6-Shogaol as a Novel Thioredoxin Reductase Inhibitor Induces Oxidative-Stress-Mediated Apoptosis in HeLa Cells
Abstract
1. Introduction
2. Results
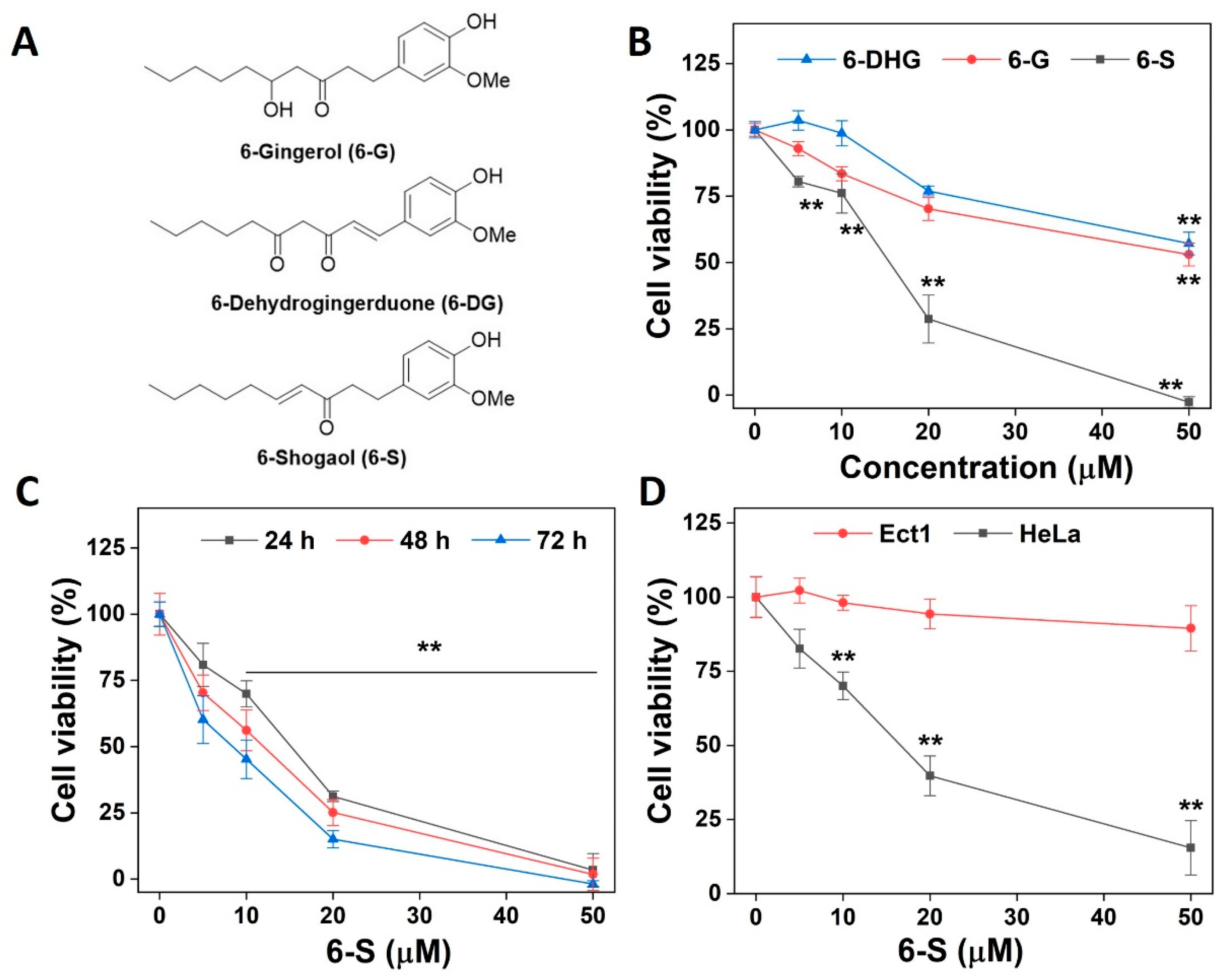
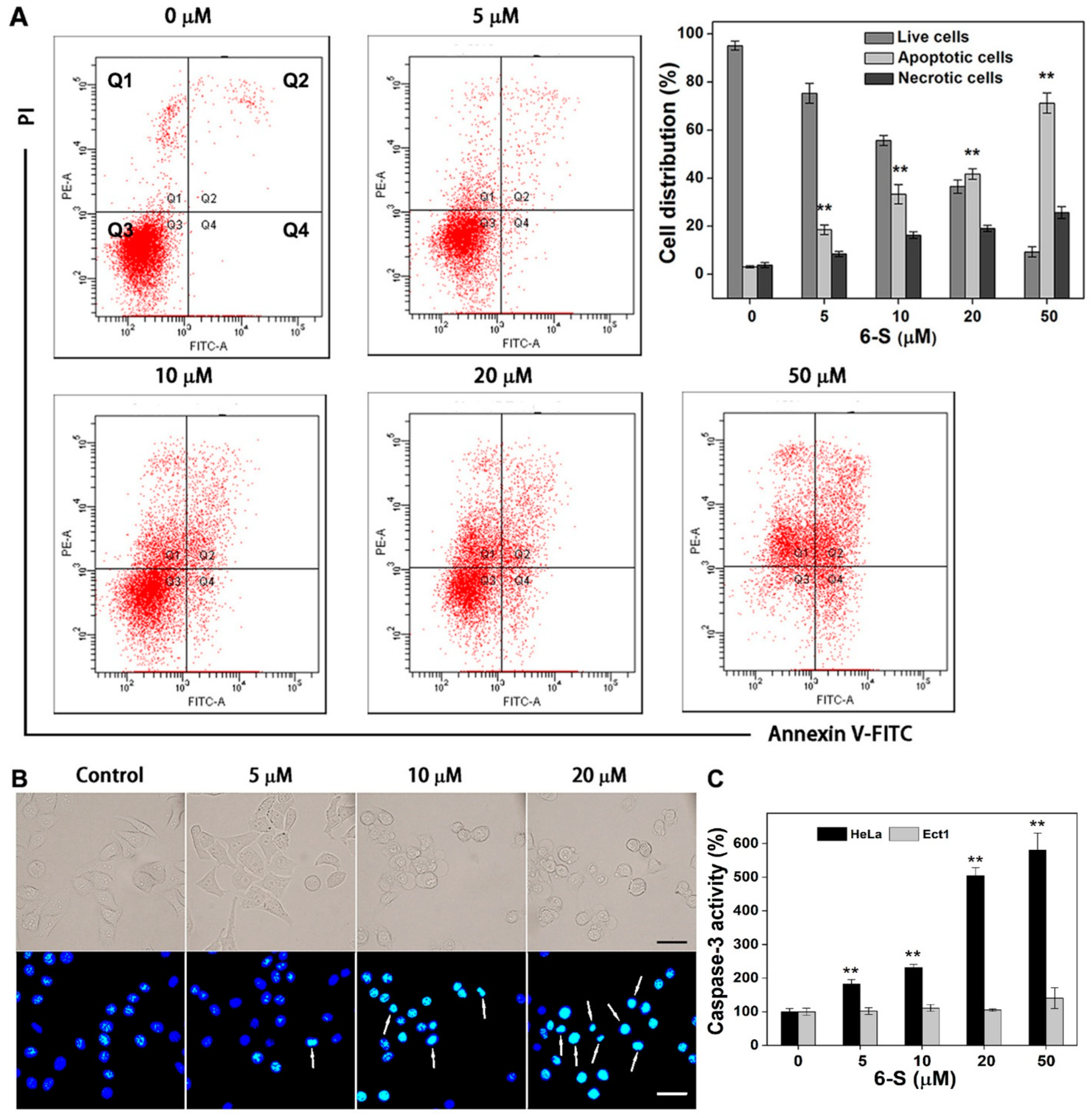
2.1. Induction of Cell Death by 6-S
2.2. Inhibition of Purified TrxR Activity by 6-S
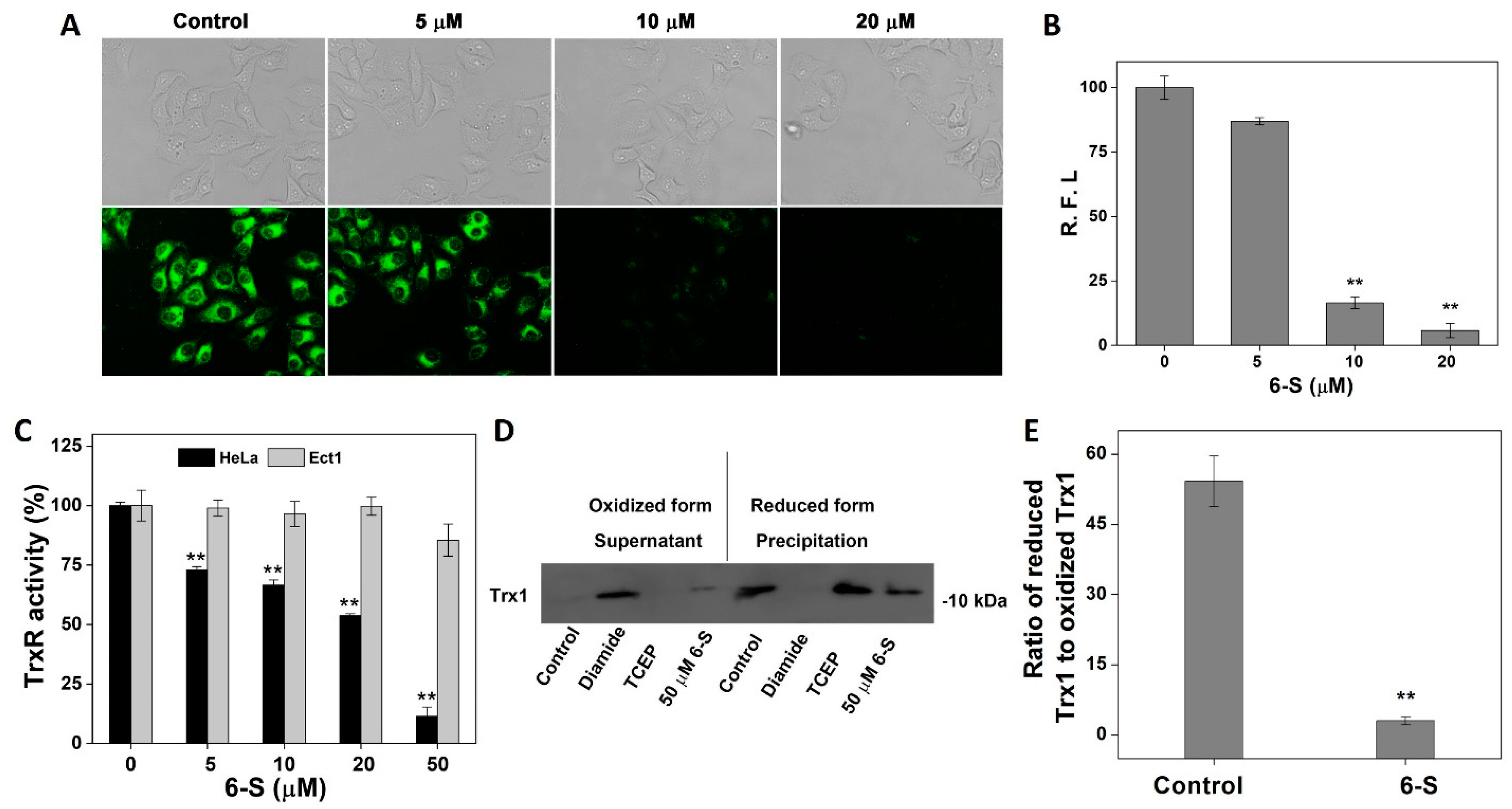
2.3. Influence of 6-S on Intracellular TrxR and Trx
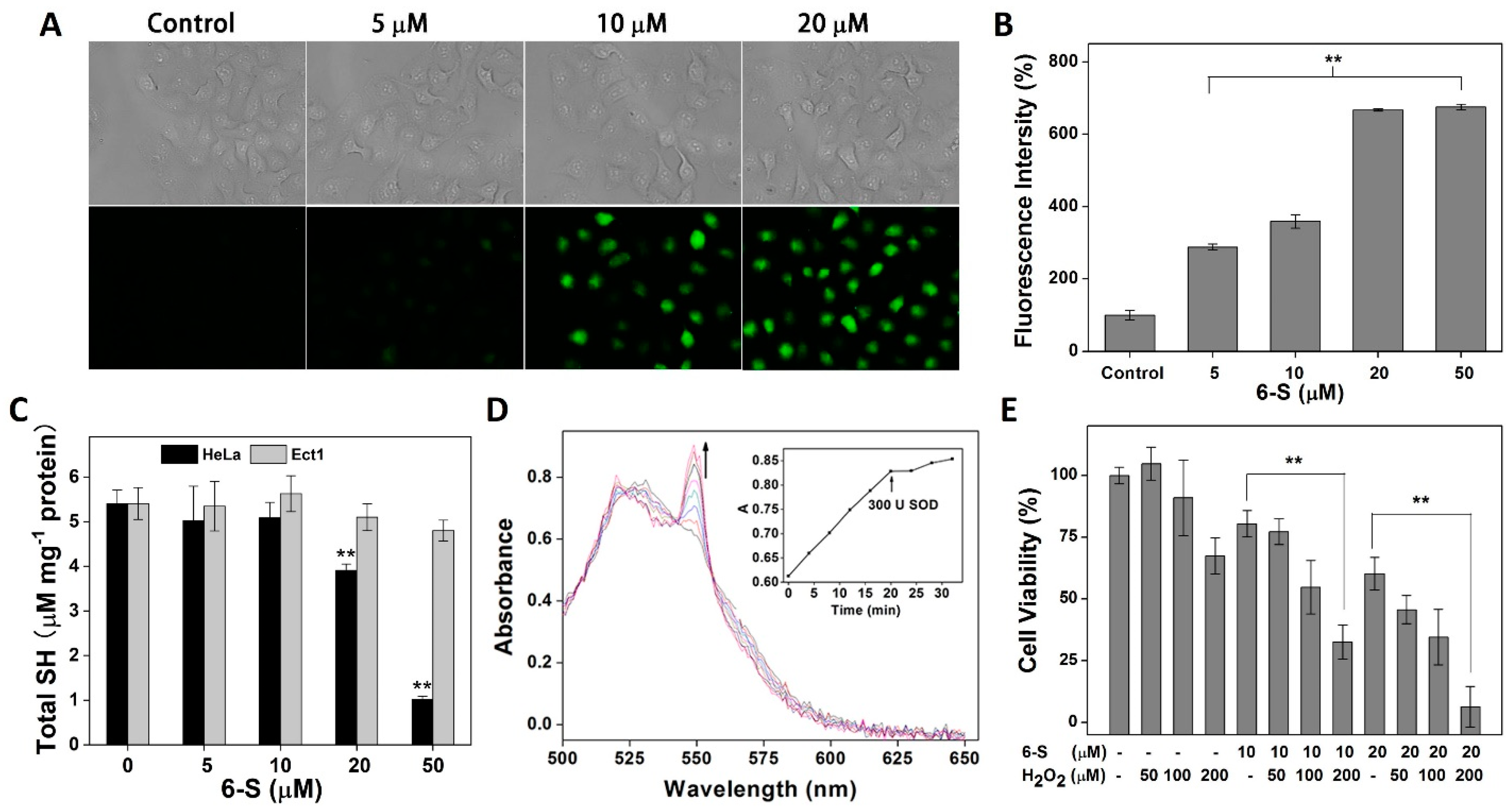
2.4. Promotion of Oxidative Stress by 6-S
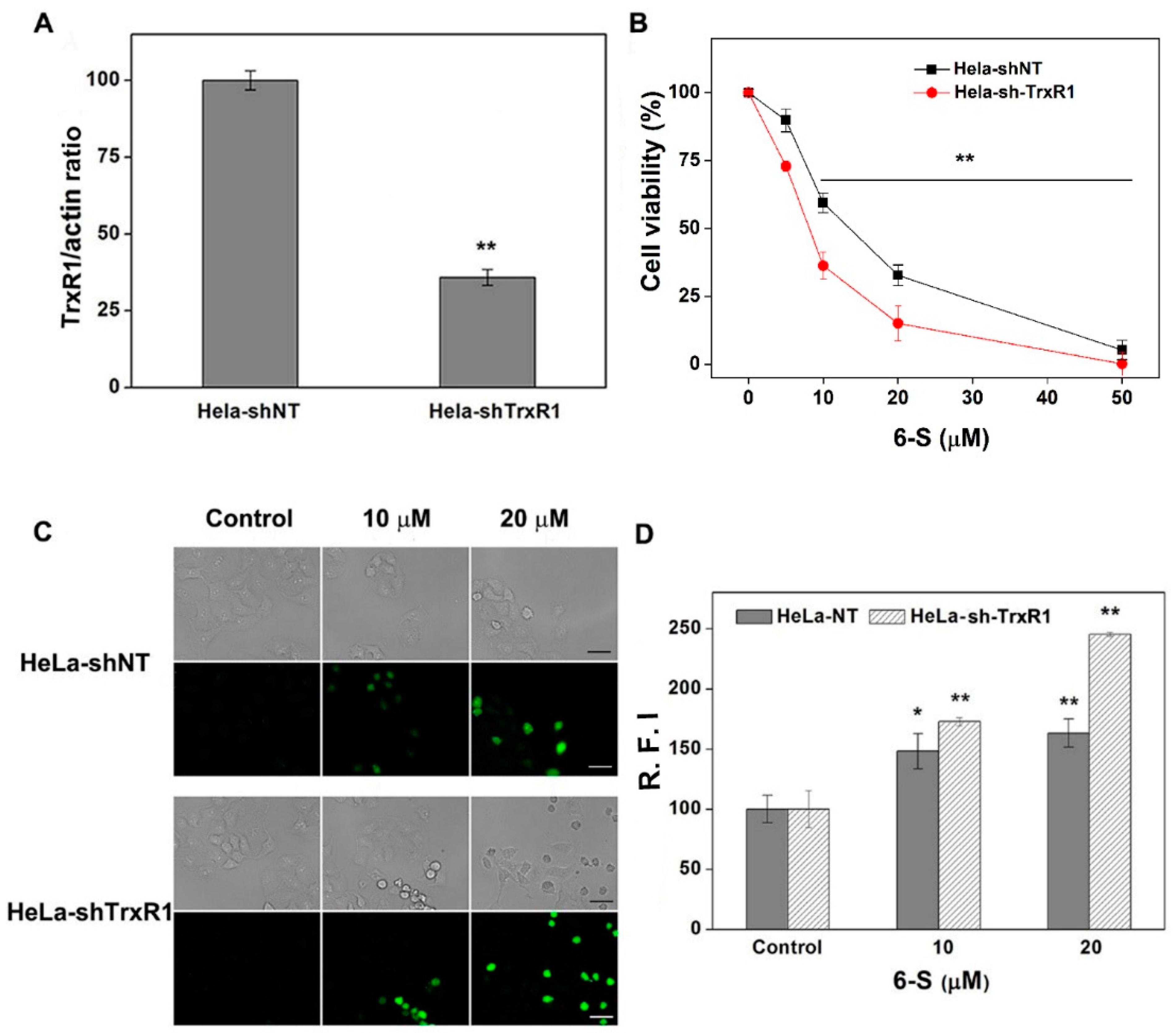
2.5. Involvement of TrxR for the Cellular Action of 6-S
2.6. Function of NAC and GSH in 6-S-Induced Cell Death
2.7. Induction of Apoptosis by 6-S in HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Enzymes
4.2. Cell Culture
4.3. MTT Assay
4.4. TrxR Activity Assays In Vitro
4.4.1. DTNB Assay
4.4.2. Endpoint Insulin Reduction Assay
4.5. GR Assay
4.6. Molecular Docking
4.7. Determination of Cellular TrxR Activity
4.8. Imaging Cellular TrxR Activity Using TRFS-Green
4.9. Intracellular ROS Detection
4.10. NADPH Oxidase Assay and Cytochrome C Reduction Assay
4.11. Assessment of Intracellular Thiol Levels
4.12. Determination of the Trx Redox State
4.13. Annexin V/Propidium Iodide (PI) Staining
4.14. Hoechst 33342 Staining
4.15. Measurement of Caspase-3 Activity
4.16. Statistics
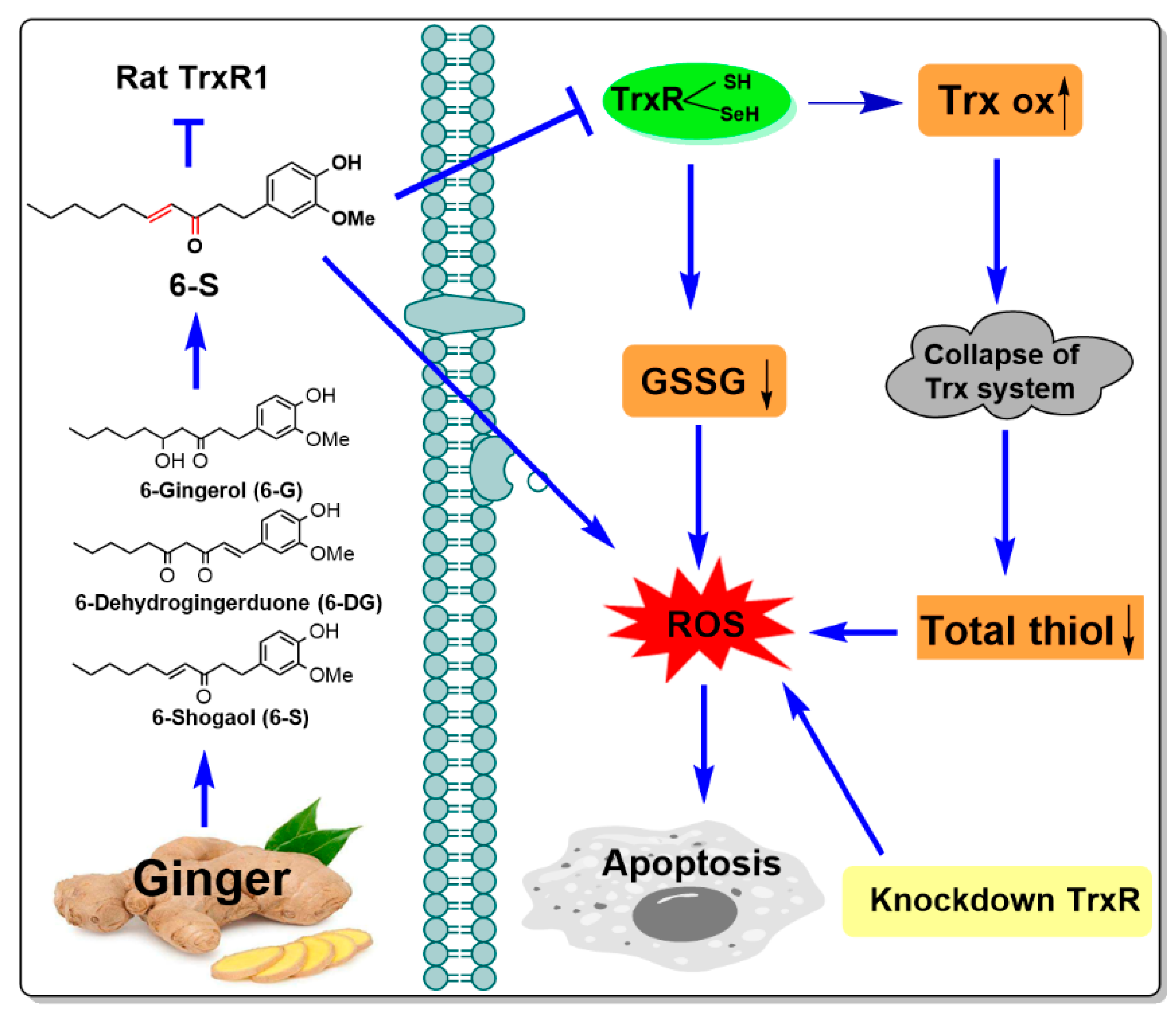
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volkova, L.V.; Pashov, A.I.; Omelchuk, N.N. Cervical carcinoma: Oncobiology and biomarkers. Int. J. Mol. Sci. 2021, 22, 12571. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Clarke, M.A. Cervical cancer screening-past, present, and future. Cancer Epidem. Biomar. 2021, 30, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system, Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar]
- Zhang, J.; Duan, D.; Osama, A.; Fang, J. Natural molecules targeting Thioredoxin system and their therapeutic potential. Antioxid. Redox Signal. 2021, 34, 1083–1107. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, L.; Song, Y.; Wang, B.; Zhang, B.; Cui, X.; Hu, G.; Liu, Y.; Wu, J.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase. Free Radic. Biol. Med. 2012, 52, 257–265. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Li, X.; Han, X.; Liu, R.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase as potential anticancer agents: An update. Med. Res. Rev. 2019, 39, 5–39. [Google Scholar] [CrossRef]
- Chupakhin, E.; Krasavin, M. Thioredoxin reductase inhibitors: Updated patent review (2017-present). Expert Opin. Ther. Pat. 2021, 31, 745–758. [Google Scholar] [CrossRef]
- White, B. Ginger: An overview. Am. Fam. Physician 2007, 75, 1689–1691. [Google Scholar]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef]
- Annamalai, G.; Kathiresan, S.; Kannappan, N. [6]-Shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependant apoptosis through activation of proapoptotic factors in Hep-2 cells. Biomed. Pharmacother. 2016, 82, 226–236. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.; Zhao, Y.; Yang, C.; Clark, A.; Leung, T.; Chen, X.; Sang, S. Synthesis, evaluation, and metabolism of novel [6]-shogaol derivatives as potent Nrf2 activators. Free Radic. Biol. Med. 2016, 95, 243–254. [Google Scholar] [CrossRef]
- Bischoff-Kont, I.; Furst, R. Benefits of ginger and its constituent 6-shogaol in inhibiting inflammatory processes. Pharmaceuticals 2021, 14, 571. [Google Scholar] [CrossRef]
- Park, G.; Oh, D.S.; Lee, M.G.; Lee, C.E.; Kim, Y.U. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol. Appl. Pharm. 2016, 310, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Yao, J.; Liu, Y.; Duan, D.; Zhang, X.; Fang, J. Activation of Nrf2 target enzymes conferring protection against oxidative stress in PC12 cells by ginger principal constituent 6-shogaol. Food Funct. 2015, 6, 2813–2823. [Google Scholar] [CrossRef]
- Wu, H.; Hsieh, M.C.; Lo, C.Y.; Liu, C.B.; Sang, S.; Ho, C.T.; Pan, M.H. 6-Shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Mol. Nutr. Food Res. 2010, 54, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kim, M.O.; Kim, K.R. Anticancer effects of 6-shogaol via the AKT signaling pathway in oral squamous cell carcinoma. J. Appl. Oral. Sci. 2021, 29, e20210209. [Google Scholar] [CrossRef]
- Dorcheh, S.N.; Rahgozar, S.; Talei, D. 6-Shogaol induces apoptosis in acute lymphoblastic leukaemia cells by targeting p53 signalling pathway and generation of reactive oxygen species. J. Cell Mol. Med. 2021, 25, 6148–6160. [Google Scholar] [CrossRef]
- Warin, R.F.; Chen, H.; Soroka, D.N.; Zhu, Y.; Sang, S. Induction of lung cancer cell apoptosis through a p53 pathway by [6]-shogaol and its cysteine-conjugated metabolite M2. J. Agric. Food Chem. 2014, 62, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Bawadood, A.S.; Al-Abbasi, F.A.; Anwar, F.; El-Halawany, A.M.; Al-Abd, A.M. 6-Shogaol suppresses the growth of breast cancer cells by inducing apoptosis and suppressing autophagy via targeting notch signaling pathway. Biomed. Pharmacother. 2020, 128, 110302. [Google Scholar] [CrossRef]
- Saha, A.; Blando, J.; Silver, E.; Beltran, L.; Sessler, J.; DiGiovanni, J. 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-kappaB signaling. Cancer Prev. Res. 2014, 7, 627–638. [Google Scholar] [CrossRef]
- Nedungadi, D.; Binoy, A.; Pandurangan, N.; Pal, S.; Nair, B.G.; Mishra, N. 6-Shogaol induces caspase-independent paraptosis in cancer cells via proteasomal inhibition. Exp. Cell Res. 2018, 364, 243–251. [Google Scholar] [CrossRef]
- Pan, M.H.; Hsieh, M.C.; Kuo, J.M.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol. Nutr. Food Res. 2008, 52, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Han, M.A.; Woo, S.M.; Min, K.J.; Kim, S.; Park, J.W.; Kim, D.E.; Kim, S.H.; Choi, Y.H.; Kwon, T.K. 6-Shogaol enhances renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated cytochrome C release and down-regulation of c-FLIP(L) expression. Chem. Biol. Interact. 2015, 228, 69–78. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, T.Z.; Liu, Y.W.; Tseng, W.C.; Liu, R.H.; Lu, F.J.; Lin, Y.S.; Kuo, S.H.; Chen, C.H. 6-shogaol (alkanone from ginger) induces apoptotic cell death of human hepatoma p53 mutant Mahlavu subline via an oxidative stress-mediated caspase-dependent mechanism. J. Agric. Food Chem. 2007, 55, 948–954. [Google Scholar] [CrossRef]
- Fang, J.; Lu, J.; Holmgren, A. Thioredoxin reductase is irreversibly modified by curcumin: A novel molecular mechanism for its anticancer activity. J. Biol. Chem. 2005, 280, 25284–25290. [Google Scholar] [CrossRef] [PubMed]
- Cenas, N.; Nivinskas, H.; Anusevicius, Z.; Sarlauskas, J.; Lederer, F.; Arner, E.S. Interactions of quinones with thioredoxin reductase: A challenge to the antioxidant role of the mammalian selenoprotein. J. Biol. Chem. 2004, 279, 2583–2592. [Google Scholar] [CrossRef]
- Lu, J.; Papp, L.; Fang, J.; Rodriguez-Nieto, S.; Zhivotovsky, B.; Holmgren, A. Inhibition of Mammalian thioredoxin reductase by some flavonoids: Implications for myricetin and quercetin anticancer activity. Cancer Res. 2006, 66, 4410–4418. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, B.; Yao, J.; Liu, Y.; Fang, J. Shikonin targets cytosolic thioredoxin reductase to induce ROS-mediated apoptosis in human promyelocytic leukemia HL-60 cells. Free Radic. Biol. Med. 2014, 70, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Fang, J. Targeting thioredoxin reductase by micheliolide contributes to radiosensitizing and inducing apoptosis of HeLa cells. Free Radic. Biol. Med. 2022, 186, 99–109. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Sunjic, S.; Zarkovic, N. The NRF2, Thioredoxin, and glutathione system in tumorigenesis and anticancer therapies. Antioxidants 2020, 9, 1151. [Google Scholar] [CrossRef]
- Desagher, S.; Martinou, J.C. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000, 10, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin system for cancer therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.S.; Holmgren, A. The thioredoxin system in cancer-introduction to a thematic volume of seminars in cancer biology, Semin. Cancer. Biol. 2006, 16, 419. [Google Scholar] [CrossRef]
- Shao, L.; Diccianni, M.B.; Tanaka, T.; Gribi, R.; Yu, A.L.; Pullen, J.D.; Camitta, B.M.; Yu, J. Thioredoxin expression in primary T-cell acute lymphoblastic leukemia and its therapeutic implication. Cancer Res. 2001, 61, 7333–7338. [Google Scholar]
- JRaffel; Bhattacharyya, A.K.; Gallegos, A.; Cui, H.; Einspahr, J.G.; Alberts, D.S.; Powis, G. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J. Lab. Clin. Med. 2003, 142, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Arner, E.S.; Holmgren, A. Structure and mechanism of mammalian thioredoxin reductase: The active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc. Natl. Acad. Sci. USA 2000, 97, 5854–5859. [Google Scholar] [CrossRef]
- Montero, A.J.; Jassem, J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs 2011, 71, 1385–1396. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Hedstrom, E.; Eriksson, S.; Zawacka-Pankau, J.; Arner, E.S.; Selivanova, G. p53-dependent inhibition of TrxR1 contributes to the tumor-specific induction of apoptosis by RITA. Cell Cycle 2009, 8, 3584–3591. [Google Scholar] [CrossRef]
- Saxena, G.; Chen, J.; Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010, 285, 3997–4005. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Yao, J.; Zhang, B.; Peng, S.; Ma, H.; Song, Y.; Fang, J. Dithiaarsanes induce oxidative stress-mediated apoptosis in HL-60 cells by selectively targeting thioredoxin reductase. J. Med. Chem. 2014, 57, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Arner, E.S.; Holmgren, A. Measurement of thioredoxin and thioredoxin reductase. Curr. Protoc. Toxicol. 2001, 7, Unit7.4. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, B.; Meng, X.; Yao, J.; Fang, J. Synthesis of piperlongumine analogues and discovery of nuclear factor erythroid 2-related factor 2 (Nrf2) activators as potential neuroprotective agents. J. Med. Chem. 2015, 58, 5242–5255. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B. Measurement of glutathione reductase activity. Curr. Protoc. Toxicol. 2001, 7, Unit7.2. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, D.; Liu, Y.; Ge, C.; Cui, X.; Sun, J.; Fang, J. Highly selective off-on fluorescent probe for imaging thioredoxin reductase in living cells. J. Am. Chem. Soc. 2014, 136, 226–233. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Zhang, L.; Cui, Y.; Liu, X.; Fang, J. A small molecule probe reveals declined mitochondrial thioredoxin reductase activity in a Parkinson’s disease model. Chem. Commun. 2016, 52, 2296–2299. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Duan, D.; Yao, J.; Gao, K.; Fang, J. Inhibition of thioredoxin reductase by alantolactone prompts oxidative stress-mediated apoptosis of HeLa cells. Biochem. Pharmacol. 2016, 102, 34–44. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Yu, S.; Zhang, J.; Zhang, J. 6-Shogaol as a Novel Thioredoxin Reductase Inhibitor Induces Oxidative-Stress-Mediated Apoptosis in HeLa Cells. Int. J. Mol. Sci. 2023, 24, 4966. https://doi.org/10.3390/ijms24054966
Peng S, Yu S, Zhang J, Zhang J. 6-Shogaol as a Novel Thioredoxin Reductase Inhibitor Induces Oxidative-Stress-Mediated Apoptosis in HeLa Cells. International Journal of Molecular Sciences. 2023; 24(5):4966. https://doi.org/10.3390/ijms24054966
Chicago/Turabian StylePeng, Shoujiao, Shaopeng Yu, Junmin Zhang, and Jiange Zhang. 2023. "6-Shogaol as a Novel Thioredoxin Reductase Inhibitor Induces Oxidative-Stress-Mediated Apoptosis in HeLa Cells" International Journal of Molecular Sciences 24, no. 5: 4966. https://doi.org/10.3390/ijms24054966
APA StylePeng, S., Yu, S., Zhang, J., & Zhang, J. (2023). 6-Shogaol as a Novel Thioredoxin Reductase Inhibitor Induces Oxidative-Stress-Mediated Apoptosis in HeLa Cells. International Journal of Molecular Sciences, 24(5), 4966. https://doi.org/10.3390/ijms24054966






