In Situ Formation of Injectable Gelatin Methacryloyl (GelMA) Hydrogels for Effective Intraocular Delivery of Triamcinolone Acetonide
Abstract
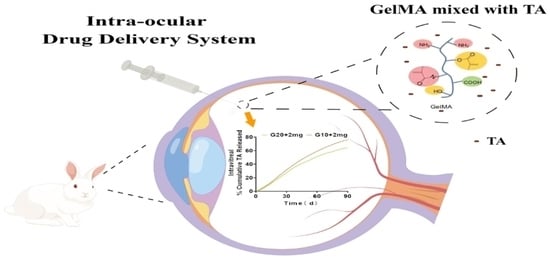
:1. Introduction
2. Result
2.1. Properties of GelMA-TA
2.2. Ex Vivo Permeation Study
2.3. In Vitro Assessment of Cytocompatibility
2.4. In Vivo Release of TA from Implants
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the GelMA Hydrogels Solutions and TA-Loaded GelMA Solution
4.3. Scanning Electron Microscope Imaging
4.4. Swelling Measurement
4.5. In Vitro Degradation Study of GelMA
4.6. In Vitro Drug Release
4.7. In Vitro Cell Studies
4.7.1. Cell Cultures
4.7.2. Assessment of HRPEs Cell Viability
4.7.3. HRPE Migration Function
4.7.4. UV Radiation Procedure
4.7.5. Quantitative Real-Time PCR (qRT-PCR)
4.8. Animal Studies
4.8.1. Intravitreal Gel Injection
4.8.2. Slit-Lamp, Color Fundus Photos, and Optical Coherence Tomography Evaluation for Ocular Media and Retinal Tissue
4.8.3. Assessment of Retinal Function by Electroretinography (ERG)
4.8.4. Intraocular Pressure (IOP) Measurements
4.8.5. Histopathological Examination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DDS | drug delivery system |
| GelMA | gelatin methacryloyl |
| HRPE | human retinal pigmental epithelial |
| HE | hematoxylin and eosin |
| HPLC | high performance liquid chromatography |
| IV | intravitreal |
| IOP | intraocular pressure |
| LAP | lithium phenyl-2,4,6-trimethybenzoylphosphinate |
| OCT | optical coherence tomography |
| PLA | poly(lactide) |
| PEG | poly(ethylene glycol) |
| PLGA | polylactic-co-glycolic acid |
| qRT-PCR | quantitative real-time PCR |
| SEM | scanning electron microscope |
| TA | triamcinolone acetonide |
References
- Janoria, K.G.; Gunda, S.; Boddu, S.H.; Mitra, A.K. Novel approaches to retinal drug delivery. Expert Opin. Drug Deliv. 2007, 4, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Hayashi, H. Intravitreal versus retrobulbar injections of triamcinolone for macular edema associated with branch retinal vein occlusion. Am. J. Ophthalmol. 2005, 139, 972–982. [Google Scholar] [CrossRef]
- Maris, P.J., Jr.; Correnti, A.J.; Donnenfeld, E.D. Intracameral triamcinolone acetonide as treatment for endothelial allograft rejection after penetrating keratoplasty. Cornea 2008, 27, 847–850. [Google Scholar] [CrossRef]
- Lin, R.C.; Sanduja, N.; Hariprasad, S.M. Successful treatment of postoperative fungal endophthalmitis using intravitreal and intracameral voriconazole. J. Ocul. Pharm. Ther. 2008, 24, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Velez, G.; Whitcup, S.M. New developments in sustained release drug delivery for the treatment of intraocular disease. Br. J. Ophthalmol. 1999, 83, 1225–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano, A.G.R.; de Fátima Pereira, A.; de Faria, L.G.A.; Fialho, S.L.; de Oliveira Patricio, P.S.; da Silva-Cunha, A.; Fulgêncio, G.O.; da Silva, G.R.; Pianetti, G.A. Etoposide-Loaded Poly(Lactic-co-Glycolic Acid) Intravitreal Implants: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2018, 19, 1652–1661. [Google Scholar] [CrossRef]
- McAvoy, K.; Jones, D.; Thakur, R.R.S. Synthesis and Characterisation of Photocrosslinked poly(ethylene glycol) diacrylate Implants for Sustained Ocular Drug Delivery. Pharm. Res. 2018, 35, 36. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.; Hashmi, U.; Riaz, R.; Rukh Abbas, S. Chitosan coated liposomes (CCL) containing triamcinolone acetonide for sustained delivery: A potential topical treatment for posterior segment diseases. Int. J. Biol. Macromol. 2020, 143, 483–491. [Google Scholar] [CrossRef]
- Blatsios, G.; Tzimas, A.S.; Mattheolabakis, G.; Panagi, Z.; Avgoustakis, K.; Gartaganis, S.P. Development of biodegradable controlled release scleral systems of triamcinolone acetonide. Curr. Eye Res. 2010, 35, 916–924. [Google Scholar] [CrossRef]
- Haller, J.A.; Bandello, F.; Belfort, R., Jr.; Blumenkranz, M.S.; Gillies, M.; Heier, J.; Loewenstein, A.; Yoon, Y.H.; Jacques, M.L.; Jiao, J.; et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010, 117, 1134–1146.e3. [Google Scholar] [CrossRef]
- Callanan, D.G.; Jaffe, G.J.; Martin, D.F.; Pearson, P.A.; Comstock, T.L. Treatment of posterior uveitis with a fluocinolone acetonide implant: Three-year clinical trial results. Arch Ophthalmol. 2008, 126, 1191–1201. [Google Scholar]
- Soares, D.C.F.; de Paula Oliveira, D.C.; Barcelos, L.S.; Barbosa, A.S.; Vieira, L.C.; Townsend, D.M.; Rubello, D.; de Barros, A.L.B.; Duarte, L.P.; Silva-Cunha, A. Antiangiogenic activity of PLGA-Lupeol implants for potential intravitreal applications. Biomed. Pharm. 2017, 92, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Fialho, S.L.; da Silva, G.R.; Silva-Cunha, A.; Zhao, M.; Behar-Cohen, F. Ocular safety of Intravitreal Clindamycin Hydrochloride Released by PLGA Implants. Pharm. Res. 2017, 34, 1083–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, H.H.; Shin, Y.N.; Cho, M.H.; Khang, G.; Lee, H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials 2007, 28, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Sun, W.; Fang, J.; Lee, K.; Li, S.; Gu, Z.; Dokmeci, M.R.; Khademhosseini, A. Biodegradable Gelatin Methacryloyl Microneedles for Transdermal Drug Delivery. Adv. Healthc. Mater. 2019, 8, e1801054. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Du, X.; Li, W.; Wang, Q.; He, D.; Yuan, J. Natural polymer-derived photocurable bioadhesive hydrogels for sutureless keratoplasty. Bioact. Mater. 2022, 8, 196–209. [Google Scholar] [CrossRef]
- Komez, A.; Baran, E.T.; Erdem, U.; Hasirci, N.; Hasirci, V. Construction of a patterned hydrogel-fibrous mat bilayer structure to mimic choroid and Bruch’s membrane layers of retina. J. Biomed. Mater. Res. A 2016, 104, 2166–2177. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chan-Park, M.B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 2010, 31, 1158–1170. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedram, P.; Mazio, C.; Imparato, G.; Netti, P.A.; Salerno, A. Bioinspired Design of Novel Microscaffolds for Fibroblast Guidance toward In Vitro Tissue Building. ACS Appl. Mater. Interfaces 2021, 13, 9589–9603. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, J.A.; Souza-Filho, A.A.; Oliveira, A.G. Intravitreal Bioerudivel sustained-release triamcinolone microspheres system (RETAAC). Preliminary report of its potential usefulnes for the treatment of diabetic macular edema. Arch Soc. Esp. Oftalmol. 2006, 81, 675–677, 679–681. [Google Scholar]
- Macky, T.A.; Oelkers, C.; Rix, U.; Heredia, M.L.; Künzel, E.; Wimberly, M.; Rohrer, B.; Crosson, C.E.; Rohr, J. Synthesis, pharmacokinetics, efficacy, and rat retinal toxicity of a novel mitomycin C-triamcinolone acetonide conjugate. J. Med. Chem. 2002, 45, 1122–1127. [Google Scholar] [CrossRef]
- Kompella, U.B.; Bandi, N.; Ayalasomayajula, S.P. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1192–1201. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, L.B.M.; Cocchi, M.; Piemonte, V. Drug Delivery with Membranes Systems. In Current Trends and Future Evelopments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; Volume 2019, pp. 2291–2309. [Google Scholar]
- Abedini, F.E.M.; Roozbehani, A.H.; Domb, A.J.; Hosseinkhani, H. Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polym. Adv. Technol. 2018, 29, 2564–2573. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharm. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Gilchrist, A.E.L.S.; Hu, Y.; Harley, B.A.C. Mesenchymal stromal cell remodeling of a gelatin hydrogel microenvironment defines an artificial hematopoietic stem cell niche. BioRxiv 2018, 289553. [Google Scholar] [CrossRef] [Green Version]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef]
- Miri, A.K.; Hosseinabadi, H.G.; Cecen, B.; Hassan, S.; Zhang, Y.S. Permeability mapping of gelatin methacryloyl hydrogels. Acta Biomater. 2018, 77, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Pahoff, S.; Bock, N.; Hutmacher, D.W. Gelatin Methacryloyl Hydrogels Control the Localized Delivery of Albumin-Bound Paclitaxel. Polymers 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.; Hutmacher, D.W.; Melchels, F.P.; Klein, T.J.; Malda, J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Doty, A.C.; Weinstein, D.G.; Hirota, K.; Olsen, K.F.; Ackermann, R.; Wang, Y.; Choi, S.; Schwendeman, S.P. Mechanisms of in vivo release of triamcinolone acetonide from PLGA microspheres. J. Control. Release 2017, 256, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Audren, F.; Tod, M.; Massin, P.; Benosman, R.; Haouchine, B.; Erginay, A.; Caulin, C.; Gaudric, A.; Bergmann, J.F. Pharmacokinetic-pharmacodynamic modeling of the effect of triamcinolone acetonide on central macular thickness in patients with diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3435–3441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.F.; Gao, Y.F.; Xie, H.T.; Wang, H.J. Pharmacokinetics and retinal toxicity of various doses of intravitreal triamcinolone acetonide in rabbits. Mol. Vis. 2014, 20, 629–636. [Google Scholar]
- A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2008, 115, 1447–1449, 1449.e1441-1410. [CrossRef] [Green Version]
- Aref, A.A.; Scott, I.U.; Oden, N.L.; Ip, M.S.; Blodi, B.A.; VanVeldhuisen, P.C. Incidence, Risk Factors, and Timing of Elevated Intraocular Pressure After Intravitreal Triamcinolone Acetonide Injection for Macular Edema Secondary to Retinal Vein Occlusion: SCORE Study Report 15. JAMA Ophthalmol. 2015, 133, 1022–1029. [Google Scholar] [CrossRef]
- Krouwels, A.; Melchels, F.P.W.; van Rijen, M.H.P.; Öner, F.C.; Dhert, W.J.A.; Tryfonidou, M.A.; Creemers, L.B. Comparing Hydrogels for Human Nucleus Pulposus Regeneration: Role of Osmolarity During Expansion. Tissue Eng. Part C Methods 2018, 24, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Allen, B.N.; Wiley, L.A.; Tucker, B.A.; Worthington, K.S. Development of High-Resolution Three-Dimensional-Printed Extracellular Matrix Scaffolds and Their Compatibility with Pluripotent Stem Cells and Early Retinal Cells. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2020, 36, 42–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.B.; Schlichtenbrede, F. Visual acuity and intraocular pressure after high-dose intravitreal triamcinolone acetonide in selected ocular diseases. Eye 2008, 22, 869–873. [Google Scholar] [CrossRef] [Green Version]
- Im, L.; Allingham, R.R.; Singh, I.; Stinnett, S.; Fekrat, S. A prospective study of early intraocular pressure changes after a single intravitreal triamcinolone injection. J. Glaucoma. 2008, 17, 128–132. [Google Scholar] [CrossRef]
- Ozkiriş, A.; Erkiliç, K. Complications of intravitreal injection of triamcinolone acetonide. Can. J. Ophthalmol. 2005, 40, 63–68. [Google Scholar] [CrossRef]
- Wang, B.; Díaz-Payno, P.J.; Browe, D.C.; Freeman, F.E.; Nulty, J.; Burdis, R.; Kelly, D.J. Affinity-bound growth factor within sulfated interpenetrating network bioinks for bioprinting cartilaginous tissues. Acta Biomater. 2021, 128, 130–142. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [Green Version]









| Group Denomination | GelMA Concentration (%) | Dose (mg) | Mean Release (μg) of 30 d | Cumulative Release of 30 d | Peak Release (μg) |
|---|---|---|---|---|---|
| G10 + TA 1 mg | 10 | 1 | 14.14 ± 2.11 | 42.42 ± 1.66 | 16.50 ± 0.98 |
| G10 + TA 2 mg | 10 | 2 | 21.01 ± 3.92 | 31.51 ± 1.18 | 24.79 ± 2.48 |
| G10 + TA 4 mg | 10 | 4 | 36.73 ± 9.97 | 27.55 ± 1.59 | 45.16 ± 3.12 |
| G10 + TA 8 mg | 10 | 8 | 62.14 ± 12.69 | 23.30 ± 1.86 | 75.76 ± 8.33 |
| G20 + TA 1 mg | 20 | 1 | 11.71 ± 1.78 | 35.14 ± 1.29 | 14.95 ± 1.37 |
| G20 + TA 2 mg | 20 | 2 | 18.12 ± 4.58 | 22.86 ± 0.87 | 27.3 ± 0.93 |
| G20 + TA 4 mg | 20 | 4 | 26.91 ± 7.59 | 20.18 ± 1.32 | 36.91 ± 3.69 |
| G20 + TA 8 mg | 20 | 8 | 36.26 ± 9.20 | 13.23 ± 0.88 | 50.78 ± 1.94 |
| TA 1 mg | 0 | 1 | 28.67 ± 13.31 | 88.23 ± 1.94 | 46.90 ± 1.55 |
| TA 2 mg | 0 | 2 | 55.18 ± 4.62 | 82.77 ± 1.81 | 61.57 ± 0.82 |
| TA 4 mg | 0 | 4 | 78.01 ± 13.33 | 58.51 ± 0.24 | 100.21 ± 4.83 |
| TA 8 mg | 0 | 8 | 94.18 ± 20.06 | 35.32 ± 0.27 | 114.91 ± 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Zhao, X.; Ren, Z.; Yang, B.; Wang, X.; Hu, A.; Hu, J. In Situ Formation of Injectable Gelatin Methacryloyl (GelMA) Hydrogels for Effective Intraocular Delivery of Triamcinolone Acetonide. Int. J. Mol. Sci. 2023, 24, 4957. https://doi.org/10.3390/ijms24054957
Shen C, Zhao X, Ren Z, Yang B, Wang X, Hu A, Hu J. In Situ Formation of Injectable Gelatin Methacryloyl (GelMA) Hydrogels for Effective Intraocular Delivery of Triamcinolone Acetonide. International Journal of Molecular Sciences. 2023; 24(5):4957. https://doi.org/10.3390/ijms24054957
Chicago/Turabian StyleShen, Chaolan, Xuan Zhao, Zewen Ren, Bing Yang, Xiaohui Wang, Andina Hu, and Jie Hu. 2023. "In Situ Formation of Injectable Gelatin Methacryloyl (GelMA) Hydrogels for Effective Intraocular Delivery of Triamcinolone Acetonide" International Journal of Molecular Sciences 24, no. 5: 4957. https://doi.org/10.3390/ijms24054957
APA StyleShen, C., Zhao, X., Ren, Z., Yang, B., Wang, X., Hu, A., & Hu, J. (2023). In Situ Formation of Injectable Gelatin Methacryloyl (GelMA) Hydrogels for Effective Intraocular Delivery of Triamcinolone Acetonide. International Journal of Molecular Sciences, 24(5), 4957. https://doi.org/10.3390/ijms24054957