The Role of Histone Modification in DNA Replication-Coupled Nucleosome Assembly and Cancer
Abstract
:1. Introduction
2. Posttranslational Modifications of Histones
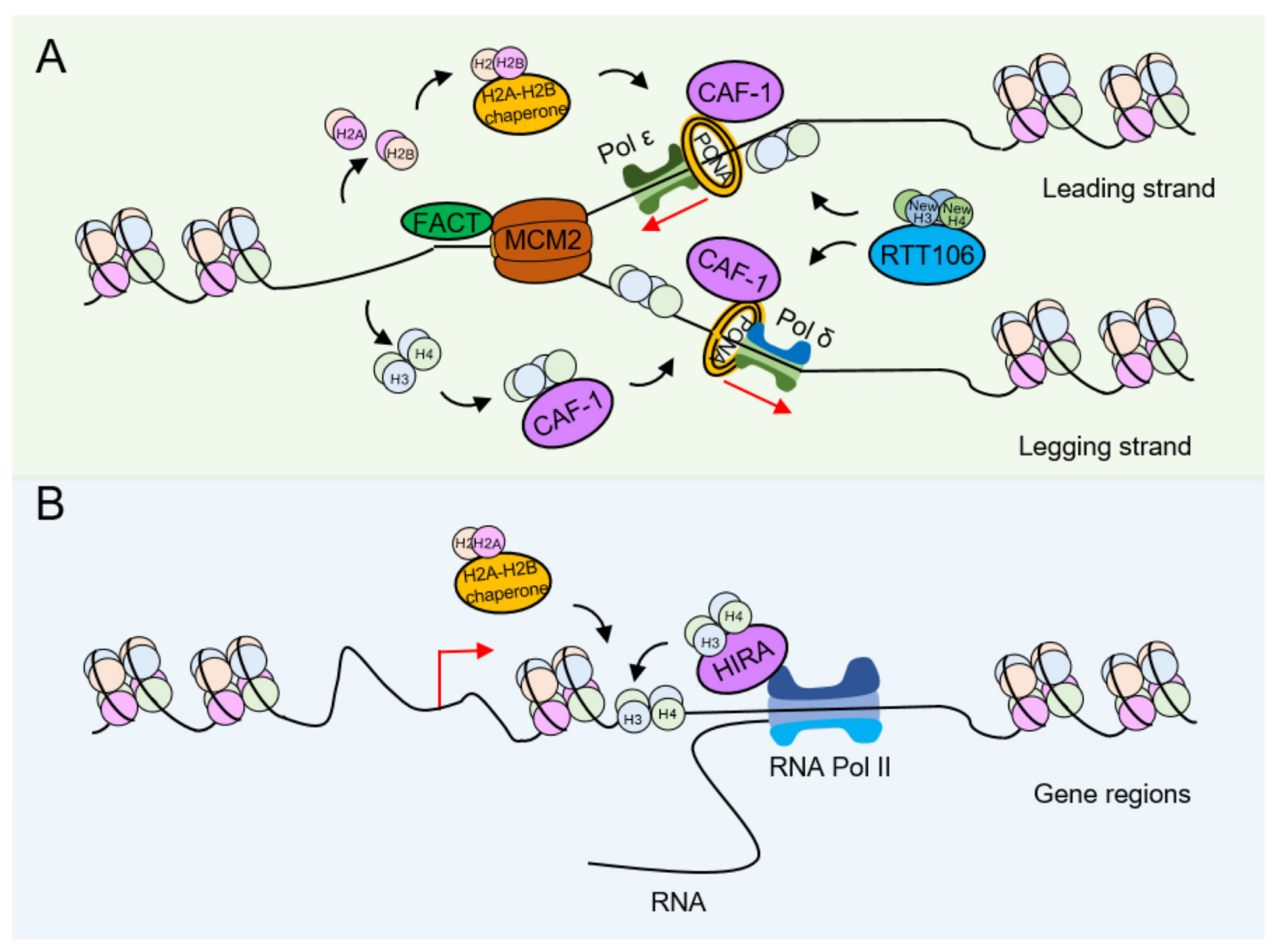
3. Nucleosome Assembly
4. The Role of Histone Modification in Nucleosome Assembly
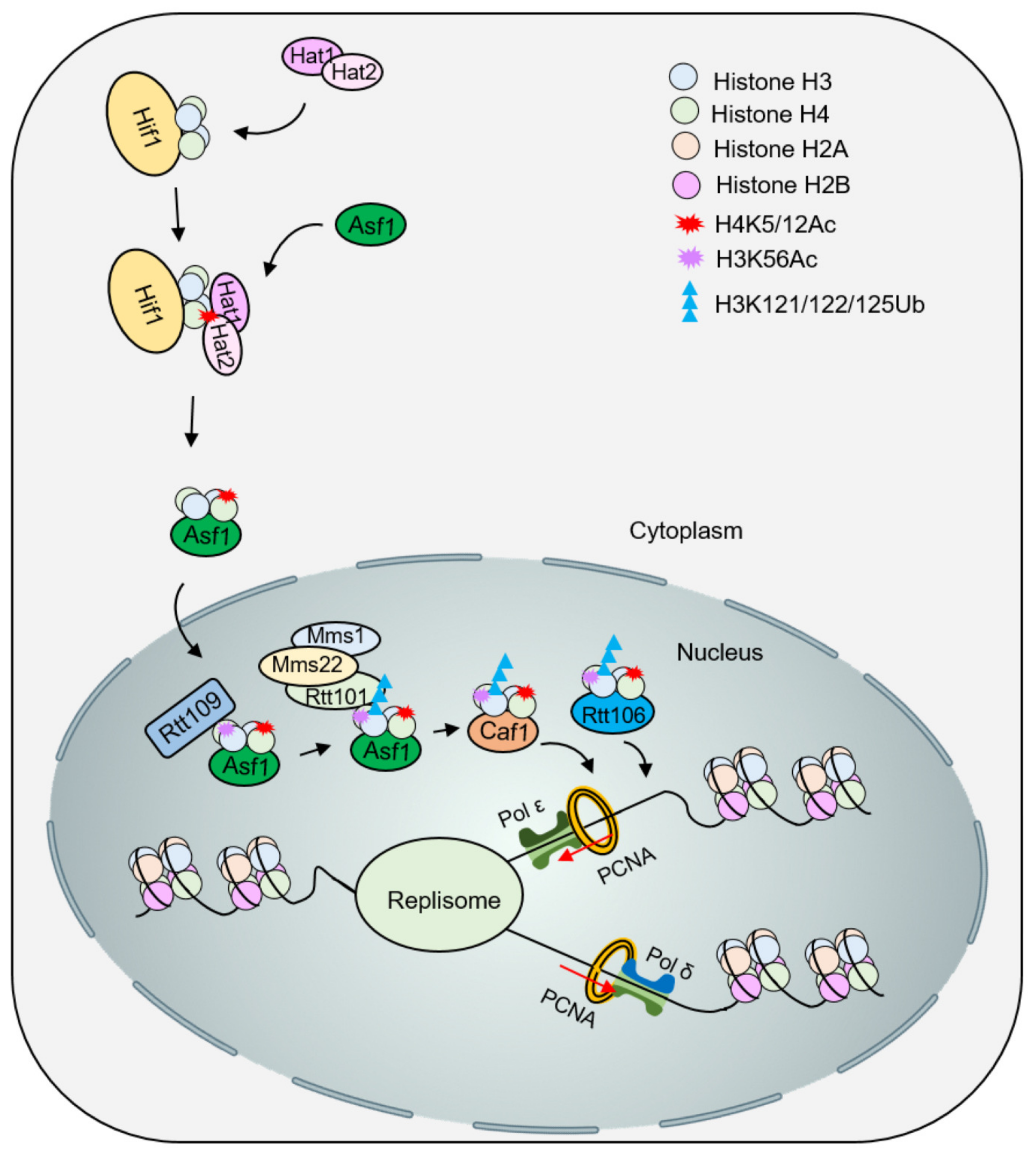
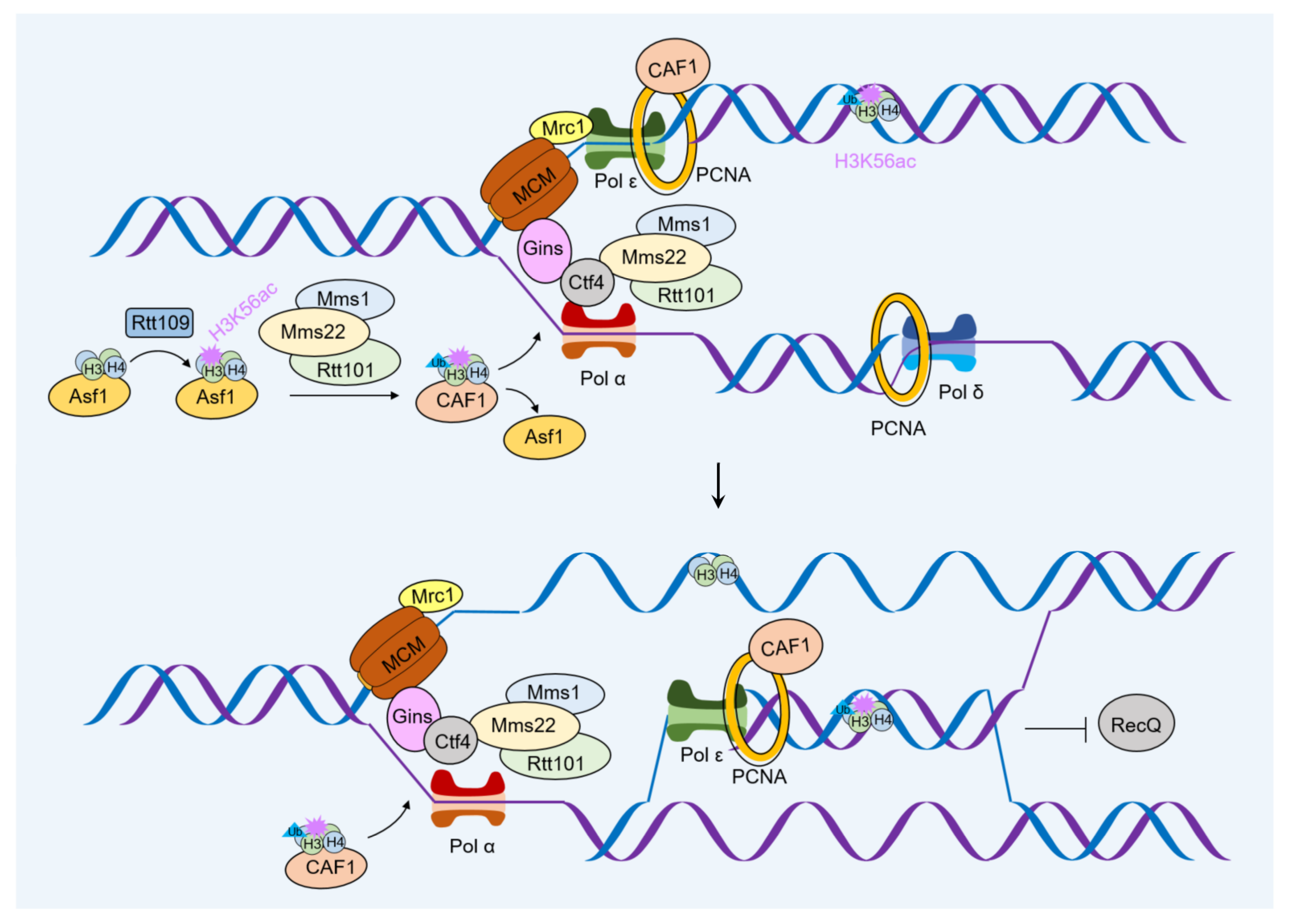
4.1. Influence of Histone Modification on the Deposition of Newly Synthesized Histones
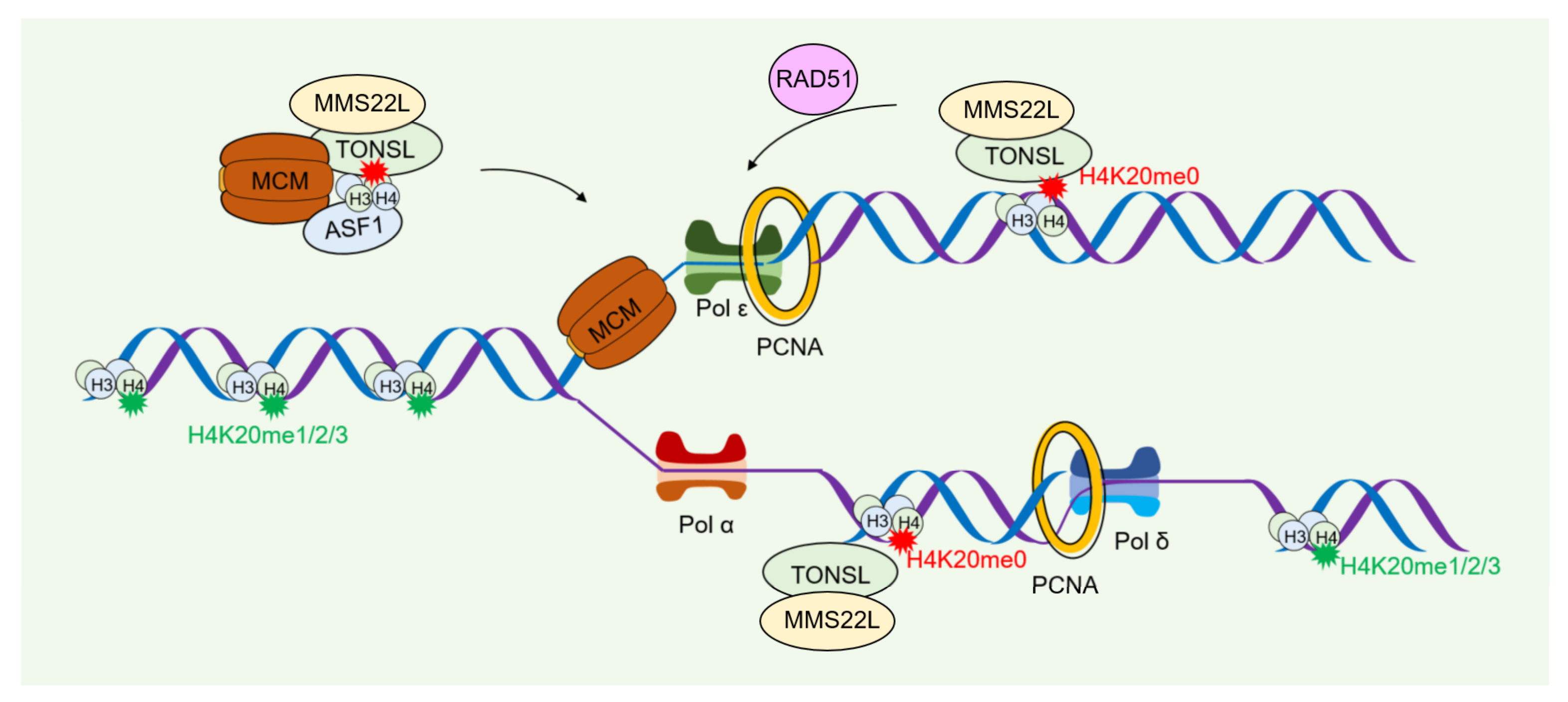
4.2. Role of Histone Modification in DNA Damage Repair during Nucleosome Assembly
5. Histone Modification and Cancer
5.1. Histone Methylation in Cancer
| Gene | Tumor | Role | Reference |
|---|---|---|---|
| SETD1A | Colorectal cancer, Lung cancer, Gastric cancer | Oncogenic | [114,115,116] |
| SETD1B | Pancancer | Suppressor | [117] |
| MLL1 | Breast cancer, Cervical carcinoma, Acute myeloid leukemia | Oncogenic | [118,119,120] |
| MLL2 | Bland cancer, Prostate cancer | Suppressor | [121,122] |
| MLL3 | Nasopharyngeal carcinoma, Breast cancer, Pancreatic cancer, Colorectal cancer | Ambiguous | [123,124,125,126] |
| MLL4 | Lung cancer, Medulloblastoma, | Suppressor | [127,128] |
| SMYD2 | Lung cancer, Cervical cancer | Oncogene | [129,130] |
| SET7 | Glioma, Colorectal cancer, Lung cancer | Suppressor | [131,132,133] |
| SET9 | Glioma, Lung cancer, Breast cancer | Suppressor | [131,133,134] |
| SMYD3 | Pancreatic cancer, Lung cancer, Breast cancer | Overexpression | [135,136,137] |
| SUV39H1 | Cervical cancer, Prostate cancer, Melanoma | Oncogene | [138,139,140] |
| SUV39H2 | Colorectal cancer, Osteosarcoma, Lung cancer | Oncogene | [141,142,143] |
| G9A | Colorectal cancer, Bladder cancer, Lung cancer, Breast cancer | Oncogene | [144,145,146,147] |
| SETDB1 | Lung cancer, Gastric cancer, Colorectal cancer | Oncogenic | [148,149,150] |
| PRDM3 | Pancreatic ductal adenocarcinoma | Suppressor | [151] |
| PRDM16 | Kidney cancer, Prostrate cancer, Lung cancer | Suppressor | [152,153,154] |
| EZH1 | Breast cancer, Hepatocellular carcinoma | Oncogenic | [155,156] |
| EZH2 | Lung cancer, Hepatocellular carcinoma, Breast cancer, Gastric cancer, Colorectal cancer | Oncogene | [156,157,158,159,160] |
| SETD2 | Prostate cancer, Pancreatic cancer, Leukemogenesis, Hepatocellular carcinoma | Suppressor | [161,162,163,164] |
| NSD1 | Pancreatic cancer, Laryngeal cancer | Oncogenic | [165,166] |
| NSD2 | Lung cancer, Colorectal cancer, Breast cancer, Renal cancer, Osteosarcoma, Prostate cancer | Oncogene | [167,168,169,170,171,172] |
| NSD3 | Lung cancer, Breast cancer, Colorectal cancer, Pancreatic cancer | Oncogene | [173,174,175] |
| SETD3 | Breast cancer, Hepatocellular carcinoma | Overexpression | [176,177] |
| ASH1L | Prostate cancer, Leukemia | Oncogenic | [178,179] |
| SETMAR | Acute myeloid leukemia | Suppressor | [180] |
| PRDM9 | Pancancer | Overexpression | [181] |
| DOT1L | Prostate cancer, Colorectal cancer, Gastric cancer, Ovarian cancer, Breast cancer, Acute myeloid leukemia | Overexpression | [119,182,183,184,185,186] |
| SET8 | Prostate cancer, Hepatocellular carcinoma, Breast cancer | Oncogenic | [187,188,189] |
| SUV4-20H2 | Hepatocellular carcinoma, Breast cancer | Suppressor | [190,191] |
5.2. Histone Acetylation in Cancer
5.3. Histone Ubiquitination in Cancer
5.4. Histone Phosphorylation in Cancer
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luger, K.; Mader, A.W.; Richmond, R.K.; Sargent, D.F.; Richmond, T.J. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997, 389, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Coradin, M.; Porter, E.G.; Garcia, B.A. Accelerating the Field of Epigenetic Histone Modification Through Mass Spectrometry-Based Approaches. Mol. Cell Proteom. 2021, 20, 100006. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y. Mapping histone modification-dependent protein interactions with chemical proteomics. Trends Biochem. Sci. 2022, 47, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; Jiang, H.; Yang, P.; Lu, H. Fishing the PTM proteome with chemical approaches using functional solid phases. Chem. Soc. Rev. 2015, 44, 8260–8287. [Google Scholar] [CrossRef]
- Allfrey, V.G.; Faulkner, R.; Mirsky, A.E. Acetylation and Methylation of Histones and Their Possible Role in the Regulation of Rna Synthesis. Proc. Natl. Acad. Sci. USA 1964, 51, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Grunstein, M.; Allis, C.D. Genetics, Biochemistry, and "Simple" Organisms Converge to Unlock Secrets in Histone Biology: The 2018 Albert Lasker Basic Medical Research Award. JAMA 2018, 320, 1233–1234. [Google Scholar] [CrossRef]
- Yun, M.; Wu, J.; Workman, J.L.; Li, B. Readers of histone modifications. Cell Res. 2011, 21, 564–578. [Google Scholar] [CrossRef] [Green Version]
- Prakash, K.; Fournier, D. Histone Code and Higher-Order Chromatin Folding: A Hypothesis. Genom. Comput. Biol. 2017, 3, e41. [Google Scholar] [CrossRef] [Green Version]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [Green Version]
- Soffers, J.H.; Li, X.; Abmayr, S.M.; Workman, J.L. Reading and Interpreting the Histone Acylation Code. Genom. Proteom. Bioinform. 2016, 14, 329–332. [Google Scholar] [CrossRef]
- Arnaudo, A.M.; Garcia, B.A. Proteomic characterization of novel histone post-translational modifications. Epigenet. Chromatin 2013, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Blackledge, N.P.; Klose, R.J. Histone lysine methylation: An epigenetic modification? Epigenomics 2010, 2, 151–161. [Google Scholar] [CrossRef]
- Wang, X.; Hayes, J.J. Site-specific binding affinities within the H2B tail domain indicate specific effects of lysine acetylation. J. Biol. Chem. 2007, 282, 32867–32876. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Nair, D.; Guan, X.; Rastogi, N.; Freitas, M.A.; Parthun, M.R. Sites of acetylation on newly synthesized histone H4 are required for chromatin assembly and DNA damage response signaling. Mol. Cell. Biol. 2013, 33, 3286–3298. [Google Scholar] [CrossRef] [Green Version]
- Osley, M.A. Regulation of histone H2A and H2B ubiquitylation. Brief. Funct. Genom. 2006, 5, 179–189. [Google Scholar] [CrossRef]
- Weake, V.M.; Workman, J.L. Histone ubiquitination: Triggering gene activity. Mol. Cell 2008, 29, 653–663. [Google Scholar] [CrossRef]
- Burgess, R.J.; Zhang, Z. Histones, histone chaperones and nucleosome assembly. Protein Cell 2010, 1, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.; Kumar, A.; Sharma, A. Structural comparisons reveal diverse binding modes between nucleosome assembly proteins and histones. Epigenet. Chromatin 2022, 15, 20. [Google Scholar] [CrossRef]
- Sauer, P.V.; Gu, Y.; Liu, W.H.; Mattiroli, F.; Panne, D.; Luger, K.; Churchill, M.E. Mechanistic insights into histone deposition and nucleosome assembly by the chromatin assembly factor-1. Nucleic Acids Res. 2018, 46, 9907–9917. [Google Scholar] [CrossRef] [Green Version]
- Groth, A.; Rocha, W.; Verreault, A.; Almouzni, G. Chromatin challenges during DNA replication and repair. Cell 2007, 128, 721–733. [Google Scholar] [CrossRef] [Green Version]
- Burgess, R.J.; Zhang, Z. Histone chaperones in nucleosome assembly and human disease. Nat. Struct. Mol. Biol. 2013, 20, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, X.; Feng, J.; Leng, H.; Li, S.; Xiao, J.; Liu, S.; Xu, Z.; Xu, J.; Li, D.; et al. The Histone Chaperone FACT Contributes to DNA Replication-Coupled Nucleosome Assembly. Cell Rep. 2016, 16, 3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, C.; Tyler, J.K.; Churchill, M.E. The histone shuffle: Histone chaperones in an energetic dance. Trends Biochem. Sci. 2010, 35, 476–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabert, C.; Groth, A. Chromatin replication and epigenome maintenance. Nat. Rev. Mol. Cell Biol. 2012, 13, 153–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziato, A.T. Assembling chromatin: The long and winding road. Biochim. Biophys. Acta 2013, 1819, 196–210. [Google Scholar] [CrossRef]
- Gurard-Levin, Z.A.; Quivy, J.P.; Almouzni, G. Histone chaperones: Assisting histone traffic and nucleosome dynamics. Annu. Rev. Biochem. 2014, 83, 487–517. [Google Scholar] [CrossRef]
- Osley, M.A. The regulation of histone synthesis in the cell cycle. Annu. Rev. Biochem. 1991, 60, 827–861. [Google Scholar] [CrossRef]
- Marzluff, W.F.; Duronio, R.J. Histone mRNA expression: Multiple levels of cell cycle regulation and important developmental consequences. Curr. Opin. Cell Biol. 2002, 14, 692–699. [Google Scholar] [CrossRef]
- Ling, X.; Harkness, T.A.; Schultz, M.C.; Fisher-Adams, G.; Grunstein, M. Yeast histone H3 and H4 amino termini are important for nucleosome assembly in vivo and in vitro: Redundant and position-independent functions in assembly but not in gene regulation. Genes Dev. 1996, 10, 686–699. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Yang, W.S.; Zhang, L.; Liu, C.P.; Xu, R.M. Topography of histone H3-H4 interaction with the Hat1-Hat2 acetyltransferase complex. Genes Dev. 2022, 36, 408–413. [Google Scholar] [CrossRef]
- Fillingham, J.; Recht, J.; Silva, A.C.; Suter, B.; Emili, A.; Stagljar, I.; Krogan, N.J.; Allis, C.D.; Keogh, M.C.; Greenblatt, J.F. Chaperone control of the activity and specificity of the histone H3 acetyltransferase Rtt109. Mol. Cell. Biol. 2008, 28, 4342–4353. [Google Scholar] [CrossRef] [Green Version]
- Burgess, R.J.; Zhou, H.; Han, J.; Zhang, Z. A role for Gcn5 in replication-coupled nucleosome assembly. Mol. Cell 2010, 37, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Ai, X.; Eugeni, E.E.; Zhang, L.; Carpenter, L.R.; Jelinek, M.A.; Freitas, M.A.; Parthun, M.R. Histone H4 lysine 91 acetylation a core domain modification associated with chromatin assembly. Mol. Cell 2005, 18, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhou, H.; Li, Z.; Xu, R.M.; Zhang, Z. Acetylation of lysine 56 of histone H3 catalyzed by RTT109 and regulated by ASF1 is required for replisome integrity. J. Biol. Chem. 2007, 282, 28587–28596. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhou, H.; Horazdovsky, B.; Zhang, K.; Xu, R.M.; Zhang, Z. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 2007, 315, 653–655. [Google Scholar] [CrossRef]
- Jasencakova, Z.; Scharf, A.N.; Ask, K.; Corpet, A.; Imhof, A.; Almouzni, G.; Groth, A. Replication stress interferes with histone recycling and predeposition marking of new histones. Mol. Cell 2010, 37, 736–743. [Google Scholar] [CrossRef]
- Tsubota, T.; Berndsen, C.E.; Erkmann, J.A.; Smith, C.L.; Yang, L.; Freitas, M.A.; Denu, J.M.; Kaufman, P.D. Histone H3-K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol. Cell 2007, 25, 703–712. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhou, H.; Wurtele, H.; Davies, B.; Horazdovsky, B.; Verreault, A.; Zhang, Z. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 2008, 134, 244–255. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhang, H.; Zhang, H.; Wang, Z.; Zhou, H.; Zhang, Z. A Cul4 E3 ubiquitin ligase regulates histone hand-off during nucleosome assembly. Cell 2013, 155, 817–829. [Google Scholar] [CrossRef] [Green Version]
- Rodriges Blanko, E.; Kadyrova, L.Y.; Kadyrov, F.A. DNA Mismatch Repair Interacts with CAF-1- and ASF1A-H3-H4-dependent Histone (H3-H4)2 Tetramer Deposition. J. Biol. Chem. 2016, 291, 9203–9217. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.H.; Roemer, S.C.; Zhou, Y.; Shen, Z.J.; Dennehey, B.K.; Balsbaugh, J.L.; Liddle, J.C.; Nemkov, T.; Ahn, N.G.; Hansen, K.C.; et al. The Cac1 subunit of histone chaperone CAF-1 organizes CAF-1-H3/H4 architecture and tetramerizes histones. eLife 2016, 5, e18023. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Setiaputra, D.; Jung, T.; Chung, J.; Leitner, A.; Yoon, J.; Aebersold, R.; Hebert, H.; Yip, C.K.; Song, J.J. Molecular Architecture of Yeast Chromatin Assembly Factor 1. Sci. Rep. 2016, 6, 26702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Gao, Y.; Li, J.; Burgess, R.; Han, J.; Liang, H.; Zhang, Z.; Liu, Y. A DNA binding winged helix domain in CAF-1 functions with PCNA to stabilize CAF-1 at replication forks. Nucleic Acids Res. 2016, 44, 5083–5094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolef Ben-Shahar, T.; Castillo, A.G.; Osborne, M.J.; Borden, K.L.; Kornblatt, J.; Verreault, A. Two fundamentally distinct PCNA interaction peptides contribute to chromatin assembly factor 1 function. Mol. Cell. Biol. 2009, 29, 6353–6365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.H.; Roemer, S.C.; Port, A.M.; Churchill, M.E.A. CAF-1-induced oligomerization of histones H3/H4 and mutually exclusive interactions with Asf1 guide H3/H4 transitions among histone chaperones and DNA. Nucleic Acids Res. 2017, 45, 9809. [Google Scholar] [CrossRef] [Green Version]
- Soniat, M.; Cagatay, T.; Chook, Y.M. Recognition Elements in the Histone H3 and H4 Tails for Seven Different Importins. J. Biol. Chem. 2016, 291, 21171–21183. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Hu, Q.; Li, Q.; Thompson, J.R.; Cui, G.; Fazly, A.; Davies, B.A.; Botuyan, M.V.; Zhang, Z.; Mer, G. Structural basis for recognition of H3K56-acetylated histone H3-H4 by the chaperone Rtt106. Nature 2012, 483, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Fazly, A.; Li, Q.; Hu, Q.; Mer, G.; Horazdovsky, B.; Zhang, Z. Histone chaperone Rtt106 promotes nucleosome formation using (H3-H4)2 tetramers. J. Biol. Chem. 2012, 287, 10753–10760. [Google Scholar] [CrossRef] [Green Version]
- Piquet, S.; Le Parc, F.; Bai, S.K.; Chevallier, O.; Adam, S.; Polo, S.E. The Histone Chaperone FACT Coordinates H2A.X-Dependent Signaling and Repair of DNA Damage. Mol. Cell 2018, 72, 888–901.e7. [Google Scholar] [CrossRef] [Green Version]
- Marechal, A.; Zou, L. RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 2015, 25, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xu, Z.; Leng, H.; Zheng, P.; Yang, J.; Chen, K.; Feng, J.; Li, Q. RPA binds histone H3-H4 and functions in DNA replication-coupled nucleosome assembly. Science 2017, 355, 415–420. [Google Scholar] [CrossRef]
- Osakabe, A.; Tachiwana, H.; Matsunaga, T.; Shiga, T.; Nozawa, R.S.; Obuse, C.; Kurumizaka, H. Nucleosome formation activity of human somatic nuclear autoantigenic sperm protein (sNASP). J. Biol. Chem. 2010, 285, 11913–11921. [Google Scholar] [CrossRef] [Green Version]
- Apta-Smith, M.J.; Hernandez-Fernaud, J.R.; Bowman, A.J. Evidence for the nuclear import of histones H3.1 and H4 as monomers. EMBO J. 2018, 37, e98714. [Google Scholar] [CrossRef]
- Ask, K.; Jasencakova, Z.; Menard, P.; Feng, Y.; Almouzni, G.; Groth, A. Codanin-1, mutated in the anaemic disease CDAI, regulates Asf1 function in S-phase histone supply. EMBO J. 2012, 31, 2013–2023. [Google Scholar] [CrossRef]
- Tang, Y.; Holbert, M.A.; Wurtele, H.; Meeth, K.; Rocha, W.; Gharib, M.; Jiang, E.; Thibault, P.; Verreault, A.; Cole, P.A.; et al. Fungal Rtt109 histone acetyltransferase is an unexpected structural homolog of metazoan p300/CBP. Nat. Struct. Mol. Biol. 2008, 15, 738–745. [Google Scholar] [CrossRef]
- Saxena, S.; Zou, L. Hallmarks of DNA replication stress. Mol. Cell 2022, 82, 2298–2314. [Google Scholar] [CrossRef]
- Ashour, M.E.; Mosammaparast, N. Mechanisms of damage tolerance and repair during DNA replication. Nucleic Acids Res. 2021, 49, 3033–3047. [Google Scholar] [CrossRef]
- Uckelmann, M.; Sixma, T.K. Histone ubiquitination in the DNA damage response. DNA Repair 2017, 56, 92–101. [Google Scholar] [CrossRef]
- Cortez, D. Replication-Coupled DNA Repair. Mol. Cell 2019, 74, 866–876. [Google Scholar] [CrossRef] [Green Version]
- Prado, F. Homologous recombination maintenance of genome integrity during DNA damage tolerance. Mol. Cell Oncol. 2014, 1, e957039. [Google Scholar] [CrossRef] [Green Version]
- Sale, J.E.; Lehmann, A.R.; Woodgate, R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat. Rev. Mol. Cell Biol. 2012, 13, 141–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.K.; Hendzel, M.J. The relationship between histone posttranslational modification and DNA damage signaling and repair. Int. J. Radiat. Biol. 2019, 95, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Shi, G.; Shan, C.M.; Li, Z.; Zhu, B.; Jia, S.; Li, Q.; Zhang, Z. Mechanisms of chromatin-based epigenetic inheritance. Sci. China Life Sci. 2022, 65, 2162–2190. [Google Scholar] [CrossRef] [PubMed]
- Burgers, P.M.J.; Kunkel, T.A. Eukaryotic DNA Replication Fork. Annu. Rev. Biochem. 2017, 86, 417–438. [Google Scholar] [CrossRef]
- Guilliam, T.A.; Yeeles, J.T.P. An updated perspective on the polymerase division of labor during eukaryotic DNA replication. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 469–481. [Google Scholar] [CrossRef]
- Buser, R.; Kellner, V.; Melnik, A.; Wilson-Zbinden, C.; Schellhaas, R.; Kastner, L.; Piwko, W.; Dees, M.; Picotti, P.; Maric, M.; et al. The Replisome-Coupled E3 Ubiquitin Ligase Rtt101Mms22 Counteracts Mrc1 Function to Tolerate Genotoxic Stress. PLoS Genet. 2016, 12, e1005843. [Google Scholar] [CrossRef] [Green Version]
- Piwko, W.; Mlejnkova, L.J.; Mutreja, K.; Ranjha, L.; Stafa, D.; Smirnov, A.; Brodersen, M.M.; Zellweger, R.; Sturzenegger, A.; Janscak, P.; et al. The MMS22L-TONSL heterodimer directly promotes RAD51-dependent recombination upon replication stress. EMBO J. 2016, 35, 2584–2601. [Google Scholar] [CrossRef] [Green Version]
- Daboussi, F.; Courbet, S.; Benhamou, S.; Kannouche, P.; Zdzienicka, M.Z.; Debatisse, M.; Lopez, B.S. A homologous recombination defect affects replication-fork progression in mammalian cells. J. Cell Sci. 2008, 121, 162–166. [Google Scholar] [CrossRef] [Green Version]
- Tjeertes, J.V.; Miller, K.M.; Jackson, S.P. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 2009, 28, 1878–1889. [Google Scholar] [CrossRef] [Green Version]
- Saredi, G.; Huang, H.; Hammond, C.M.; Alabert, C.; Bekker-Jensen, S.; Forne, I.; Reveron-Gomez, N.; Foster, B.M.; Mlejnkova, L.; Bartke, T.; et al. H4K20me0 marks post-replicative chromatin and recruits the TONSL-MMS22L DNA repair complex. Nature 2016, 534, 714–718. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, L.; Panier, S.; Wildenhain, J.; Tkach, J.M.; Al-Hakim, A.; Landry, M.C.; Escribano-Diaz, C.; Szilard, R.K.; Young, J.T.; Munro, M.; et al. The MMS22L-TONSL complex mediates recovery from replication stress and homologous recombination. Mol. Cell 2010, 40, 619–631. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, A.; Spicuglia, S.; Lasonder, E.; Vermeulen, M.; Campsteijn, C.; Stunnenberg, H.G.; Logie, C. Characterization of lysine 56 of histone H3 as an acetylation site in Saccharomyces cerevisiae. J. Biol. Chem. 2005, 280, 25949–25952. [Google Scholar] [CrossRef] [Green Version]
- Maas, N.L.; Miller, K.M.; DeFazio, L.G.; Toczyski, D.P. Cell cycle and checkpoint regulation of histone H3 K56 acetylation by Hst3 and Hst4. Mol. Cell 2006, 23, 109–119. [Google Scholar] [CrossRef]
- Masumoto, H.; Hawke, D.; Kobayashi, R.; Verreault, A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature 2005, 436, 294–298. [Google Scholar] [CrossRef]
- Collins, S.R.; Miller, K.M.; Maas, N.L.; Roguev, A.; Fillingham, J.; Chu, C.S.; Schuldiner, M.; Gebbia, M.; Recht, J.; Shales, M.; et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 2007, 446, 806–810. [Google Scholar] [CrossRef]
- Wurtele, H.; Kaiser, G.S.; Bacal, J.; St-Hilaire, E.; Lee, E.H.; Tsao, S.; Dorn, J.; Maddox, P.; Lisby, M.; Pasero, P.; et al. Histone H3 lysine 56 acetylation and the response to DNA replication fork damage. Mol. Cell. Biol. 2012, 32, 154–172. [Google Scholar] [CrossRef] [Green Version]
- Tessarz, P.; Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef]
- Clemente-Ruiz, M.; Gonzalez-Prieto, R.; Prado, F. Histone H3K56 acetylation, CAF1, and Rtt106 coordinate nucleosome assembly and stability of advancing replication forks. PLoS Genet. 2011, 7, e1002376. [Google Scholar] [CrossRef] [Green Version]
- Luke, B.; Versini, G.; Jaquenoud, M.; Zaidi, I.W.; Kurz, T.; Pintard, L.; Pasero, P.; Peter, M. The cullin Rtt101p promotes replication fork progression through damaged DNA and natural pause sites. Curr. Biol. 2006, 16, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Duro, E.; Vaisica, J.A.; Brown, G.W.; Rouse, J. Budding yeast Mms22 and Mms1 regulate homologous recombination induced by replisome blockage. DNA Repair 2008, 7, 811–818. [Google Scholar] [CrossRef]
- Erkmann, J.A.; Kaufman, P.D. A negatively charged residue in place of histone H3K56 supports chromatin assembly factor association but not genotoxic stress resistance. DNA Repair 2009, 8, 1371–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Li, Q.; McCullough, L.; Kettelkamp, C.; Formosa, T.; Zhang, Z. Ubiquitylation of FACT by the cullin-E3 ligase Rtt101 connects FACT to DNA replication. Genes Dev. 2010, 24, 1485–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhai, L.; Xu, J.; Joo, H.Y.; Jackson, S.; Erdjument-Bromage, H.; Tempst, P.; Xiong, Y.; Zhang, Y. Histone H3 and H4 ubiquitylation by the CUL4-DDB-ROC1 ubiquitin ligase facilitates cellular response to DNA damage. Mol. Cell 2006, 22, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; McCall, C.M.; Ohta, T.; Xiong, Y. Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat. Cell Biol. 2004, 6, 1003–1009. [Google Scholar] [CrossRef]
- Formosa, T. The role of FACT in making and breaking nucleosomes. Biochim. Biophys. Acta 2013, 1819, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Gambus, A.; Jones, R.C.; Sanchez-Diaz, A.; Kanemaki, M.; van Deursen, F.; Edmondson, R.D.; Labib, K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 2006, 8, 358–366. [Google Scholar] [CrossRef]
- Murawska, M.; Schauer, T.; Matsuda, A.; Wilson, M.D.; Pysik, T.; Wojcik, F.; Muir, T.W.; Hiraoka, Y.; Straub, T.; Ladurner, A.G. The Chaperone FACT and Histone H2B Ubiquitination Maintain S. pombe Genome Architecture through Genic and Subtelomeric Functions. Mol. Cell 2020, 77, 501–513.e7. [Google Scholar] [CrossRef]
- Moyal, L.; Lerenthal, Y.; Gana-Weisz, M.; Mass, G.; So, S.; Wang, S.Y.; Eppink, B.; Chung, Y.M.; Shalev, G.; Shema, E.; et al. Requirement of ATM-dependent monoubiquitylation of histone H2B for timely repair of DNA double-strand breaks. Mol. Cell 2011, 41, 529–542. [Google Scholar] [CrossRef]
- Chandrasekharan, M.B.; Huang, F.; Sun, Z.W. Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc. Natl. Acad. Sci. USA 2009, 106, 16686–16691. [Google Scholar] [CrossRef] [Green Version]
- Fleming, A.B.; Kao, C.F.; Hillyer, C.; Pikaart, M.; Osley, M.A. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol. Cell 2008, 31, 57–66. [Google Scholar] [CrossRef]
- Wang, P.; Yang, W.; Zhao, S.; Nashun, B. Regulation of chromatin structure and function: Insights into the histone chaperone FACT. Cell Cycle 2021, 20, 465–479. [Google Scholar] [CrossRef]
- Le, S.; Davis, C.; Konopka, J.B.; Sternglanz, R. Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast 1997, 13, 1029–1042. [Google Scholar] [CrossRef]
- Tyler, J.K.; Adams, C.R.; Chen, S.R.; Kobayashi, R.; Kamakaka, R.T.; Kadonaga, J.T. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 1999, 402, 555–560. [Google Scholar] [CrossRef]
- Groth, A.; Corpet, A.; Cook, A.J.; Roche, D.; Bartek, J.; Lukas, J.; Almouzni, G. Regulation of replication fork progression through histone supply and demand. Science 2007, 318, 1928–1931. [Google Scholar] [CrossRef]
- Sanematsu, F.; Takami, Y.; Barman, H.K.; Fukagawa, T.; Ono, T.; Shibahara, K.I.; Nakayama, T. Asf1 is required for viability and chromatin assembly during DNA replication in vertebrate cells. J. Biol. Chem. 2006, 281, 13817–13827. [Google Scholar] [CrossRef] [Green Version]
- Pietrobon, V.; Freon, K.; Hardy, J.; Costes, A.; Iraqui, I.; Ochsenbein, F.; Lambert, S.A. The chromatin assembly factor 1 promotes Rad51-dependent template switches at replication forks by counteracting D-loop disassembly by the RecQ-type helicase Rqh1. PLoS Biol. 2014, 12, e1001968. [Google Scholar] [CrossRef] [Green Version]
- Jiao, R.; Bachrati, C.Z.; Pedrazzi, G.; Kuster, P.; Petkovic, M.; Li, J.L.; Egli, D.; Hickson, I.D.; Stagljar, I. Physical and functional interaction between the Bloom’s syndrome gene product and the largest subunit of chromatin assembly factor 1. Mol. Cell. Biol. 2004, 24, 4710–4719. [Google Scholar] [CrossRef] [Green Version]
- Lazo, P.A. Targeting Histone Epigenetic Modifications and DNA Damage Responses in Synthetic Lethality Strategies in Cancer? Cancers 2022, 14, 4050. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [Green Version]
- Nacev, B.A.; Feng, L.; Bagert, J.D.; Lemiesz, A.E.; Gao, J.; Soshnev, A.A.; Kundra, R.; Schultz, N.; Muir, T.W.; Allis, C.D. The expanding landscape of ‘oncohistone’ mutations in human cancers. Nature 2019, 567, 473–478. [Google Scholar] [CrossRef]
- Espiritu, D.; Gribkova, A.K.; Gupta, S.; Shaytan, A.K.; Panchenko, A.R. Molecular Mechanisms of Oncogenesis through the Lens of Nucleosomes and Histones. J. Phys. Chem. B 2021, 125, 3963–3976. [Google Scholar] [CrossRef] [PubMed]
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Aliev, G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin. Cancer Biol. 2022, 83, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Z.; Jia, J.; Du, T.; Zhang, N.; Tang, Y.; Fang, Y.; Fang, D. Overview of Histone Modification. In Histone Mutations and Cancer; Advances in Experimental Medicine and Biology; Springer: Singapore, 2021; Volume 1283, pp. 1–16. [Google Scholar] [CrossRef] [PubMed]
- McCleland, M.L.; Soukup, T.M.; Liu, S.D.; Esensten, J.H.; de Sousa e Melo, F.; Yaylaoglu, M.; Warming, S.; Roose-Girma, M.; Firestein, R. Cdk8 deletion in the Apc(Min) murine tumour model represses EZH2 activity and accelerates tumourigenesis. J. Pathol. 2015, 237, 508–519. [Google Scholar] [CrossRef]
- Mohammad, F.; Helin, K. Oncohistones: Drivers of pediatric cancers. Genes Dev. 2017, 31, 2313–2324. [Google Scholar] [CrossRef] [Green Version]
- Gsell, C.; Richly, H.; Coin, F.; Naegeli, H. A chromatin scaffold for DNA damage recognition: How histone methyltransferases prime nucleosomes for repair of ultraviolet light-induced lesions. Nucleic Acids Res. 2020, 48, 1652–1668. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zhou, Y.; Zhuang, X.; Zhu, Y.; Wu, Z.; Lu, Y.; Li, S.; Zeng, Y.; Lu, Q.R.; Huo, Y.; et al. HDAC3 Deficiency Promotes Liver Cancer through a Defect in H3K9ac/H3K9me3 Transition. Cancer Res. 2019, 79, 3676–3688. [Google Scholar] [CrossRef]
- Michalak, E.M.; Burr, M.L.; Bannister, A.J.; Dawson, M.A. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Duan, R.; Du, W.; Guo, W. EZH2: A novel target for cancer treatment. J. Hematol. Oncol. 2020, 13, 104. [Google Scholar] [CrossRef]
- Lima-Fernandes, E.; Murison, A.; da Silva Medina, T.; Wang, Y.; Ma, A.; Leung, C.; Luciani, G.M.; Haynes, J.; Pollett, A.; Zeller, C.; et al. Targeting bivalency de-represses Indian Hedgehog and inhibits self-renewal of colorectal cancer-initiating cells. Nat. Commun. 2019, 10, 1436. [Google Scholar] [CrossRef] [Green Version]
- Ntziachristos, P.; Tsirigos, A.; Van Vlierberghe, P.; Nedjic, J.; Trimarchi, T.; Flaherty, M.S.; Ferres-Marco, D.; da Ros, V.; Tang, Z.; Siegle, J.; et al. Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat. Med. 2012, 18, 298–301. [Google Scholar] [CrossRef]
- Shimizu, T.; Kubovcakova, L.; Nienhold, R.; Zmajkovic, J.; Meyer, S.C.; Hao-Shen, H.; Geier, F.; Dirnhofer, S.; Guglielmelli, P.; Vannucchi, A.M.; et al. Loss of Ezh2 synergizes with JAK2-V617F in initiating myeloproliferative neoplasms and promoting myelofibrosis. J. Exp. Med. 2016, 213, 1479–1496. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wang, Z.; Zhou, J.; Huang, J.; Zhou, L.; Luo, J.; Wan, Y.Y.; Long, H.; Zhu, B. EZH2 Inhibitor GSK126 Suppresses Antitumor Immunity by Driving Production of Myeloid-Derived Suppressor Cells. Cancer Res. 2019, 79, 2009–2020. [Google Scholar] [CrossRef]
- Fang, L.; Teng, H.; Wang, Y.; Liao, G.; Weng, L.; Li, Y.; Wang, X.; Jin, J.; Jiao, C.; Chen, L.; et al. SET1A-Mediated Mono-Methylation at K342 Regulates YAP Activation by Blocking Its Nuclear Export and Promotes Tumorigenesis. Cancer Cell 2018, 34, 103–118.e9. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Liu, J.; Li, K.; Yang, G.; Chen, S.; Wu, J.; Xie, X.; Ren, H.; Pang, Y. An SETD1A/Wnt/beta-catenin feedback loop promotes NSCLC development. J. Exp. Clin. Cancer Res. 2021, 40, 318. [Google Scholar] [CrossRef]
- Wu, J.; Chai, H.; Xu, X.; Yu, J.; Gu, Y. Histone methyltransferase SETD1A interacts with HIF1alpha to enhance glycolysis and promote cancer progression in gastric cancer. Mol. Oncol. 2020, 14, 1397–1409. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Demeulemeester, J.; Wedge, D.C.; Vollan, H.K.M.; Pitt, J.J.; Russnes, H.G.; Pandey, B.P.; Nilsen, G.; Nord, S.; Bignell, G.R.; et al. Pan-cancer analysis of homozygous deletions in primary tumours uncovers rare tumour suppressors. Nat. Commun. 2017, 8, 1221. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Hong, F.; Li, D.; Jin, Y.; Kon, L.; Xu, Z.; He, H.; Xie, Q. Long non-coding RNA ROR recruits histone transmethylase MLL1 to up-regulate TIMP3 expression and promote breast cancer progression. J. Transl. Med. 2021, 19, 95. [Google Scholar] [CrossRef]
- Riedel, S.S.; Haladyna, J.N.; Bezzant, M.; Stevens, B.; Pollyea, D.A.; Sinha, A.U.; Armstrong, S.A.; Wei, Q.; Pollock, R.M.; Daigle, S.R.; et al. MLL1 and DOT1L cooperate with meningioma-1 to induce acute myeloid leukemia. J. Clin. Investig. 2016, 126, 1438–1450. [Google Scholar] [CrossRef] [Green Version]
- Qiang, R.; Cai, N.; Wang, X.; Wang, L.; Cui, K.; Wang, X.; Li, X. MLL1 promotes cervical carcinoma cell tumorigenesis and metastasis through interaction with β-catenin. OncoTargets Ther. 2016, 9, 6631–6640. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Li, C.; Fan, Z.; Liu, H.; Zhang, X.; Cai, Z.; Xu, L.; Luo, J.; Huang, Y.; He, L.; et al. Single-cell Sequencing Reveals Variants in ARID1A, GPRC5A and MLL2 Driving Self-renewal of Human Bladder Cancer Stem Cells. Eur. Urol. 2017, 71, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, C.S.; Wu, Y.M.; Robinson, D.R.; Cao, X.; Dhanasekaran, S.M.; Khan, A.P.; Quist, M.J.; Jing, X.; Lonigro, R.J.; Brenner, J.C.; et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012, 487, 239–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, S.; Wang, Z.; Chen, Y.; Li, R. SPLUNC1 and MLL3 regulate cancer stem cells in nasopharyngeal carcinoma. J. BUON 2019, 24, 1700–1705. [Google Scholar] [PubMed]
- Kim, S.S.; Lee, M.H.; Lee, M.O. Histone methyltransferases regulate the transcriptional expression of ERalpha and the proliferation of tamoxifen-resistant breast cancer cells. Breast Cancer Res. Treat. 2020, 180, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, J.; Yue, B.; Liang, L.; Zhang, S.; Chen, Y. Long non-coding RNA DANCR regulate MLL3 and thereby it determines the progression of pancreatic cancer. J. BUON 2020, 25, 1954–1959. [Google Scholar]
- Larsson, C.; Cordeddu, L.; Siggens, L.; Pandzic, T.; Kundu, S.; He, L.; Ali, M.A.; Pristovsek, N.; Hartman, K.; Ekwall, K.; et al. Restoration of KMT2C/MLL3 in human colorectal cancer cells reinforces genome-wide H3K4me1 profiles and influences cell growth and gene expression. Clin. Epigenet. 2020, 12, 74. [Google Scholar] [CrossRef]
- Alam, H.; Tang, M.; Maitituoheti, M.; Dhar, S.S.; Kumar, M.; Han, C.Y.; Ambati, C.R.; Amin, S.B.; Gu, B.; Chen, T.Y.; et al. KMT2D Deficiency Impairs Super-Enhancers to Confer a Glycolytic Vulnerability in Lung Cancer. Cancer Cell 2020, 37, 599–617.e7. [Google Scholar] [CrossRef]
- Dhar, S.S.; Zhao, D.; Lin, T.; Gu, B.; Pal, K.; Wu, S.J.; Alam, H.; Lv, J.; Yun, K.; Gopalakrishnan, V.; et al. MLL4 Is Required to Maintain Broad H3K4me3 Peaks and Super-Enhancers at Tumor Suppressor Genes. Mol. Cell 2018, 70, 825–841.e6. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Kou, F.; Ji, Z.; Li, B.; Zhang, B.; Guo, Y.; Yang, L. SMYD2 promotes tumorigenesis and metastasis of lung adenocarcinoma through RPS7. Cell Death Dis. 2021, 12, 439. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, G.; Guo, Y.; Cao, Y.; Niu, S.; Fan, X.; Zhang, J. SMYD2 suppresses p53 activity to promote glucose metabolism in cervical cancer. Exp. Cell Res. 2021, 404, 112649. [Google Scholar] [CrossRef]
- Li, C.; Feng, S.Y.; Chen, L. SET7/9 promotes H3K4me3 at lncRNA DRAIC promoter to modulate growth and metastasis of glioma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12241–12250. [Google Scholar] [CrossRef]
- Zhang, S.L.; Du, X.; Tan, L.N.; Deng, F.H.; Zhou, B.Y.; Zhou, H.J.; Zhu, H.Y.; Chu, Y.; Liu, D.L.; Tan, Y.Y. SET7 interacts with HDAC6 and suppresses the development of colon cancer through inactivation of HDAC6. Am. J. Transl. Res. 2020, 12, 602–611. [Google Scholar]
- Daks, A.; Mamontova, V.; Fedorova, O.; Petukhov, A.; Shuvalov, O.; Parfenyev, S.; Netsvetay, S.; Venina, A.; Kizenko, A.; Imyanitov, E.; et al. Set7/9 controls proliferation and genotoxic drug resistance of NSCLC cells. Biochem. Biophys. Res. Commun. 2021, 572, 41–48. [Google Scholar] [CrossRef]
- Montenegro, M.F.; Sanchez-Del-Campo, L.; Gonzalez-Guerrero, R.; Martinez-Barba, E.; Pinero-Madrona, A.; Cabezas-Herrera, J.; Rodriguez-Lopez, J.N. Tumor suppressor SET9 guides the epigenetic plasticity of breast cancer cells and serves as an early-stage biomarker for predicting metastasis. Oncogene 2016, 35, 6143–6152. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Pan, Y.; Ma, X.; Liu, L.; Wang, W.; You, W. SMYD3 overexpression indicates poor prognosis and promotes cell proliferation, migration and invasion in non-small cell lung cancer. Int. J. Oncol. 2020, 57, 756–766. [Google Scholar] [CrossRef]
- Zhu, C.L.; Huang, Q. Overexpression of the SMYD3 Promotes Proliferation, Migration, and Invasion of Pancreatic Cancer. Dig. Dis. Sci. 2020, 65, 489–499. [Google Scholar] [CrossRef]
- Fenizia, C.; Bottino, C.; Corbetta, S.; Fittipaldi, R.; Floris, P.; Gaudenzi, G.; Carra, S.; Cotelli, F.; Vitale, G.; Caretti, G. SMYD3 promotes the epithelial-mesenchymal transition in breast cancer. Nucleic Acids Res. 2019, 47, 1278–1293. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Tian, S.; Zhao, M.; Yang, T.; Quan, S.; Yang, Q.; Song, L.; Yang, X. SUV39H1-DNMT3A-mediated epigenetic regulation of Tim-3 and galectin-9 in the cervical cancer. Cancer Cell Int. 2020, 20, 325. [Google Scholar] [CrossRef]
- Yu, T.; Wang, C.; Yang, J.; Guo, Y.; Wu, Y.; Li, X. Metformin inhibits SUV39H1-mediated migration of prostate cancer cells. Oncogenesis 2017, 6, e324. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.; Kim, J.Y.; Lim, S.C.; Lee, K.Y.; Kim, O.; Choi, H.S. SUV39H1/DNMT3A-dependent methylation of the RB1 promoter stimulates PIN1 expression and melanoma development. FASEB J. 2018, 32, 5647–5660. [Google Scholar] [CrossRef]
- Shuai, W.; Wu, J.; Chen, S.; Liu, R.; Ye, Z.; Kuang, C.; Fu, X.; Wang, G.; Li, Y.; Peng, Q.; et al. SUV39H2 promotes colorectal cancer proliferation and metastasis via tri-methylation of the SLIT1 promoter. Cancer Lett. 2018, 422, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Liu, G.; Liu, L. Histone methyltransferase SUV39H2 regulates LSD1-dependent CDH1 expression and promotes epithelial mesenchymal transition of osteosarcoma. Cancer Cell Int. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, B.; Wang, J.; Xiong, Y.; Wang, K.; Qi, Y.; Sun, H.; Wu, L.; Yang, L. Identification of SUV39H2 as a potential oncogene in lung adenocarcinoma. Clin. Epigenet. 2018, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Bergin, C.J.; Zouggar, A.; Haebe, J.R.; Masibag, A.N.; Desrochers, F.M.; Reilley, S.Y.; Agrawal, G.; Benoit, Y.D. G9a controls pluripotent-like identity and tumor-initiating function in human colorectal cancer. Oncogene 2021, 40, 1191–1202. [Google Scholar] [CrossRef]
- Segovia, C.; San Jose-Eneriz, E.; Munera-Maravilla, E.; Martinez-Fernandez, M.; Garate, L.; Miranda, E.; Vilas-Zornoza, A.; Lodewijk, I.; Rubio, C.; Segrelles, C.; et al. Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat. Med. 2019, 25, 1073–1081. [Google Scholar] [CrossRef]
- Pangeni, R.P.; Yang, L.; Zhang, K.; Wang, J.; Li, W.; Guo, C.; Yun, X.; Sun, T.; Wang, J.; Raz, D.J. G9a regulates tumorigenicity and stemness through genome-wide DNA methylation reprogramming in non-small cell lung cancer. Clin. Epigenet. 2020, 12, 88. [Google Scholar] [CrossRef]
- Casciello, F.; Al-Ejeh, F.; Kelly, G.; Brennan, D.J.; Ngiow, S.F.; Young, A.; Stoll, T.; Windloch, K.; Hill, M.M.; Smyth, M.J.; et al. G9a drives hypoxia-mediated gene repression for breast cancer cell survival and tumorigenesis. Proc. Natl. Acad. Sci. USA 2017, 114, 7077–7082. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Long, J.; Gao, Y.; Zhang, W.; Han, F.; Xu, C.; Sun, L.; Yang, S.C.; Lan, J.; Hou, Z.; et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat. Cell Biol. 2019, 21, 214–225. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Y.; Liang, X.; Li, T.; Shao, W.; Liu, F.; Cui, X.; Wang, Y.; Lv, L.; Chai, L.; et al. SETDB1 promotes gastric carcinogenesis and metastasis via upregulation of CCND1 and MMP9 expression. J. Pathol. 2021, 253, 148–159. [Google Scholar] [CrossRef]
- Hou, Z.; Sun, L.; Xu, F.; Hu, F.; Lan, J.; Song, D.; Feng, Y.; Wang, J.; Luo, X.; Hu, J.; et al. Blocking histone methyltransferase SETDB1 inhibits tumorigenesis and enhances cetuximab sensitivity in colorectal cancer. Cancer Lett. 2020, 487, 63–73. [Google Scholar] [CrossRef]
- Ye, J.; Huang, A.; Wang, H.; Zhang, A.M.Y.; Huang, X.; Lan, Q.; Sato, T.; Goyama, S.; Kurokawa, M.; Deng, C.; et al. PRDM3 attenuates pancreatitis and pancreatic tumorigenesis by regulating inflammatory response. Cell Death Dis. 2020, 11, 187. [Google Scholar] [CrossRef] [Green Version]
- Kundu, A.; Nam, H.; Shelar, S.; Chandrashekar, D.S.; Brinkley, G.; Karki, S.; Mitchell, T.; Livi, C.B.; Buckhaults, P.; Kirkman, R.; et al. PRDM16 suppresses HIF-targeted gene expression in kidney cancer. J. Exp. Med. 2020, 217, e20191005. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.; Yan, C.; Hao, J.; Zhang, C.; Wang, P.; Zhao, C.; Cai, S.; Meng, B.; Zhang, A.; Li, L. PRDM16, negatively regulated by miR-372-3p, suppresses cell proliferation and invasion in prostate cancer. Andrologia 2022, e14529. [Google Scholar] [CrossRef]
- Fei, L.R.; Huang, W.J.; Wang, Y.; Lei, L.; Li, Z.H.; Zheng, Y.W.; Wang, Z.; Yang, M.Q.; Liu, C.C.; Xu, H.T. PRDM16 functions as a suppressor of lung adenocarcinoma metastasis. J. Exp. Clin. Cancer Res. 2019, 38, 35. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Yang, Y.; Wu, H. MicroRNA-765 alleviates the malignant progression of breast cancer via interacting with EZH1. Am. J. Transl. Res. 2019, 11, 4500–4507. [Google Scholar]
- Kusakabe, Y.; Chiba, T.; Oshima, M.; Koide, S.; Rizq, O.; Aoyama, K.; Ao, J.; Kaneko, T.; Kanzaki, H.; Kanayama, K.; et al. EZH1/2 inhibition augments the anti-tumor effects of sorafenib in hepatocellular carcinoma. Sci. Rep. 2021, 11, 21396. [Google Scholar] [CrossRef]
- Wan, L.; Li, X.; Shen, H.; Bai, X. Quantitative analysis of EZH2 expression and its correlations with lung cancer patients’ clinical pathological characteristics. Clin. Transl. Oncol. 2013, 15, 132–138. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Lu, J.; Huang, B.; Wang, Y.; Dong, M.; Fan, D.; Li, H.; Gao, Y.; Hou, P.; et al. Methylation of EZH2 by PRMT1 regulates its stability and promotes breast cancer metastasis. Cell Death Differ. 2020, 27, 3226–3242. [Google Scholar] [CrossRef]
- Pan, Y.M.; Wang, C.G.; Zhu, M.; Xing, R.; Cui, J.T.; Li, W.M.; Yu, D.D.; Wang, S.B.; Zhu, W.; Ye, Y.J.; et al. STAT3 signaling drives EZH2 transcriptional activation and mediates poor prognosis in gastric cancer. Mol. Cancer 2016, 15, 79. [Google Scholar] [CrossRef] [Green Version]
- Cheraghi, S.; Asadzadeh, H.; Javadi, G. Dysregulated Expression of Long Non-Coding RNA MINCR and EZH2 in Colorectal Cancer. Iran. Biomed. J. 2022, 26, 64–69. [Google Scholar] [CrossRef]
- Yuan, H.; Han, Y.; Wang, X.; Li, N.; Liu, Q.; Yin, Y.; Wang, H.; Pan, L.; Li, L.; Song, K.; et al. SETD2 Restricts Prostate Cancer Metastasis by Integrating EZH2 and AMPK Signaling Pathways. Cancer Cell 2020, 38, 350–365.e7. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Lu, P.; Yang, Y.; He, R.; Zhang, L.; Shi, J.; Wu, J.; Yang, M.; Zhang, Z.G.; Wang, L.W.; et al. Loss of Setd2 promotes Kras-induced acinar-to-ductal metaplasia and epithelia-mesenchymal transition during pancreatic carcinogenesis. Gut 2020, 69, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Song, J.; Hu, C.L.; Chen, S.B.; Zhang, Q.; Xu, C.H.; Wu, J.C.; Hou, D.; Sun, M.; Zhang, Y.L.; et al. SETD2 deficiency accelerates MDS-associated leukemogenesis via S100a9 in NHD13 mice and predicts poor prognosis in MDS. Blood 2020, 135, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.K.; McCutcheon, J.N.; Rao, G.; Liu, S.V.; Pommier, Y.; Skrzypski, M.; Zhang, Y.W.; Giaccone, G. Acquired SETD2 mutation and impaired CREB1 activation confer cisplatin resistance in metastatic non-small cell lung cancer. Oncogene 2019, 38, 180–193. [Google Scholar] [CrossRef]
- Ettel, M.; Zhao, L.; Schechter, S.; Shi, J. Expression and prognostic value of NSD1 and SETD2 in pancreatic ductal adenocarcinoma and its precursor lesions. Pathology 2019, 51, 392–398. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, D.; Zeng, L.; Li, Y.; Hausmann, S.; Ghosh, D.; Yuan, G.; Nguyen, T.N.; Lyu, R.; Caporicci, M.; Morales Benitez, A.; et al. NSD2 dimethylation at H3K36 promotes lung adenocarcinoma pathogenesis. Mol. Cell 2021, 81, 4481–4492.e9. [Google Scholar] [CrossRef]
- Zhao, L.H.; Li, Q.; Huang, Z.J.; Sun, M.X.; Lu, J.J.; Zhang, X.H.; Li, G.; Wu, F. Identification of histone methyltransferase NSD2 as an important oncogenic gene in colorectal cancer. Cell Death Dis. 2021, 12, 974. [Google Scholar] [CrossRef]
- Gao, B.; Liu, X.; Li, Z.; Zhao, L.; Pan, Y. Overexpression of EZH2/NSD2 Histone Methyltransferase Axis Predicts Poor Prognosis and Accelerates Tumor Progression in Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 600514. [Google Scholar] [CrossRef]
- Lu, M.H.; Fan, M.F.; Yu, X.D. NSD2 promotes osteosarcoma cell proliferation and metastasis by inhibiting E-cadherin expression. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 928–936. [Google Scholar]
- Han, X.; Piao, L.; Xu, X.; Luo, F.; Liu, Z.; He, X. NSD2 Promotes Renal Cancer Progression Through Stimulating Akt/Erk Signaling. Cancer Manag. Res. 2020, 12, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Aytes, A.; Giacobbe, A.; Mitrofanova, A.; Ruggero, K.; Cyrta, J.; Arriaga, J.; Palomero, L.; Farran-Matas, S.; Rubin, M.A.; Shen, M.M.; et al. NSD2 is a conserved driver of metastatic prostate cancer progression. Nat. Commun. 2018, 9, 5201. [Google Scholar] [CrossRef] [Green Version]
- Jeong, G.Y.; Park, M.K.; Choi, H.J.; An, H.W.; Park, Y.U.; Choi, H.J.; Park, J.; Kim, H.Y.; Son, T.; Lee, H.; et al. NSD3-Induced Methylation of H3K36 Activates NOTCH Signaling to Drive Breast Tumor Initiation and Metastatic Progression. Cancer Res. 2021, 81, 77–90. [Google Scholar] [CrossRef]
- Yi, L.; Yi, L.; Liu, Q.; Li, C. Downregulation of NSD3 (WHSC1L1) inhibits cell proliferation and migration via ERK1/2 deactivation and decreasing CAPG expression in colorectal cancer cells. OncoTargets Ther. 2019, 12, 3933–3943. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Xie, J.; Cai, S.; Wang, Q.; Feng, Z.; Li, Y.; Lu, J.J.; Chen, W.; Ye, Z. Elevated expression of nuclear receptor-binding SET domain 3 promotes pancreatic cancer cell growth. Cell Death Dis. 2021, 12, 913. [Google Scholar] [CrossRef]
- Hassan, N.; Rutsch, N.; Gyorffy, B.; Espinoza-Sanchez, N.A.; Gotte, M. SETD3 acts as a prognostic marker in breast cancer patients and modulates the viability and invasion of breast cancer cells. Sci. Rep. 2020, 10, 2262. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Wang, Y.; Dong, L.; Che, T.; Zhao, H.; Yan, X.; Lin, Z. Stabilization of SETD3 by deubiquitinase USP27 enhances cell proliferation and hepatocellular carcinoma progression. Cell. Mol. Life Sci. 2022, 79, 70. [Google Scholar] [CrossRef]
- Yu, M.; Jia, Y.; Ma, Z.; Ji, D.; Wang, C.; Liang, Y.; Zhang, Q.; Yi, H.; Zeng, L. Structural insight into ASH1L PHD finger recognizing methylated histone H3K4 and promoting cell growth in prostate cancer. Front. Oncol. 2022, 12, 906807. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Q.; Wong, S.H.; Huang, M.; Klein, B.J.; Shen, J.; Ikenouye, L.; Onishi, M.; Schneidawind, D.; Buechele, C.; et al. ASH1L Links Histone H3 Lysine 36 Dimethylation to MLL Leukemia. Cancer Discov. 2016, 6, 770–783. [Google Scholar] [CrossRef] [Green Version]
- Jeyaratnam, D.C.; Baduin, B.S.; Hansen, M.C.; Hansen, M.; Jorgensen, J.M.; Aggerholm, A.; Ommen, H.B.; Hokland, P.; Nyvold, C.G. Delineation of known and new transcript variants of the SETMAR (Metnase) gene and the expression profile in hematologic neoplasms. Exp. Hematol. 2014, 42, 448–456.e4. [Google Scholar] [CrossRef]
- Houle, A.A.; Gibling, H.; Lamaze, F.C.; Edgington, H.A.; Soave, D.; Fave, M.J.; Agbessi, M.; Bruat, V.; Stein, L.D.; Awadalla, P. Aberrant PRDM9 expression impacts the pan-cancer genomic landscape. Genome Res. 2018, 28, 1611–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatapalli, R.; Sagar, V.; Rodriguez, Y.; Zhao, J.C.; Unno, K.; Pamarthy, S.; Lysy, B.; Anker, J.; Han, H.; Yoo, Y.A.; et al. Histone methyltransferase DOT1L coordinates AR and MYC stability in prostate cancer. Nat. Commun. 2020, 11, 4153. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, Q.; Zhu, Q.; Lu, X.; Li, M.; Hou, T.; Li, Z.; Tang, M.; Li, Y.; Wang, H.; et al. CBP mediated DOT1L acetylation confers DOT1L stability and promotes cancer metastasis. Theranostics 2020, 10, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wei, Z.; Wang, Q.; Zhang, X.; Tao, X.; Wu, N.; Liu, X.; Qian, J. The role of DOT1L in the proliferation and prognosis of gastric cancer. Biosci. Rep. 2020, 40, BSR20193515. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, H.; Xu, B.; Jiang, D.; Huang, S.; Yu, H.; Wu, Z.; Wu, Q. Depletion of H3K79 methyltransferase Dot1L promotes cell invasion and cancer stem-like cell property in ovarian cancer. Am. J. Transl. Res. 2019, 11, 1145–1153. [Google Scholar]
- Kurani, H.; Razavipour, S.F.; Harikumar, K.B.; Dunworth, M.; Ewald, A.J.; Nasir, A.; Pearson, G.; Van Booven, D.; Zhou, Z.; Azzam, D.; et al. DOT1L Is a Novel Cancer Stem Cell Target for Triple-Negative Breast Cancer. Clin. Cancer Res. 2022, 28, 1948–1965. [Google Scholar] [CrossRef]
- Hou, L.; Li, Q.; Yu, Y.; Li, M.; Zhang, D. SET8 induces epithelial-mesenchymal transition and enhances prostate cancer cell metastasis by cooperating with ZEB1. Mol. Med. Rep. 2016, 13, 1681–1688. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Qiao, K.; Du, Y.; Zhang, X.; Cheng, H.; Peng, L.; Guo, Z. Downregulation of histone methyltransferase SET8 inhibits progression of hepatocellular carcinoma. Sci. Rep. 2020, 10, 4490. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Zhang, X.; Song, F.; Liu, Q.; Dai, H.; Zheng, H.; Cui, P.; Zhang, L.; Zhang, W.; Chen, K. A functional single nucleotide polymorphism of SET8 is prognostic for breast cancer. Oncotarget 2016, 7, 34277–34287. [Google Scholar] [CrossRef] [Green Version]
- Pogribny, I.P.; Ross, S.A.; Tryndyak, V.P.; Pogribna, M.; Poirier, L.A.; Karpinets, T.V. Histone H3 lysine 9 and H4 lysine 20 trimethylation and the expression of Suv4-20h2 and Suv-39h1 histone methyltransferases in hepatocarcinogenesis induced by methyl deficiency in rats. Carcinogenesis 2006, 27, 1180–1186. [Google Scholar] [CrossRef]
- Tryndyak, V.P.; Kovalchuk, O.; Pogribny, I.P. Loss of DNA methylation and histone H4 lysine 20 trimethylation in human breast cancer cells is associated with aberrant expression of DNA methyltransferase 1, Suv4-20h2 histone methyltransferase and methyl-binding proteins. Cancer Biol. Ther. 2006, 5, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Guan, K.L. Acetyl-CoA, protein acetylation, and liver cancer. Mol. Cell 2022, 82, 4196–4198. [Google Scholar] [CrossRef]
- Verdone, L.; Caserta, M.; Di Mauro, E. Role of histone acetylation in the control of gene expression. Biochem. Cell Biol. 2005, 83, 344–353. [Google Scholar] [CrossRef]
- Klein, B.J.; Jang, S.M.; Lachance, C.; Mi, W.; Lyu, J.; Sakuraba, S.; Krajewski, K.; Wang, W.W.; Sidoli, S.; Liu, J.; et al. Histone H3K23-specific acetylation by MORF is coupled to H3K14 acylation. Nat. Commun. 2019, 10, 4724. [Google Scholar] [CrossRef] [Green Version]
- Dang, F.; Wei, W. Targeting the acetylation signaling pathway in cancer therapy. Semin. Cancer Biol. 2022, 85, 209–218. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Li, J.; Yang, F.; Wu, H.; Dai, F.; Hu, M.; Lu, X.; Peng, Y.; Liu, M.; et al. Antitumor action of a novel histone deacetylase inhibitor, YF479, in breast cancer. Neoplasia 2014, 16, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Guo, P.; Chen, W.; Li, H.; Li, M.; Li, L. The Histone Acetylation Modifications of Breast Cancer and their Therapeutic Implications. Pathol. Oncol. Res. 2018, 24, 807–813. [Google Scholar] [CrossRef]
- Yang, G.; Yuan, Y.; Yuan, H.; Wang, J.; Yun, H.; Geng, Y.; Zhao, M.; Li, L.; Weng, Y.; Liu, Z.; et al. Histone acetyltransferase 1 is a succinyltransferase for histones and non-histones and promotes tumorigenesis. EMBO Rep. 2021, 22, e50967. [Google Scholar] [CrossRef]
- Yin, Y.W.; Jin, H.J.; Zhao, W.; Gao, B.; Fang, J.; Wei, J.; Zhang, D.D.; Zhang, J.; Fang, D. The Histone Acetyltransferase GCN5 Expression Is Elevated and Regulated by c-Myc and E2F1 Transcription Factors in Human Colon Cancer. Gene Expr. 2015, 16, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Stemmler, M.P. PCAF, ISX, and BRD4: A maleficent alliance serving lung cancer malignancy. EMBO Rep. 2020, 21, e49766. [Google Scholar] [CrossRef]
- Cheng, Y.W.; Zeng, F.M.; Li, D.J.; Wang, S.H.; He, J.Z.; Guo, Z.C.; Nie, P.J.; Wu, Z.Y.; Shi, W.Q.; Wen, B.; et al. P300/CBP-associated factor (PCAF)-mediated acetylation of Fascin at lysine 471 inhibits its actin-bundling activity and tumor metastasis in esophageal cancer. Cancer Commun. 2021, 41, 1398–1416. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.N.; Avery, V.M.; Carrasco-Pozo, C. Metabolic Roles of Androgen Receptor and Tip60 in Androgen-Dependent Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 6622. [Google Scholar] [CrossRef] [PubMed]
- McGuire, A.; Casey, M.C.; Shalaby, A.; Kalinina, O.; Curran, C.; Webber, M.; Callagy, G.; Holian, E.; Bourke, E.; Kerin, M.J.; et al. Quantifying Tip60 (Kat5) stratifies breast cancer. Sci. Rep. 2019, 9, 3819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Wang, Y.; Wei, T.; Zhao, X.; Li, F.; Li, Y.; Wang, F.; Cai, Y.; Jin, J. KAT8/MOF-Mediated Anti-Cancer Mechanism of Gemcitabine in Human Bladder Cancer Cells. Biomol. Ther. 2021, 29, 184–194. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, K.; Wang, C.; Wang, S.; Sun, H.; Liu, W.; Wang, X.; Niu, J.; Cong, S.Y.; Zhou, X.; et al. Histone acetyltransferase MOF is involved in suppression of endometrial cancer and maintenance of ERalpha stability. Biochem. Biophys. Res. Commun. 2019, 509, 541–548. [Google Scholar] [CrossRef]
- Guo, R.; Liang, Y.; Zou, B.; Li, D.; Wu, Z.; Xie, F.; Zhang, X.; Li, X. The Histone Acetyltransferase MOF Regulates SIRT1 Expression to Suppress Renal Cell Carcinoma Progression. Front. Oncol. 2022, 12, 842967. [Google Scholar] [CrossRef]
- Hemming, M.L.; Benson, M.R.; Loycano, M.A.; Anderson, J.A.; Andersen, J.L.; Taddei, M.L.; Krivtsov, A.V.; Aubrey, B.J.; Cutler, J.A.; Hatton, C.; et al. MOZ and Menin-MLL Complexes Are Complementary Regulators of Chromatin Association and Transcriptional Output in Gastrointestinal Stromal Tumor. Cancer Discov. 2022, 12, 1804–1823. [Google Scholar] [CrossRef]
- Yokoyama, A. Role of the MOZ/MLL-mediated transcriptional activation system for self-renewal in normal hematopoiesis and leukemogenesis. FEBS J. 2021, 289, 7987–8002. [Google Scholar] [CrossRef]
- Baell, J.B.; Leaver, D.J.; Hermans, S.J.; Kelly, G.L.; Brennan, M.S.; Downer, N.L.; Nguyen, N.; Wichmann, J.; McRae, H.M.; Yang, Y.; et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature 2018, 560, 253–257. [Google Scholar] [CrossRef]
- Chen, T.F.; Hao, H.F.; Zhang, Y.; Chen, X.Y.; Zhao, H.S.; Yang, R.; Li, P.; Qiu, L.X.; Sang, Y.H.; Xu, C.; et al. HBO1 induces histone acetylation and is important for non-small cell lung cancer cell growth. Int. J. Biol. Sci. 2022, 18, 3313–3323. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Ling, Z.Y.; Zhu, Y.R.; Shi, C.; Wang, Y.; Zhang, X.Y.; Zhang, Z.Q.; Jiang, Q.; Chen, M.B.; Yang, S.; et al. The histone acetyltransferase HBO1 functions as a novel oncogenic gene in osteosarcoma. Theranostics 2021, 11, 4599–4615. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Deng, L.; Chen, G.; Liu, Y. HBO1 overexpression is important for hepatocellular carcinoma cell growth. Cell Death Dis. 2021, 12, 549. [Google Scholar] [CrossRef]
- Iizuka, M.; Susa, T.; Takahashi, Y.; Tamamori-Adachi, M.; Kajitani, T.; Okinaga, H.; Fukusato, T.; Okazaki, T. Histone acetyltransferase Hbo1 destabilizes estrogen receptor α by ubiquitination and modulates proliferation of breast cancers. Cancer Sci. 2013, 104, 1647–1655. [Google Scholar] [CrossRef]
- Gruber, M.; Ferrone, L.; Puhr, M.; Santer, F.R.; Furlan, T.; Eder, I.E.; Sampson, N.; Schäfer, G.; Handle, F.; Culig, Z. p300 is upregulated by docetaxel and is a target in chemoresistant prostate cancer. Endocr. Relat. Cancer 2020, 27, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.; Gong, R.; Zhan, J.; Zhou, T.; Ma, Y.; Zhao, Y.; Zhang, Y.; Chen, G.; Zhang, Z.; Ma, S.; et al. p300 promotes proliferation, migration, and invasion via inducing epithelial-mesenchymal transition in non-small cell lung cancer cells. BMC Cancer 2018, 18, 641. [Google Scholar] [CrossRef]
- Clague, M.J.; Urbe, S. Integration of cellular ubiquitin and membrane traffic systems: Focus on deubiquitylases. FEBS J. 2017, 284, 1753–1766. [Google Scholar] [CrossRef] [Green Version]
- Heideker, J.; Wertz, I.E. DUBs, the regulation of cell identity and disease. Biochem. J. 2015, 467, 191. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, J. Role of deubiquitinating enzymes in DNA double-strand break repair. J. Zhejiang Univ. Sci. B 2021, 22, 63–72. [Google Scholar] [CrossRef]
- Mattiroli, F.; Penengo, L. Histone Ubiquitination: An Integrative Signaling Platform in Genome Stability. Trends Genet. 2021, 37, 566–581. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Rezaeian, A.H.; Xu, X.; Chou, P.C.; Jin, G.; Han, F.; Pan, B.S.; Wang, C.Y.; Long, J.; et al. H3 ubiquitination by NEDD4 regulates H3 acetylation and tumorigenesis. Nat. Commun. 2017, 8, 14799. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.; Subbarayalu, P.; Medina, D.; Nirzhor, S.; Timilsina, S.; Rajamanickam, S.; Eedunuri, V.K.; Gupta, Y.; Zheng, S.; Abdelfattah, N.; et al. M6A RNA Methylation Regulates Histone Ubiquitination to Support Cancer Growth and Progression. Cancer Res. 2022, 82, 1872–1889. [Google Scholar] [CrossRef] [PubMed]
- Challa, K.; Schmid, C.D.; Kitagawa, S.; Cheblal, A.; Iesmantavicius, V.; Seeber, A.; Amitai, A.; Seebacher, J.; Hauer, M.H.; Shimada, K.; et al. Damage-induced chromatome dynamics link Ubiquitin ligase and proteasome recruitment to histone loss and efficient DNA repair. Mol. Cell 2021, 81, 811–829.e6. [Google Scholar] [CrossRef] [PubMed]
- Ting, X.; Xia, L.; Yang, J.; He, L.; Si, W.; Shang, Y.; Sun, L. USP11 acts as a histone deubiquitinase functioning in chromatin reorganization during DNA repair. Nucleic Acids Res. 2019, 47, 9721–9740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerutti, H.; Casas-Mollano, J.A. Histone H3 phosphorylation: Universal code or lineage specific dialects? Epigenetics 2009, 4, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Yu, X.; Zhang, Y.; Xue, X.; Yu, Q.; Zha, Z.; Gogol, M.; Workman, J.L.; Li, S. Metabolic regulation of telomere silencing by SESAME complex-catalyzed H3T11 phosphorylation. Nat. Commun. 2021, 12, 594. [Google Scholar] [CrossRef]
- Metzger, E.; Yin, N.; Wissmann, M.; Kunowska, N.; Fischer, K.; Friedrichs, N.; Patnaik, D.; Higgins, J.M.; Potier, N.; Scheidtmann, K.H.; et al. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat. Cell Biol. 2008, 10, 53–60. [Google Scholar] [CrossRef]
- Armache, A.; Yang, S.; Martinez de Paz, A.; Robbins, L.E.; Durmaz, C.; Cheong, J.Q.; Ravishankar, A.; Daman, A.W.; Ahimovic, D.J.; Klevorn, T.; et al. Histone H3.3 phosphorylation amplifies stimulation-induced transcription. Nature 2020, 583, 852–857. [Google Scholar] [CrossRef]
- Udugama, M.; Vinod, B.; Chan, F.L.; Hii, L.; Garvie, A.; Collas, P.; Kalitsis, P.; Steer, D.; Das, P.P.; Tripathi, P.; et al. Histone H3.3 phosphorylation promotes heterochromatin formation by inhibiting H3K9/K36 histone demethylase. Nucleic Acids Res. 2022, 50, 4500–4514. [Google Scholar] [CrossRef]
- He, F.; Yu, Q.; Wang, M.; Wang, R.; Gong, X.; Ge, F.; Yu, X.; Li, S. SESAME-catalyzed H3T11 phosphorylation inhibits Dot1-catalyzed H3K79me3 to regulate autophagy and telomere silencing. Nat. Commun. 2022, 13, 7526. [Google Scholar] [CrossRef]
- Leal, J.A.; Estrada-Tobar, Z.M.; Wade, F.; Mendiola, A.J.P.; Meza, A.; Mendoza, M.; Nerenberg, P.S.; Zurita-Lopez, C.I. Phosphoserine inhibits neighboring arginine methylation in the RKS motif of histone H3. Arch. Biochem. Biophys. 2021, 698, 108716. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, S.Y.; Miller, K.M. Preserving genome integrity and function: The DNA damage response and histone modifications. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 208–241. [Google Scholar] [CrossRef]
- Zhao, Z.; Shilatifard, A. Epigenetic modifications of histones in cancer. Genome Biol. 2019, 20, 245. [Google Scholar] [CrossRef] [Green Version]
- Fults, D.W. Stemming the growth of pediatric gliomas through histone modification. Neuro Oncol. 2021, 23, 341–342. [Google Scholar] [CrossRef]
- Allis, C.D. Pursuing the Secrets of Histone Proteins: An Amazing Journey with a Remarkable Supporting Cast. Cell 2018, 175, 18–21. [Google Scholar] [CrossRef]


| Gene | Tumor | Role | Reference |
|---|---|---|---|
| HAT1 | Liver cancer, Pancreatic cancer, Cholangiocarcinoma | Overexpression | [198] |
| GCN5 | Colorectal cancer | Overexpression | [199] |
| PCAF | Lung cancer, Esophageal cancer | Ambiguous | [200,201] |
| Tip60 | Prostate cancer, Breast cancer | Ambiguous | [202,203] |
| MOF | Bladder cancer, Endometrial cancer, Renal cell carcinoma | Suppressor | [204,205,206] |
| MOZ | Gastrointestinal stromal tumor, Acute myeloid leukemia | Oncogene | [207,208] |
| MORF | lymphoma | Oncogene | [209] |
| HBO1 | Lung cancer, Osteosarcoma, Hepatocellular carcinoma, Breast cancer | Oncogenic | [210,211,212,213] |
| p300 | Esophageal cancer, Prostate cancer, Lung cancer | Oncogenic | [201,214,215] |
| CBP | Colorectal cancer | Oncogenic | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, Q.; Zhang, Y.; Han, J. The Role of Histone Modification in DNA Replication-Coupled Nucleosome Assembly and Cancer. Int. J. Mol. Sci. 2023, 24, 4939. https://doi.org/10.3390/ijms24054939
Zhang Y, Zhang Q, Zhang Y, Han J. The Role of Histone Modification in DNA Replication-Coupled Nucleosome Assembly and Cancer. International Journal of Molecular Sciences. 2023; 24(5):4939. https://doi.org/10.3390/ijms24054939
Chicago/Turabian StyleZhang, Yaguang, Qin Zhang, Yang Zhang, and Junhong Han. 2023. "The Role of Histone Modification in DNA Replication-Coupled Nucleosome Assembly and Cancer" International Journal of Molecular Sciences 24, no. 5: 4939. https://doi.org/10.3390/ijms24054939
APA StyleZhang, Y., Zhang, Q., Zhang, Y., & Han, J. (2023). The Role of Histone Modification in DNA Replication-Coupled Nucleosome Assembly and Cancer. International Journal of Molecular Sciences, 24(5), 4939. https://doi.org/10.3390/ijms24054939







