The Impact of Semicarbazide Sensitive Amine Oxidase Activity on Rat Aortic Vascular Smooth Muscle Cells
Abstract
:1. Introduction
2. Results
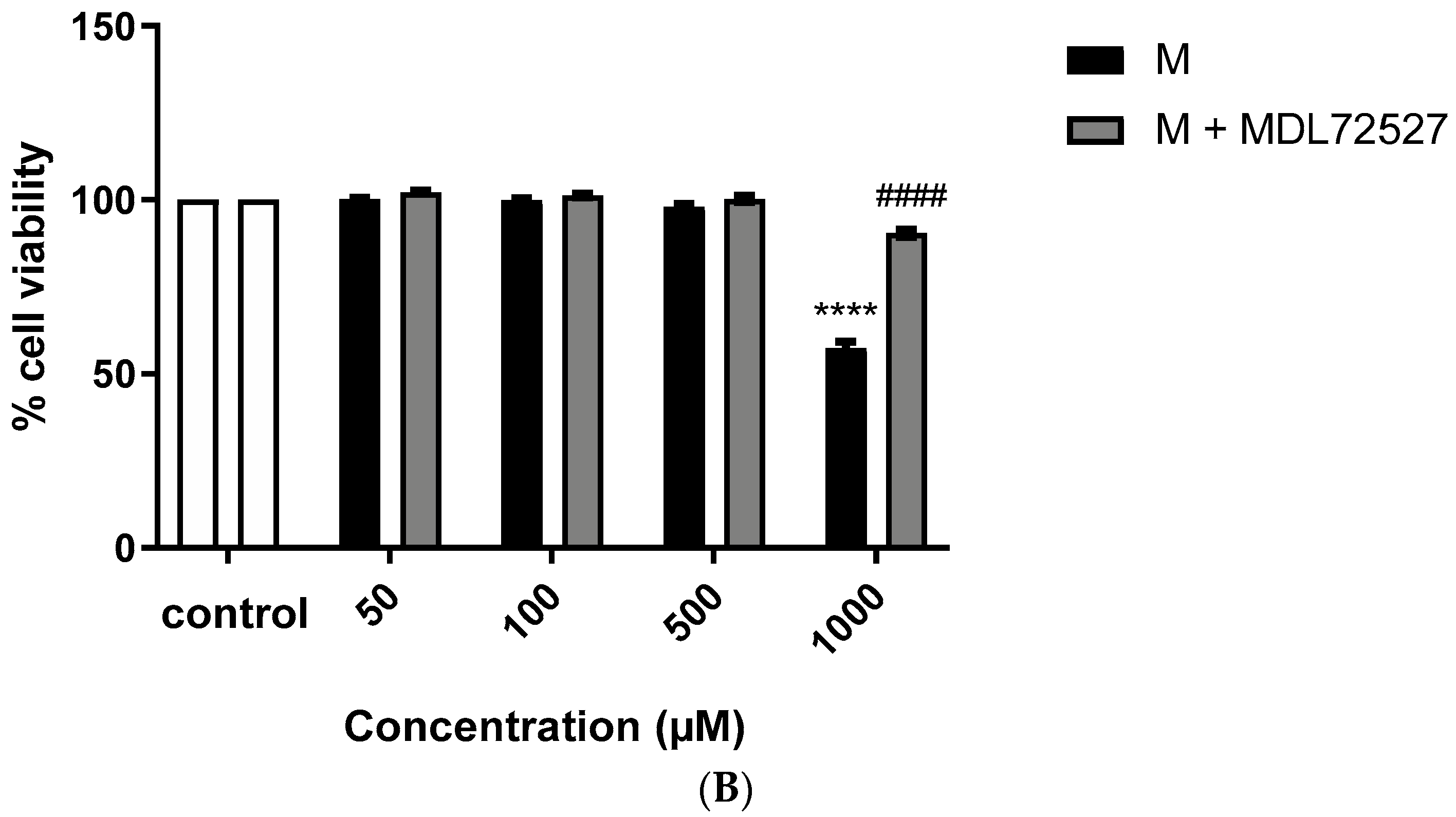
2.1. Active SSAO Induces VSMCs Death
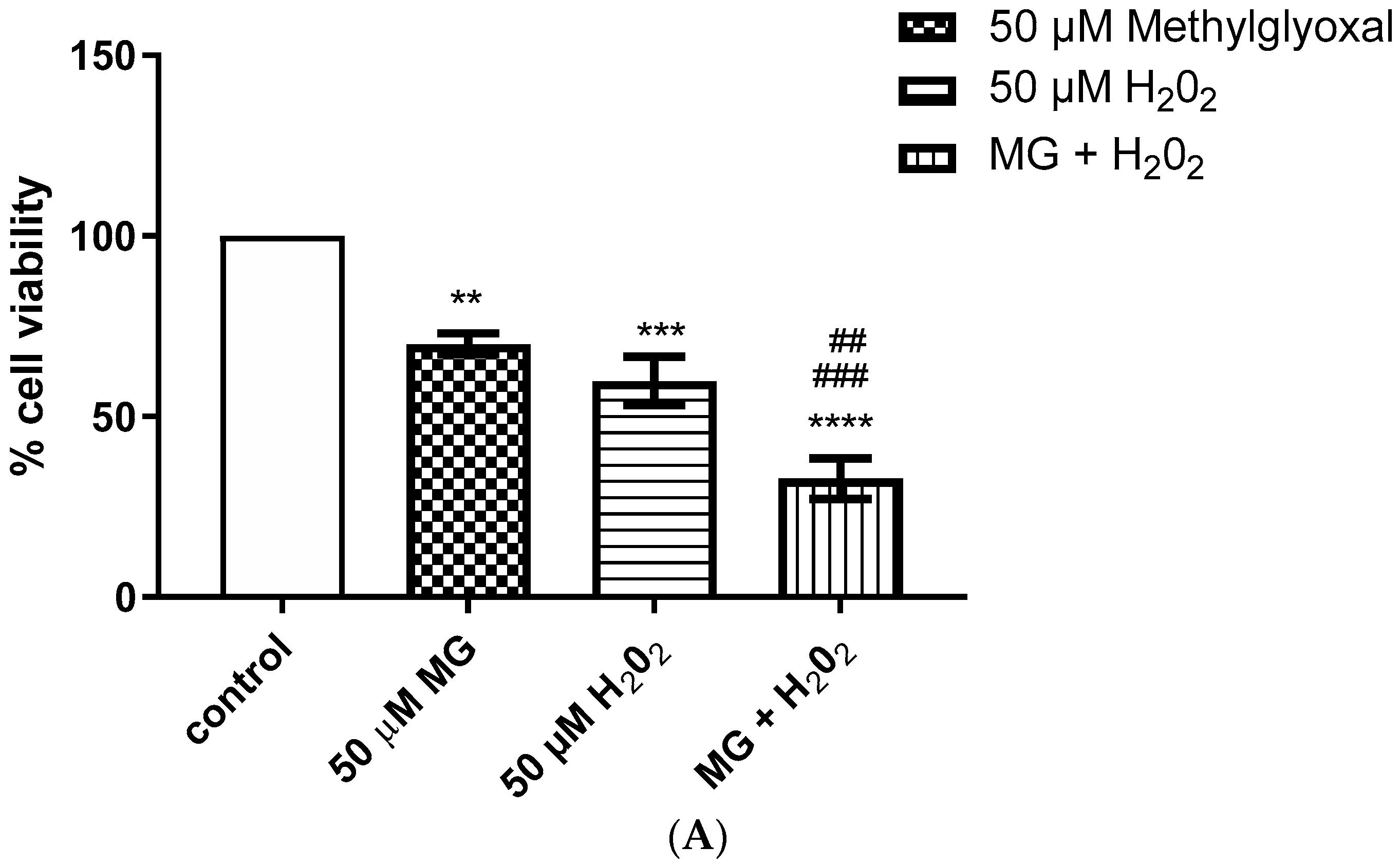
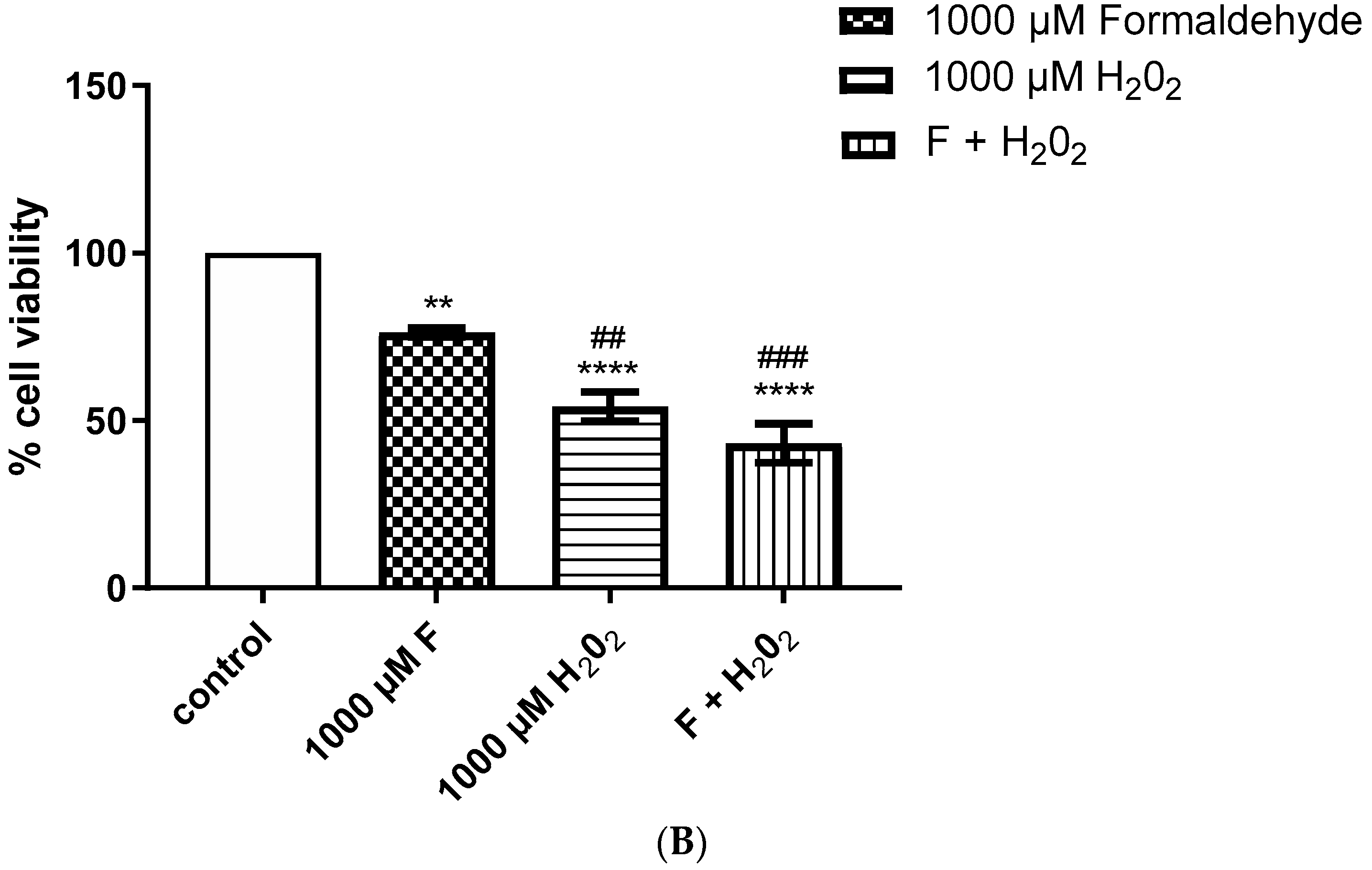
2.2. Enhanced Cytotoxic Effect Was Observed after Simultaneous Addition of Methylglyoxal and H2O2, and Formaldehyde and H2O2
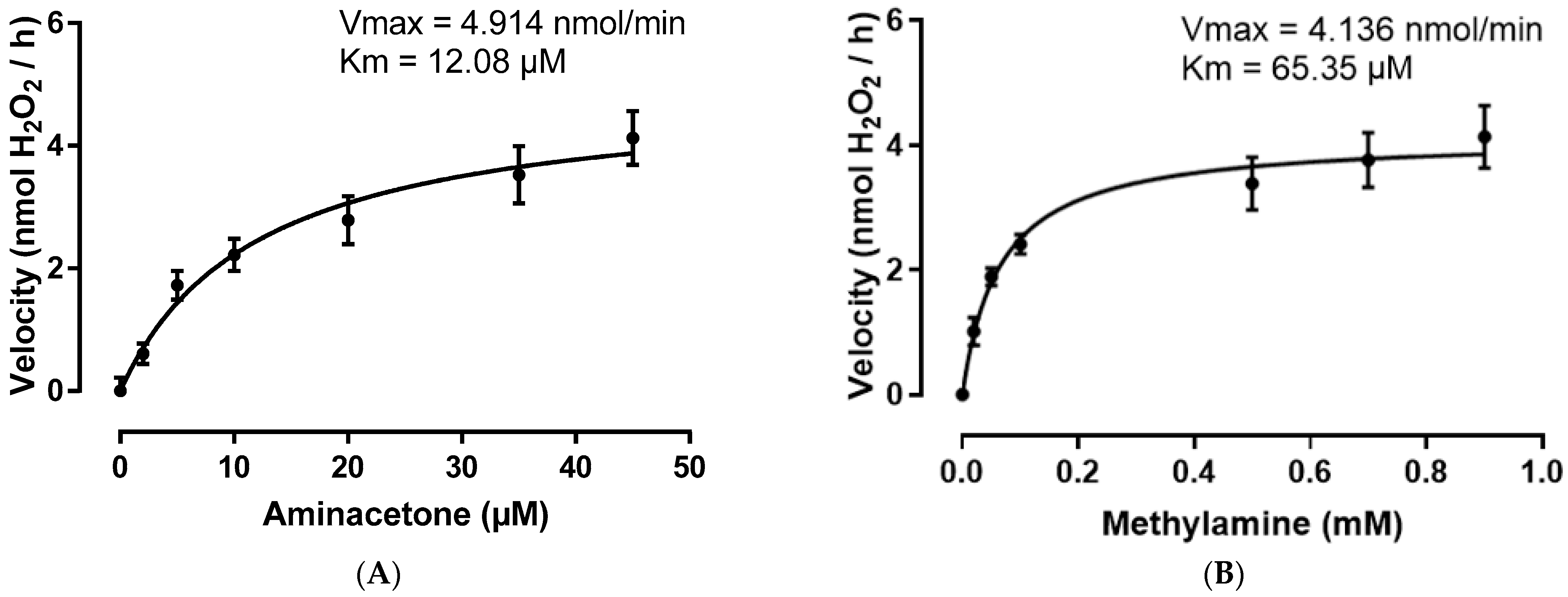
2.3. SSAO Has Higher Affinity for Aminoacetone Comparing to Methylamine and Converts Both at a Fast Rate in Rat Aortic VSMCs
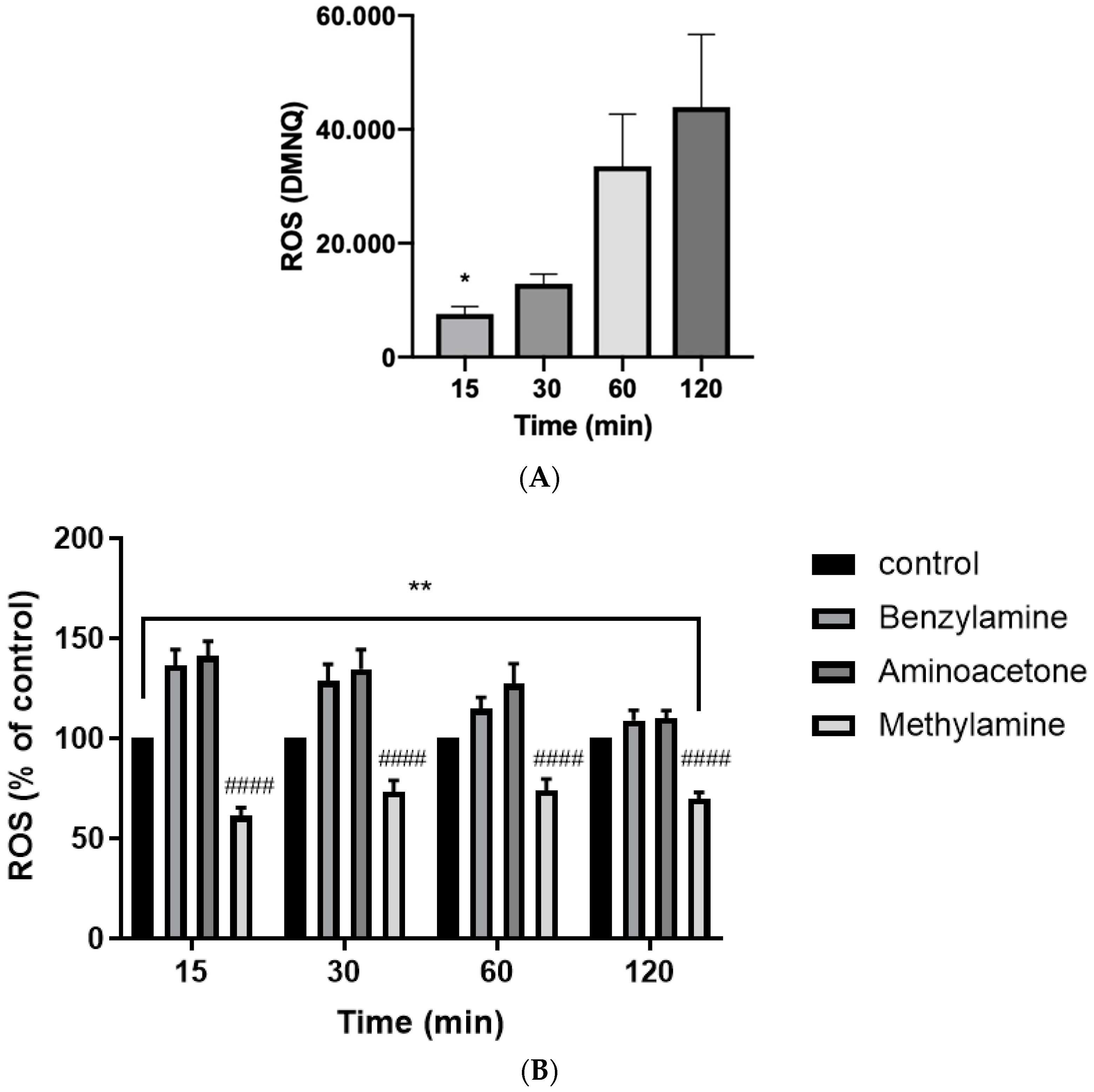
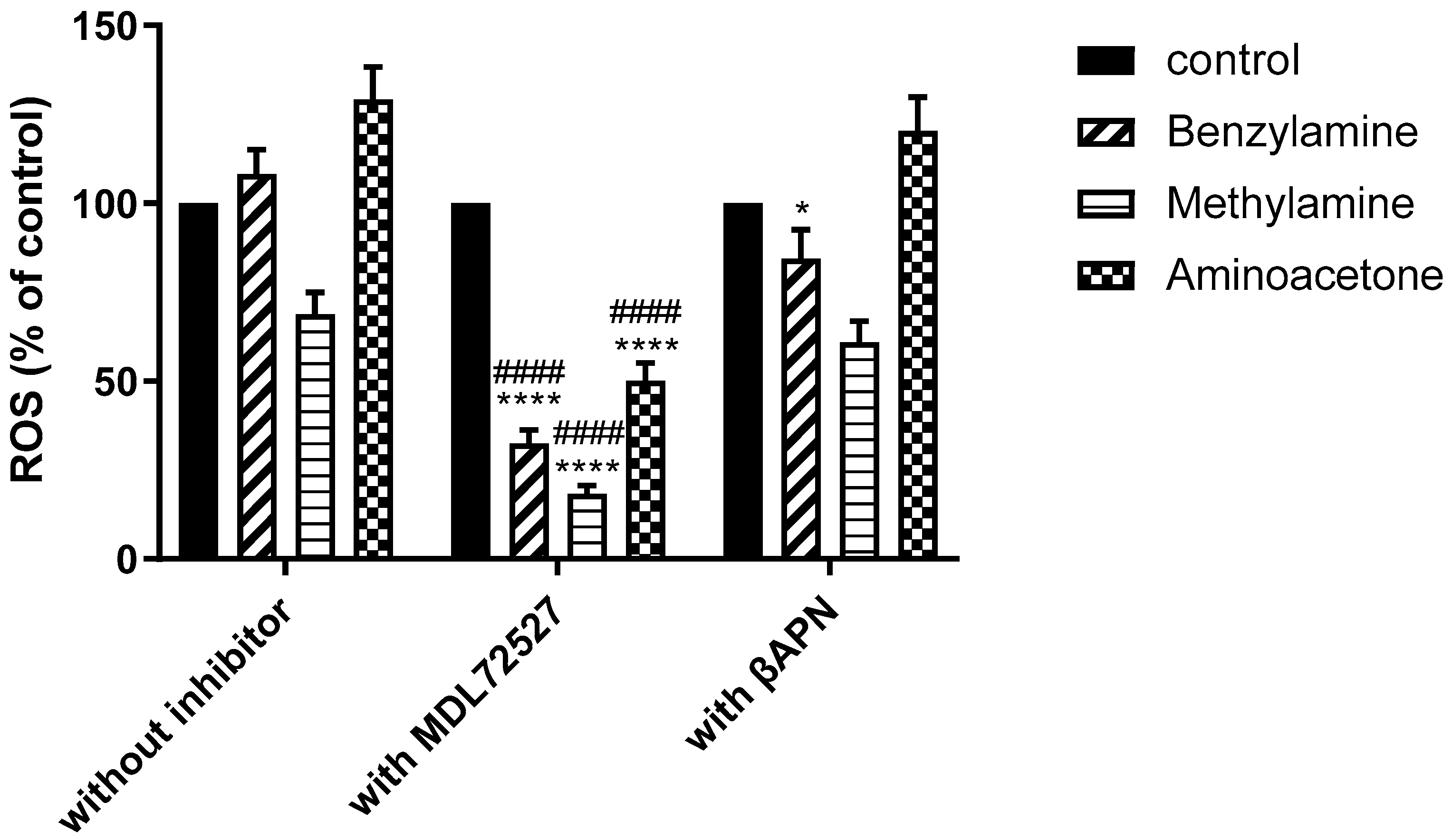
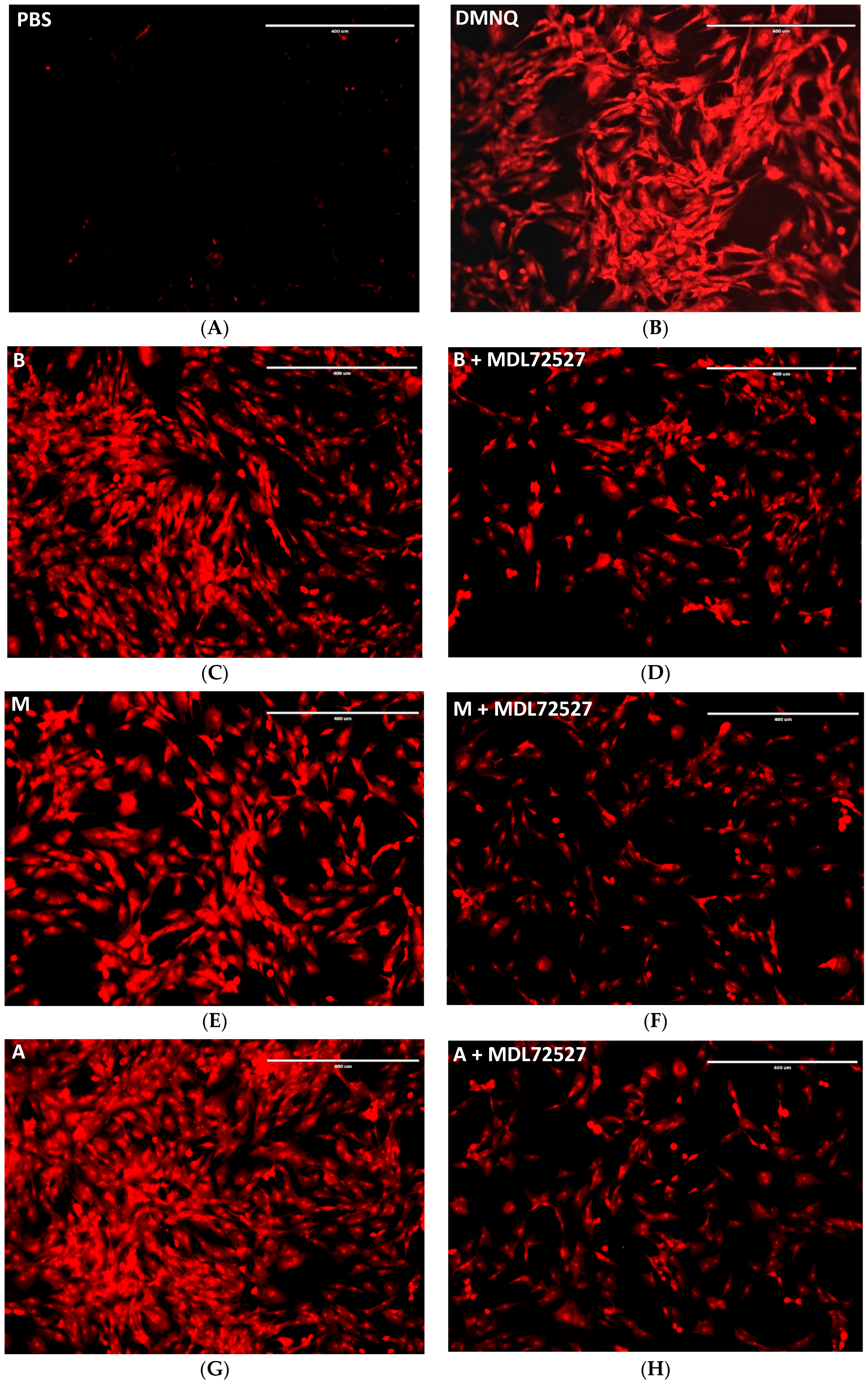
2.4. SSAO Activity Induces ROS Formation in Rat Aortic VSMCs
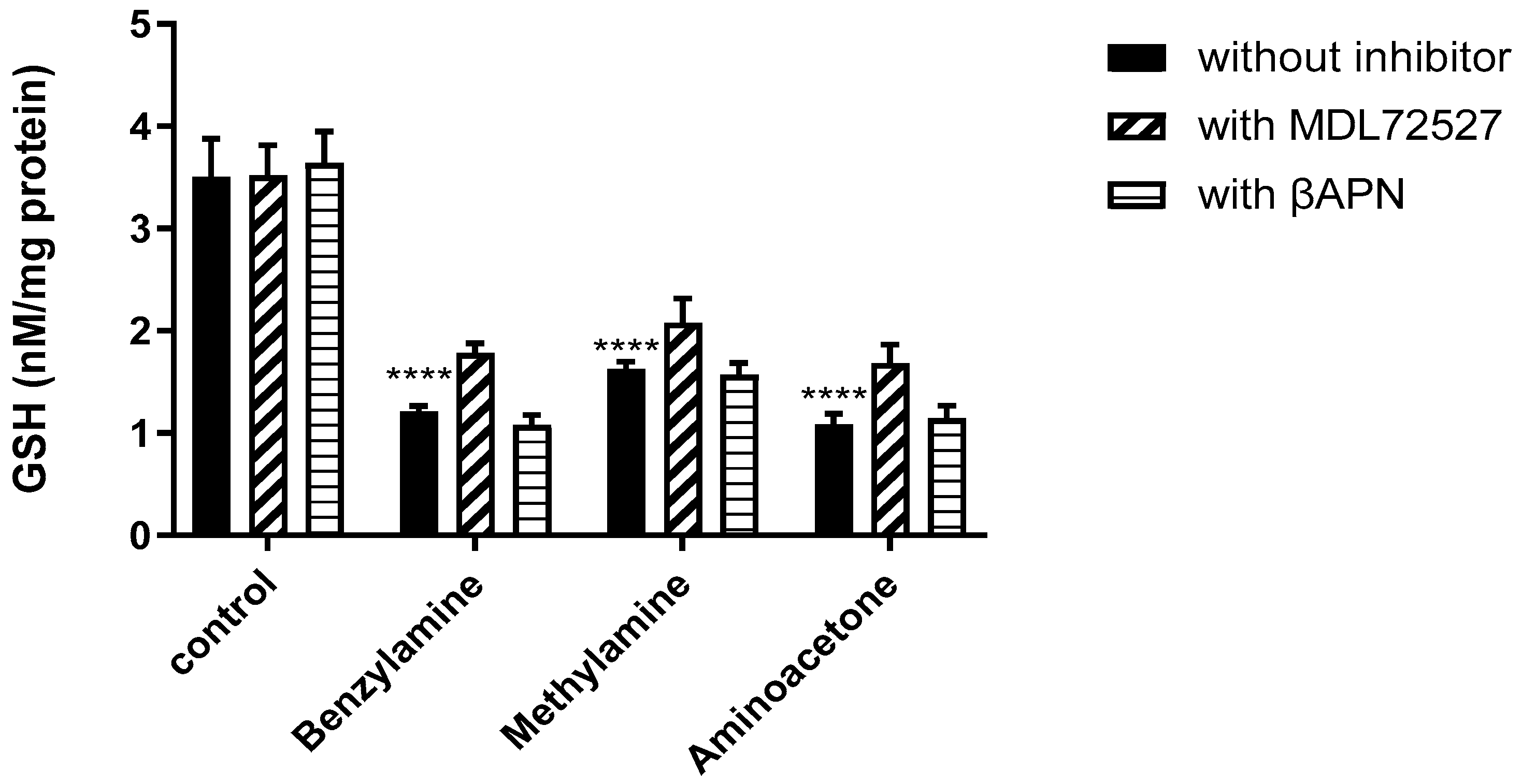
2.5. SSAO Activity Reduces Total GSH Levels in Rat Aortic VSMCs
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Cells
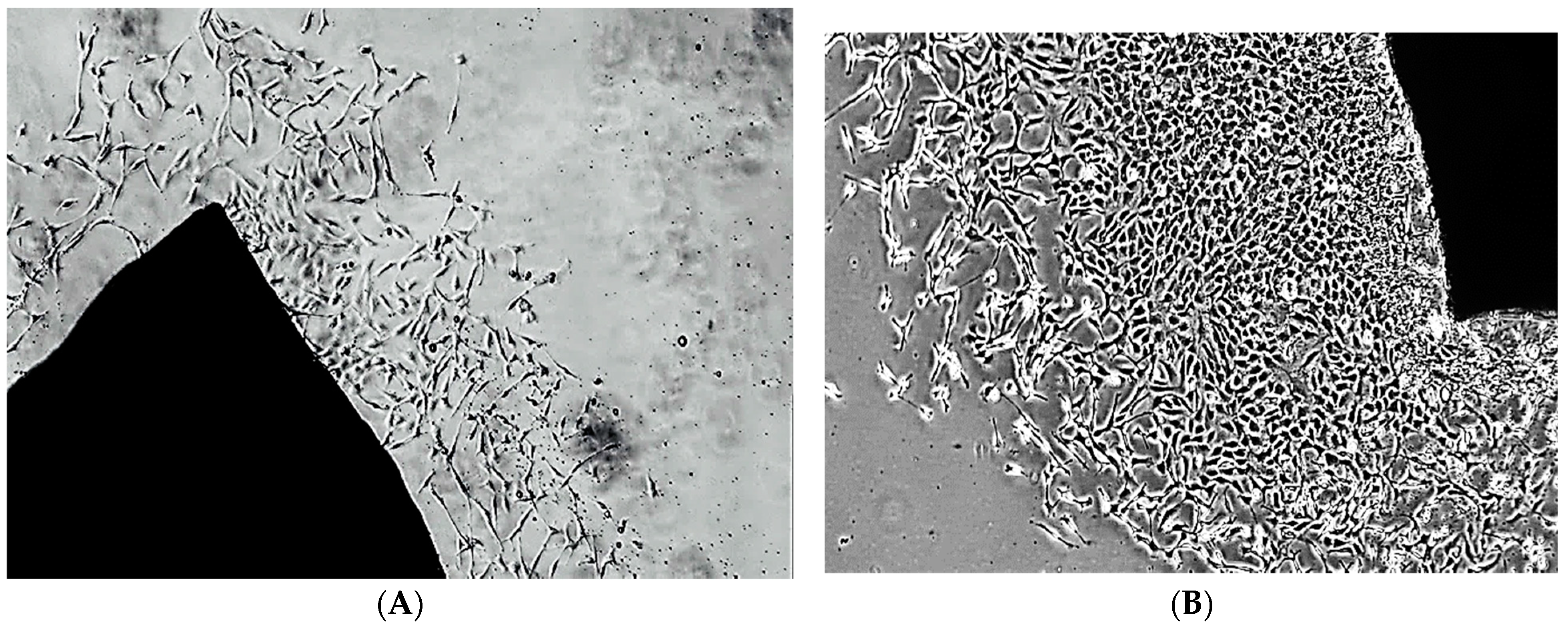
4.4. Isolation and Characterisation of Rat Aortic VSMCs
4.5. Cell Viability Assay
4.6. Amplex Red Assay
4.7. Reactive Oxygen Species (ROS) Assay
4.8. Measurement of Total Glutathione (GSH)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | Advanced glycation end products |
| AoC3 | Amine oxidase copper containing 3 |
| BCA | Bicinchoninic acid |
| βAPN | β-aminopropionitrile |
| CVD | Cardiovascular diseases |
| DMEM | Dulbecco’s modified eagle medium |
| DMNQ | 2,3-dimethoxy-1,4-naphthguinone |
| FBS | Fetal bovine serum |
| GSH | Total glutathione |
| HDL | High-density lipoprotein |
| HO-1 | Heme-oxygenase 1 |
| HRPO | Horseradish peroxidase |
| LDL | Low-density lipoprotein |
| LOX | Lysyl oxidase |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| oxLDL | Oxidized low-density lipoprotein |
| PBS | Phosphate Buffer Saline |
| ROS | Reactive oxygen species |
| SSA | Sulfosalicylic acid |
| VAP-1 | Vascular adhesion protein 1 |
| VSMCs | Vascular smooth muscle cells |
| TGRL | Triglyceride-rich lipoproteins |
Appendix A

References
- Abella, A.; Garcia-Vicente, S.; Viguerie, N.; Ros-Baro, A.; Camps, M.; Palacin, M.; Zorzano, A.; Marti, L. Adipocytes release a soluble form of VAP-1/SSAO by a metalloprotease-dependent process and in a regulated manner. Diabetologia 2004, 47, 429–438. [Google Scholar] [CrossRef] [Green Version]
- Stolen, C.M.; Yegutkin, G.G.; Kurkijärvi, R.; Bono, P.; Alitalo, K.; Jalkanen, S. Origins of Serum Semicarbazide-Sensitive Amine Oxidase. Circ. Res. 2004, 95, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Boomsma, F.; Hut, H.; Bagghoe, U.; Van Der Houwen, A.; Meiracker, A.V.D. Semicarbazide-sensitive amine oxidase (SSAO): From cell to circulation. Med. Sci. Monit. 2005, 11, RA122–RA126. [Google Scholar]
- Pannecoeck, R.; Serruys, D.; Benmeridja, L.; Delanghe, J.R.; van Geel, N.; Speeckaert, R.; Speeckaert, M.M. Vascular adhesion protein 1: Role in human pathology and application as a biomarker. Crit. Rev. Clin. Lab. Sci. 2015, 52, 284–300. [Google Scholar] [CrossRef]
- Manasieva, V.; Thakur, S.; Lione, L.A.; Patel, J.; Baydoun, A.; Skamarauskas, J. Semicarbazide-sensitive amine oxidase (SSAO) and Lysyl oxi-dase (LOX) association in rat aortic vascular smooth muscle cells. Biomolecules 2022, 11, 1563. [Google Scholar] [CrossRef]
- Rodríguez, C.; Alcudia, J.F.; Martínez-González, J.; Raposo, B.; Navarro, M.A.; Badimon, L. Lysyl oxidase (LOX) down-regulation by TNFα: A new mechanism underlying TNFα-induced endothelial dysfunction. Atherosclerosis 2004, 28, 558–564. [Google Scholar] [CrossRef]
- Rodríguez, C.; Martínez-González, J.; Raposo, B.; Alcudia, J.F.; Guadall, A.; Badimon, L. Regulation of lysyl oxidase in vascular cells: Lysyl oxidase as a new player in cardiovascular diseases. Cardiovasc. Res. 2008, 79, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Gimbrone, M.A.; Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 18, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 52, 524–533. [Google Scholar] [CrossRef]
- Vakhtangadze, T.; Singh Tak, R.; Singh, U.; Baig, M.S.; Bezsonov, E. Gender Differences in Atherosclerotic Vascular Disease: From Lipids to Clinical Outcomes. Front. Cardiovasc. Med. 2021, 8, 223–225. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Unzeta, M.; Healy, J.; O’Sullivan, M.I.; Davey, G.; Tipton, K.F. Semicarbazide-Sensitive Amine Oxidases: Enzymes with Quite a Lot to Do. NeuroToxicology 2004, 25, 303–315. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trøseid, M.; Ueland, T.; Hov, J.R.; Svardal, A.; Gregersen, I.; Dahl, C.P.; Aakhus, S.; Gude, E.; Bjørndal, B.; Halvorsen, B.; et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015, 277, 717–726. [Google Scholar] [CrossRef]
- Mente, A.; Chalcraft, K.; Ak, H.; Davis, A.D.; Lonn, E.; Miller, R.; Potter, M.A.; Yusuf, S.; Anand, S.S.; McQueen, M.J. The relationship between trimethylamine-N-oxide and prevalent cardiovascular disease in a multi-ethnic population living in Canada. Can. J. Cardiol. 2015, 31, 1189–1194. [Google Scholar] [CrossRef]
- Lever, M.; George, P.M.; Slow, S.; Bellamy, D.; Young, J.M.; Ho, M.; McEntyre, C.J.; Elmslie, J.L.; Atkinson, W.; Molyneux, S.L.; et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: An observational study. PLoS ONE. 2014, 9, e114969. [Google Scholar] [CrossRef] [Green Version]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA damage, DNA repair, aging, and neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-H.; Yu, T.-Y.; Hung, C.-S.; Yang, C.-Y.; Lin, M.-S.; Su, C.-Y.; Chen, Y.-L.; Kao, H.-L.; Chuang, L.-M.; Tsai, F.-C.; et al. Inhibition of Semicarbazide- sensitive Amine Oxidase Reduces Atherosclerosis in Cholesterol-fed New Zealand White Rabbits. Sci. Rep. 2018, 8, 9249. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-H.; Yu, T.-Y.; Tsai, F.-C.; Weston, C.-J.; Lin, M.-S.; Hung, C.-S.; Kao, H.-L.; Li, Y.-I.; Solé, M.; Unzeta, M.; et al. Inhibition of Semicarbazide-Sensitive Amine Oxidase Reduces Atherosclerosis in Apolipoprotein E-Deficient Mice. Transl. Res. 2018, 197, 12–31. [Google Scholar] [CrossRef]
- Gubisne-Haberle, D.; Hill, W.; Kazachkov, M.; Richardson, J.S.; Yu, P.H. Protein cross-linkage induced by formaldehyde derived from semicarbazide-sensitive amine oxidase-mediated deamination of methylamine. J. Pharmacol. Exp. Ther. 2004, 310, 1125–1132. [Google Scholar] [CrossRef] [Green Version]
- Dator, R.P.; Solivio, M.J.; Villalta, P.W.; Balbo, S. Bioanalytical and Mass Spectrometric Methods for Aldehyde Profiling in Biological Fluids. Toxics 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.H.; Mei-Zuo, D. Oxidative Deamination of Methylamine by Semicarbazide-Sensitive Amine Oxidase Leads to Cytotoxic Damage in Endothelial Cells. Diabetes 1993, 42, 87–93. [Google Scholar] [CrossRef]
- Hernandez, M.; Solé, M.; Boada, M.; Unzeta, M. Soluble Semicarbazide Sensitive Amine Oxidase (SSAO) catalysis induces apoptosis in vascular smooth muscle cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Mercier, N. The role of ‘‘semicarbazide-sensitive amine oxidase’’ in the arterial wall. Artery Res. 2009, 3, 141–147. [Google Scholar] [CrossRef]
- Mathys, K.C.; Ponnampalam, S.N.; Padival, S.; Nagaraj, R.H. Semicarbazide-sensitive amine oxidase in aortic smooth muscle cells mediates synthesis of a methylglyoxal-AGE: Implications for vascular complications in diabetes. Biochem. Biophys. Res. Commun. 2002, 297, 863–869. [Google Scholar] [CrossRef]
- Lin, Z.; Li, H.; Luo, H.; Zhang, Y.; Luo, W. Benzylamine and methylamine, substrates of semicarbazide-sensitive amine oxidase, attenuate inflammatory response induced by lipopolysaccharide. Int. Immunopharmacol. 2011, 2, 1079–1089. [Google Scholar] [CrossRef]
- Vidrio, H.; Medina, M.; González-Romo, P.; Lorenzana-Jiménez, M.; Díaz-Arista, P.; Baeza, A. Semicarbazide-sensitive amine oxidase substrates potentiate hydralazine hypotension: Possible role of hydrogen peroxide. J. Pharmacol. Exp. Ther. 2003, 307, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Zorzano, A.; Abella, A.; Marti, L.; Carpéné, C.; Palacín, M.; Testar, X. Semicarbazide-sensitive amine oxidase activity exerts insulin-like effects on glucose metabolism and insulin-signalling pathways in adipose cells. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2003, 47, 3–9. [Google Scholar] [CrossRef]
- Byon, C.H.; Heath, J.M.; Chen, Y. Redox signalling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016, 9, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Obata, T. Diabetes, and semicarbazide-sensitive amine oxidase (SSAO) activity: A review. Life Sci. 2006, 79, 417–422. [Google Scholar] [CrossRef]
- Xiao, W.; Loscalzo, J.; Quiles, J.L.; Sánchez-González, C.; Vera-Ramírez, L.; Giampieri, F.; Navarro-Hortal, M.D.; Llopis, J.; Battino, M.; Varela-López, A. Metabolic Responses to Reductive Stress. Antioxid. Redox Signal. 2020, 32, 1332–1347. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Li, H.; Luo, H.; Luo, W. Inhibition of Semicarbazide- Sensitive Amine Oxidase Attenuates Myocardial Ischemia-Reperfusion Injury in an In Vivo Rat Model. Life Sci. 2011, 88, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, L.; Zhi, F.; Niu, P.; Yang, M.; Zhu, X.; Diao, Y.; Li, Y.; Wang, J.; Zhao, Y. Inactivation of Semicarbazide-Sensitive Amine Oxidase Induces the Phenotypic Switch of Smooth Muscle Cells and Aggravates the Development of Atherosclerotic Lesions. Atherosclerosis 2016, 249, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Okin, D.; Medzhitov, R. The Effect of Sustained Inflammation on Hepatic Mevalonate Pathway Results in Hyperglycemia. Cell 2016, 165, 343–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Yu, P.H. Assessment of the Deamination of Aminoacetone, an Endogenous Substrate for Semicarbazide-Sensitive Amine Oxidase. Anal. Biochem. 1999, 270, 97–102. [Google Scholar] [CrossRef]
- Nayak, D.D.; Agashe, D.; Lee, M.-C.; Marx, C.J. Selection Maintains Apparently Degenerate Metabolic Pathways due to Tradeoffs in Using Methylamine for Carbon versus Nitrogen. Curr. Biol. 2016, 26, 1416–1426. [Google Scholar] [CrossRef] [Green Version]
- Sartori, A.; Garay-Malpartida, H.M.; Forni, M.F.; Schumacher, R.I.; Dutra, F.; Sogayar, M.C.; Bechara, E.J. Aminoacetone, a putative endogenous source of methylglyoxal, causes oxidative stress and death to insulin-producing rinm5f cells. Chem. Res. Toxicol. 2008, 21, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Sartori, A.; Mano, C.M.; Nascimento, O.R.; Dyszy, F.H.; Nantes, I.L.; Bechara, E.J.H. Oxidative Damage to Cytochrome C Induced by Aminoacetone. Free Radic. Biol. Med. 2010, 49, S171. [Google Scholar] [CrossRef]
- Lin, Z.; Luo, W.; Li, H.; Zhang, Y. The effect of endogenous formaldehyde on the rat aorta endothelial cells. Toxicol. Lett. 2005, 159, 134–143. [Google Scholar] [CrossRef]
- Braun, J.D.; Pastene, D.O.; Breedijk, A.; Rodriguez, A.; Hofmann, B.B.; Sticht, C.; von Ochsenstein, E.; Allgayer, H.; Born, J.V.D.; Bakker, S.; et al. Methylglyoxal down-regulates the expression of cell cycle associated genes and activates the p53 pathway in human umbilical vein endothelial cells. Sci. Rep. 2019, 9, 1152. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, Y.; He, X.; Yang, P.; Zong, T.; Sun, P.; Sun, R.; Yu, T.; Jiang, Z. The cellular function and molecular mechanism of formaldehyde in cardiovascular disease and heart development. J. Cell. Mol. Med. 2021, 25, 5358–5371. [Google Scholar] [CrossRef]
- Saito, Y.; Nishio, K.; Yoshida, Y.; Niki, E. Cytotoxic effect of formaldehyde with free radicals via increment of cellular reactive oxygen species. Toxicology 2005, 210, 235–245. [Google Scholar] [CrossRef]
- Desai, K.M.; Chang, T.; Wang, H.; Banigesh, A.; Dhar, A.; Liu, J.; Untereiner, A.; Wu, L. Oxidative stress and aging: Is methylglyoxal the hidden enemy? Can. J. Physiol. Pharmacol. 2010, 88, 10–21. [Google Scholar]
- Kim, D.; Kim, K.-A.; Kim, J.-H.; Kim, E.-H.; Bae, O.-N. Methylglyoxal-Induced Dysfunction in Brain Endothelial Cells via the Suppression of Akt/HIF-1α Pathway and Activation of Mitophagy Associated with Increased Reactive Oxygen Species. Antioxidants 2020, 9, 820. [Google Scholar] [CrossRef]
- Liu, C.; Cao, B.; Zhang, Q.; Zhang, Y.; Chen, X.; Kong, X.; Dong, Y. Inhibition of thioredoxin 2 by intracellular methylglyoxal accumulation leads to mitochondrial dysfunction and apoptosis in INS-1 cells. Endocrine 2020, 68, 103–115. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; Ciriolo, M. Glutathione: New roles in redox signalling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.J.; Bourne, L.E.; Thakur, S.; Farrington, K.; Gorog, D.A.; Orriss, I.R.; Baydoun, A.R. 2-Oxothiazolidine-4-carboxylic acid inhibits vascularcalcification via induction of glutathione synthesis. J. Cell. Physiol. 2020, 236, 2696–2705. [Google Scholar] [CrossRef]
- Franco, R.; Panayiotidis, M.I.; Cidlowski, J.A. Glutathione Depletion Is Necessary for Apoptosis in Lymphoid Cells Independent of Reactive Oxygen Species Formation. J. Biol. Chem. 2007, 282, 30452–30465. [Google Scholar] [CrossRef] [Green Version]
- Boomsma, F.; van Dijk, J.; Bhaggoe, U.M.; Bouhuizen, A.M.; Meiracker, A.H.V.D. Variation in semicarbazide-sensitive amine oxidase activity in plasma and tissues of mammals. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 126, 69–78. [Google Scholar] [CrossRef]
- Chi, J.; Meng, L.; Pan, S.; Lin, H.; Zhai, X.; Liu, L.; Zhou, C.; Jiang, C.; Guo, H. Primary Culture of Rat Aortic Vascular Smooth Muscle Cells: A New Method. Med. Sci. Monit. 2017, 23, 4014–4020. [Google Scholar] [CrossRef] [Green Version]
- Oosterhoff, L.A.; Kruitwagen, H.S.; Van Wolferen, M.E.; Van Balkom, B.W.; Mokry, M.; Lansu, N.; Dungen, N.A.V.D.; Penning, L.C.; Spanjersberg, T.C.; De Graaf, J.W.; et al. Characterization of Endothelial and Smooth Muscle Cells from Different Canine Vessels. Front. Physiol. 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay guidance manual; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Tchivilev, I.; Madamanchi, N.R.; Vendrov, A.E.; Niu, X.L.; Runge, M.S. Identification of a protective role for protein phosphatase 1cγ1 against oxidative stress-induced vascular smooth muscle cell apoptosis. J. Biol. Chem. 2008, 283, 22193–22205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulphide levels using enzymatic recycling method. Nat. Protoc. 2006, 27, 3150–3165. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manasieva, V.; Thakur, S.; Lione, L.A.; Baydoun, A.R.; Skamarauskas, J. The Impact of Semicarbazide Sensitive Amine Oxidase Activity on Rat Aortic Vascular Smooth Muscle Cells. Int. J. Mol. Sci. 2023, 24, 4946. https://doi.org/10.3390/ijms24054946
Manasieva V, Thakur S, Lione LA, Baydoun AR, Skamarauskas J. The Impact of Semicarbazide Sensitive Amine Oxidase Activity on Rat Aortic Vascular Smooth Muscle Cells. International Journal of Molecular Sciences. 2023; 24(5):4946. https://doi.org/10.3390/ijms24054946
Chicago/Turabian StyleManasieva, Vesna, Shori Thakur, Lisa A. Lione, Anwar R. Baydoun, and John Skamarauskas. 2023. "The Impact of Semicarbazide Sensitive Amine Oxidase Activity on Rat Aortic Vascular Smooth Muscle Cells" International Journal of Molecular Sciences 24, no. 5: 4946. https://doi.org/10.3390/ijms24054946
APA StyleManasieva, V., Thakur, S., Lione, L. A., Baydoun, A. R., & Skamarauskas, J. (2023). The Impact of Semicarbazide Sensitive Amine Oxidase Activity on Rat Aortic Vascular Smooth Muscle Cells. International Journal of Molecular Sciences, 24(5), 4946. https://doi.org/10.3390/ijms24054946








