B. Update on Immunosuppression in Liver Transplantation. Euroasian J. Hepatogastroenterol. 2019, 9, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Marubashi, S.; Dono, K.; Nagano, H.; Kobayashi, S.; Takeda, Y.; Umeshita, K.; Monden, M.; Doki, Y.; Mori, M. Biliary Reconstruction in Living Donor Liver Transplantation: Technical Invention and Risk Factor Analysis for Anastomotic Stricture. Transplantation 2009, 88, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.K.; Ryu, J.K.; Lee, S.H.; Park, J.K.; Yang, K.Y.; Kim, Y.-T.; Yoon, Y.B.; Lee, H.W.; Yi, N.-J.; Suh, K.S. Endoscopic Treatment for Biliary Stricture after Adult Living Donor Liver Transplantation. Liver Transplant. 2009, 15, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Shirabe, K.; Morita, K.; Soejima, Y.; Taketomi, A.; Yoshizumi, T.; Uchiyama, H.; Kayashima, H.; Hashimoto, N.; Maehara, Y. Minimal Hilar Dissection Prevents Biliary Anastomotic Stricture after Living Donor Liver Transplantation. Transplantation 2011, 92, 1147–1151. [Google Scholar] [CrossRef]
- Chok, K.S.H.; Chan, S.C.; Cheung, T.T.; Sharr, W.W.; Chan, A.C.Y.; Lo, C.M.; Fan, S.T. Bile Duct Anastomotic Stricture after Adult-to-Adult Right Lobe Living Donor Liver Transplantation. Liver Transplant. 2011, 17, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Miller, C.M.; Quintini, C.; Aucejo, F.N.; Hirose, K.; Uso, T.D.; Trenti, L.; Kelly, D.M.; Winans, C.G.; Vogt, D.P.; et al. Is Impaired Hepatic Arterial Buffer Response a Risk Factor for Biliary Anastomotic Stricture in Liver Transplant Recipients? Surgery 2010, 148, 582–588. [Google Scholar] [CrossRef]
- Rull, R.; Garcia Valdecasas, J.C.; Grande, L.; Fuster, J.; Lacy, A.M.; González, F.X.; Rimola, A.; Navasa, M.; Iglesias, C.; Visa, J. Intrahepatic Biliary Lesions after Orthotopic Liver Transplantation. Transpl. Int. 2001, 14, 129–134. [Google Scholar] [CrossRef]
- Song, G.-W.; Lee, S.-G. Living Donor Liver Transplantation. Curr. Opin. Organ. Transpl. 2014, 19, 217–222. [Google Scholar] [CrossRef]
- Neuhaus, P.; Blumhardt, G.; Bechstein, W.O.; Steffen, R.; Platz, K.-P.; Keck, H. Technique and Results of Biliary Reconstruction Using Side-to-Side Choledochocholedochostomy in 300 Orthotopic Liver Transplants. Ann. Surg. 1994, 219, 426. [Google Scholar] [CrossRef]
- Verdonk, R.C.; Buis, C.I.; Porte, R.J.; van der Jagt, E.J.; Limburg, A.J.; van den Berg, A.P.; Slooff, M.J.H.; Peeters, P.M.J.G.; de Jong, K.P.; Kleibeuker, J.H.; et al. Anastomotic Biliary Strictures after Liver Transplantation: Causes and Consequences. Liver Transplant. 2006, 12, 726–735. [Google Scholar] [CrossRef]
- Wachs, M.E.; Bak, T.E.; Karrer, F.M.; Everson, G.T.; Shrestha, R.; Trouillot, T.E.; Mandell, M.S.; Steinberg, T.G.; Kam, I. Adult living donor liver transplantation using a right hepatic lobe. Transplantation 1998, 66, 1313–1316. [Google Scholar] [CrossRef]
- Chok, K.S.H.; Lo, C.M. Systematic Review and Meta-Analysis of Studies of Biliary Reconstruction in Adult Living Donor Liver Transplantation. ANZ J. Surg. 2017, 87, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Prieto, M.; Valdivieso, A.; Gastaca, M.; Pijoan, J.I.; Ruiz, P.; Ventoso, A.; Palomares, I.; Ortiz de Urbina, J. Hepaticojejunostomy in Orthotopic Liver Transplant: A Retrospective Case Control Study. Transpl. Proc. 2019, 51, 58–61. [Google Scholar] [CrossRef]
- Kochhar, G.; Parungao, J.M.; Hanouneh, I.A.; Parsi, M.A. Biliary Complications Following Liver Transplantation. World J. Gastroenterol. 2013, 19, 2841–2846. [Google Scholar] [CrossRef]
- Greif, F.; Bronsther, O.L.; van Thiel, D.H.; Casavilla, A.; Iwatsuki, S.; Tzakis, A.; Todo, S.; Fung, J.J.; Starzl, T.E. The Incidence, Timing, and Management of Biliary Tract Complications after Orthotopic Liver Transplantation. Ann. Surg. 1994, 219, 40–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagannath, S.; Kalloo, A.N. Biliary Complications after Liver Transplantation. Curr. Treat. Options Gastroenterol. 2002, 5, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Moser, M. Management of Biliary Problems after Liver Transplantation. Liver Transplant. 2001, 7, S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.; Fleischer, B.; Alverdy, J. The Biology of Anastomotic Healing—The Unknown Overwhelms the Known. J. Gastrointest. Surg. 2020, 24, 2160–2166. [Google Scholar] [CrossRef]
- Alverdy, J.C.; Schardey, H.M. Anastomotic Leak: Toward an Understanding of Its Root Causes. J. Gastrointest. Surg. 2021, 25, 2966–2975. [Google Scholar] [CrossRef]
- Williamson, A.J.; Alverdy, J.C. Influence of the Microbiome on Anastomotic Leak. Clin. Colon Rectal Surg. 2021, 34, 439–446. [Google Scholar] [CrossRef]
- Sharma, S.; Gurakar, A.; Jabbour, N. Biliary Strictures Following Liver Transplantation: Past, Present and Preventive Strategies. Liver Transplant. 2008, 14, 759–769. [Google Scholar] [CrossRef]
- Roumilhac, D. Long-Term Results of Percutaneous Management for Anastomotic Biliary Stricture after Orthotopic Liver Transplantation. Liver Transplant. 2003, 9, 394–400. [Google Scholar] [CrossRef]
- Zajko, A.B.; Campbell, W.L.; Logsdon, G.A.; Bron, K.M.; Tzakis, A.; Esquivel, C.O.; Starzl, T.E. Cholangiographic Findings in Hepatic Artery Occlusion after Liver Transplantation. AJR Am. J. Roentgenol. 1987, 149, 485. [Google Scholar] [CrossRef] [Green Version]
- Ward, E.M.; Kiely, M.J.; Maus, T.P.; Wiesner, R.H.; Krom, R.A. Hilar Biliary Strictures after Liver Transplantation: Cholangiography and Percutaneous Treatment. Radiology 1990, 177, 259–263. [Google Scholar] [CrossRef]
- Dries, S.O.D.; Sutton, M.E.; Lisman, T.; Porte, R.J. Protection of Bile Ducts in Liver Transplantation: Looking Beyond Ischemia. Transplantation 2011, 92, 373–379. [Google Scholar] [CrossRef]
- Sanchez-Urdazpal, L.; Gores, G.J.; Ward, E.M.; Maus, T.P.; Wahlstrom, H.E.; Moore, S.B.; Wiesner, R.H.; Krom, R.A.F. Ischemic-Type Biliary Complications after Orthotopic Liver Transplantation. Hepatology 1992, 16, 49–53. [Google Scholar] [CrossRef]
- Moench, C. Prevention of Ischemic-Type Biliary Lesions by Arterial Back-Table Pressure Perfusion. Liver Transplant. 2003, 9, 285–289. [Google Scholar] [CrossRef]
- Bajer, L.; Kverka, M.; Kostovcik, M.; Macinga, P.; Dvorak, J.; Stehlikova, Z.; Brezina, J.; Wohl, P.; Spicak, J.; Drastich, P. Distinct Gut Microbiota Profiles in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis. World J. Gastroenterol. 2017, 23, 4548. [Google Scholar] [CrossRef]
- Liwinski, T.; Zenouzi, R.; John, C.; Ehlken, H.; Rühlemann, M.C.; Bang, C.; Groth, S.; Lieb, W.; Kantowski, M.; Andersen, N.; et al. Alterations of the Bile Microbiome in Primary Sclerosing Cholangitis. Gut 2020, 69, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Quraishi, M.N.; Acharjee, A.; Beggs, A.D.; Horniblow, R.; Tselepis, C.; Gkoutos, G.; Ghosh, S.; Rossiter, A.E.; Loman, N.; van Schaik, W.; et al. A Pilot Integrative Analysis of Colonic Gene Expression, Gut Microbiota, and Immune Infiltration in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Association of Disease with Bile Acid Pathways. J. Crohns Colitis 2020, 14, 935–947. [Google Scholar] [CrossRef] [Green Version]
- Iwasawa, K.; Suda, W.; Tsunoda, T.; Oikawa-Kawamoto, M.; Umetsu, S.; Inui, A.; Fujisawa, T.; Morita, H.; Sogo, T.; Hattori, M. Characterisation of the Faecal Microbiota in Japanese Patients with Paediatric-Onset Primary Sclerosing Cholangitis. Gut 2017, 66, 1344–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Ren, F.-G.; Liu, P.; Zhang, H.-K.; Zhu, H.-Y.; Feng, Z.; Zhang, X.-F.; Wang, B.; Liu, X.-M.; Zhang, X.-G.; et al. Characteristics of Fecal Microbial Communities in Patients with Non-Anastomotic Biliary Strictures after Liver Transplantation. World J. Gastroenterol. 2017, 23, 8217–8226. [Google Scholar] [CrossRef] [PubMed]
- Rühlemann, M.; Liwinski, T.; Heinsen, F.-A.; Bang, C.; Zenouzi, R.; Kummen, M.; Thingholm, L.; Tempel, M.; Lieb, W.; Karlsen, T.; et al. Consistent Alterations in Faecal Microbiomes of Patients with Primary Sclerosing Cholangitis Independent of Associated Colitis. Aliment. Pharm. Ther. 2019, 50, 580–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Bella, S.; Antonello, R.M.; Sanson, G.; Maraolo, A.E.; Giacobbe, D.R.; Sepulcri, C.; Ambretti, S.; Aschbacher, R.; Bartolini, L.; Bernardo, M.; et al. Anaerobic Bloodstream Infections in Italy (ITANAEROBY): A 5-Year Retrospective Nationwide Survey. Anaerobe 2022, 75, 102583. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bao, X.; Goel, A.; Colombel, J.-F.; Pekow, J.; Jabri, B.; Williams, K.M.; Castillo, A.; Odin, J.A.; Meckel, K.; et al. The Features of Mucosa-Associated Microbiota in Primary Sclerosing Cholangitis. Aliment. Pharm. Ther. 2016, 43, 790–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabino, J.; Vieira-Silva, S.; Machiels, K.; Joossens, M.; Falony, G.; Ballet, V.; Ferrante, M.; van Assche, G.; van der Merwe, S.; Vermeire, S.; et al. Primary Sclerosing Cholangitis Is Characterised by Intestinal Dysbiosis Independent from IBD. Gut 2016, 65, 1681–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria Expansion: A Microbial Signature of Epithelial Dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Wan, Y.-J.Y. The Role of Gut Microbiota in Liver Disease Development and Treatment. Liver Res. 2019, 3, 3–18. [Google Scholar] [CrossRef]
- Bang, J.H.; Jung, Y.; Cheon, S.; Kim, C.J.; Song, K.H.; Choe, P.G.; Park, W.B.; Kim, E.S.; Park, S.W.; Kim, H.B.; et al. Pseudomonas Aeruginosa Bacteremia in Patients with Liver Cirrhosis: A Comparison with Bacteremia Caused by Enterobacteriaceae. BMC Infect. Dis. 2013, 13, 332. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, F.; Quigley, E.M.M. Manipulation of the Microbiota for Treatment of IBS and IBD—Challenges and Controversies. Gastroenterology 2014, 146, 1554–1563. [Google Scholar] [CrossRef]
- Cani, P.D. The Gut Microbiota Manages Host Metabolism. Nat. Rev. Endocrinol. 2014, 10, 74–76. [Google Scholar] [CrossRef]
- Wang, W.; Xu, S.; Ren, Z.; Jiang, J.; Zheng, S. Gut Microbiota and Allogeneic Transplantation. J. Transl. Med. 2015, 13, 275. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-Liver Axis in Hepatic Disease. Hepatology 2014, 59, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.; Raes, J.; Pelletier, E.; le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chen, J.; Wei, Q.; Saeb-Parsy, K.; Xu, X. The Role of Ischemia/Reperfusion Injury in Early Hepatic Allograft Dysfunction. Liver Transplant. 2020, 26, 1034–1048. [Google Scholar] [CrossRef]
- Rosas-Villegas, A.; Sánchez-Tapia, M.; Avila-Nava, A.; Ramírez, V.; Tovar, A.; Torres, N. Differential Effect of Sucrose and Fructose in Combination with a High Fat Diet on Intestinal Microbiota and Kidney Oxidative Stress. Nutrients 2017, 9, 393. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, X.; Shang, Q.; Gao, Z.; Hao, F.; Guo, H.; Guo, C. Fecal Microbiota Transplantation (FMT) Could Reverse the Severity of Experimental Necrotizing Enterocolitis (NEC) via Oxidative Stress Modulation. Free Radic. Biol. Med. 2017, 108, 32–43. [Google Scholar] [CrossRef]
- Bauer, T.M.; Steinbruckner, B.; Brinkmann, F.E.; Ditzen, A.K.; Schwacha, H.; Aponte, J.J.; Pelz, K.; Kist, M.; Blum, H.E. Small Intestinal Bacterial Overgrowth in Patients with Cirrhosis: Prevalence and Relation with Spontaneous Bacterial Peritonitis. Am. J. Gastroenterol. 2001, 96, 2962–2967. [Google Scholar] [CrossRef]
- Ye, F.; Shen, H.; Li, Z.; Meng, F.; Li, L.; Yang, J.; Chen, Y.; Bo, X.; Zhang, X.; Ni, M. Influence of the Biliary System on Biliary Bacteria Revealed by Bacterial Communities of the Human Biliary and Upper Digestive Tracts. PLoS ONE 2016, 11, e0150519. [Google Scholar] [CrossRef]
- Little, R.; Wine, E.; Kamath, B.M.; Griffiths, A.M.; Ricciuto, A. Gut Microbiome in Primary Sclerosing Cholangitis: A Review. World J. Gastroenterol. 2020, 26, 2768–2780. [Google Scholar] [CrossRef]
- D’Amico, F.; Bertacco, A.; Finotti, M.; di Renzo, C.; Rodriguez-Davalos, M.I.; Gondolesi, G.E.; Cillo, U.; Mulligan, D.; Geibel, J. Bile Microbiota in Liver Transplantation: Proof of Concept Using Gene Amplification in a Heterogeneous Clinical Scenario. Front. Surg. 2021, 8, 621525. [Google Scholar] [CrossRef]
- Bodera, P.; Chcialowski, A. Immunomodulatory Effect of Probiotic Bacteria. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 58–64. [Google Scholar] [CrossRef]
- Ren, Z.; Jiang, J.; Lu, H.; Chen, X.; He, Y.; Zhang, H.; Xie, H.; Wang, W.; Zheng, S.; Zhou, L. Intestinal Microbial Variation May Predict Early Acute Rejection after Liver Transplantation in Rats. Transplantation 2014, 98, 844–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, G.; Hanauer, S.; Levitsky, J. The Role of the Intestine in the Pathogenesis of Primary Sclerosing Cholangitis: Evidence and Therapeutic Implications. Hepatology 2020, 72, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on Immunosuppression in Liver Transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Jorgenson, M.R.; Descourouez, J.L.; Siodlak, M.; Tjugum, S.; Rice, J.P.; Fernandez, L.A. Efficacy and Safety of Probiotics and Synbiotics in Liver Transplantation. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 758–768. [Google Scholar] [CrossRef]
- Shasthry, S.M. Fecal Microbiota Transplantation in Alcohol Related Liver Diseases. Clin. Mol. Hepatol. 2020, 26, 294–301. [Google Scholar] [CrossRef]
- Hassouneh, R.; Bajaj, J.S. Gut Microbiota Modulation and Fecal Transplantation: An Overview on Innovative Strategies for Hepatic Encephalopathy Treatment. J. Clin. Med. 2021, 10, 330. [Google Scholar] [CrossRef]

| Phylum | Family | Genus | Characteristics |
|---|---|---|---|
| Actinobacteria | Micrococcaceae | Rothia | Part of the oral microbiota. Increased abundance within the gut during PSC [109]. Contamination from endoscopic procedures is suspected [109]. |
| Propionibacteriaceae | Cutibacterium | Prevalent on human skin and frequently associated with skin conditions. Increased abundance in the biliary tract in patients with PSC suspected to be contamination from endoscopic procedures [110]. | |
| Bacteroidetes | Bacteroidaceae | Bacteroides | Frequent colonization of the human gut. Increased amount in the gut is found among PSC patients [111], whereas its proportion decreases in advanced cirrhosis patients [34]). |
| Prevotellaceae | Prevotella | Produce butyrate, which promotes intestinal barrier function. In PSC patients, abundance is decreased in the gut [109,111] but increased in the biliary tract [110]. | |
| Tannerellaceae | Parabacteroides | Frequent colonization of the human gut. Increased amount in the gut is found among patients with PSC [112] | |
| Staphylococcaceae | Staphylococcus | Frequent colonization of human skin, nasopharynx, and gut. Therefore, its increased abundance in the biliary tract for patients with cholangitis and other liver diseases is mostly due to contamination from endoscopic procedures [110]. | |
| Firmicutes | Enterococcaceae | Enterococcus | Frequent colonization of the gut and biliary tract. Its abundance is associated with an increase of taurolithocholic acid, a proinflammatory and cancerogenic type of bile acid, therefore frequently associated with cholangitis [110]. Increased abundance in patients with PSC both in the biliary tract and the gut [109,110,112]. In patients with biliary complications, its abundance is increased in the gut [113] but higher in patients with NAS than in patients with anastomotic stricture [13]. |
| Lachnospiraceae | Roseburia | Frequent colonization of the human gut. Can perform 7α-dehydroxylation. Produces primary amines that act as vascular adhesion protein-1, which is critical for effector cell recruitment to the liver [109]. Produces butyrate, which promotes intestinal barrier function. Decreased amount in the gut in PSC patients [109,111] | |
| Lactobacillaceae | Lactobacillus | Frequent colonization of the human digestive system and female genital system. Increased colonic colonization is found in patients with PSC and other live diseases [114]. | |
| Veillonellaceae | Veillonella | Commensal bacteria of human intestines and oral cavity. 70% of the strains are resistant to penicillin [115]. Produces primary amines that act as vascular adhesion protein 1, which is critical for effector cell recruitment to the liver [109]. In patients with PSC, there is increased colonization in the gut and the biliary tract [109,110]. | |
| Megasphaera | Can perform 7α-dehydroxylation. Produce primary amines that act as vascular adhesion protein-1, critical for effector cell recruitment to the liver [109]. Increased amount in the gut in PSC [116]. | ||
| Streptococcaceae | Streptococcus | Part of the oral microbiome. Increased amount in the gastrointestinal and biliary tract in PSC patients [109,110,112,116]. In liver LT patients, increased abundance is associated with biliary stent use due to its biofilm-forming nature [13]. It is increased in both the gastrointestinal and biliary tract for patients with NAS [13,113]. Contamination from endoscopic procedures is suspected [26]. | |
| Clostridiaceae | Clostridium | Play an important role in colonic homeostasis by influencing the Treg and the production of proinflammatory cytokines [11]. Increased amount in the gut is found among PSC patients [111,116], whereas its proportion decreases in advanced cirrhosis patients [34]. | |
| Fusobacteriota | Fusobacteriaceae | Fusobacterium | Increased amount in the gut in PSC patients and associated with CRC cancerogenesis [117]. Increased abundance is associated with biliary stent use due to its biofilm-forming nature [13]. |
| Proteobacteria | Enterobacteriaceae | Escherichia | Commensal bacteria of the gut microbiota. It can perform 7α-dehydroxylation, which converts primary bile acids to secondary bile acids [11]. It also produces primary amines that act as vascular adhesion protein 1, which is critical for effector cell recruitment to the liver [109]. It is associated with increased colonic epithelial oxygen availability, inflammation, epithelial dysfunction, and disease [118]. It is the predominant pathogen of spontaneous bacterial peritonitis in liver cirrhosis [119]. Increased abundance is found in the gut among patients with NAS [113] and end-stage liver disease [34,110,116]. |
| Klebsiella | Increased in patients with NAS [113]. In addition, it frequently causes spontaneous bacterial peritonitis [119]. | ||
| Pseudomonadaceae | Pseudomonas | A frequent cause of infections in patients with end-stage liver disease and cholangitis [120]. Increased in the gut among patients with NAS [113] | |
| Neisseriaceae | Neisseria | Linked to H2S production that can damage deoxyribonucleic acid [119]. Increased colonization in the biliary traction during PSC [110,119]. | |
| Sphingomonadaceae | Sphingomonas | Express amine oxidases and associated with aberrant homing of gut lymphocytes to the liver [111]. Increased amount in the gut in PSC patients [111]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirth, U.; Jiang, T.; Schardey, J.; Kratz, K.; Li, M.; Schirren, M.; Kühn, F.; Bazhin, A.; Werner, J.; Guba, M.; et al. The Role of Microbiota in Liver Transplantation and Liver Transplantation-Related Biliary Complications. Int. J. Mol. Sci. 2023, 24, 4841. https://doi.org/10.3390/ijms24054841
Wirth U, Jiang T, Schardey J, Kratz K, Li M, Schirren M, Kühn F, Bazhin A, Werner J, Guba M, et al. The Role of Microbiota in Liver Transplantation and Liver Transplantation-Related Biliary Complications. International Journal of Molecular Sciences. 2023; 24(5):4841. https://doi.org/10.3390/ijms24054841
Chicago/Turabian StyleWirth, Ulrich, Tianxiao Jiang, Josefine Schardey, Katharina Kratz, Mingming Li, Malte Schirren, Florian Kühn, Alexandr Bazhin, Jens Werner, Markus Guba, and et al. 2023. "The Role of Microbiota in Liver Transplantation and Liver Transplantation-Related Biliary Complications" International Journal of Molecular Sciences 24, no. 5: 4841. https://doi.org/10.3390/ijms24054841





