In Vitro Characteristics of Canine Primary Tracheal Epithelial Cells Maintained at an Air–Liquid Interface Compared to In Vivo Morphology
Abstract
1. Introduction
2. Results
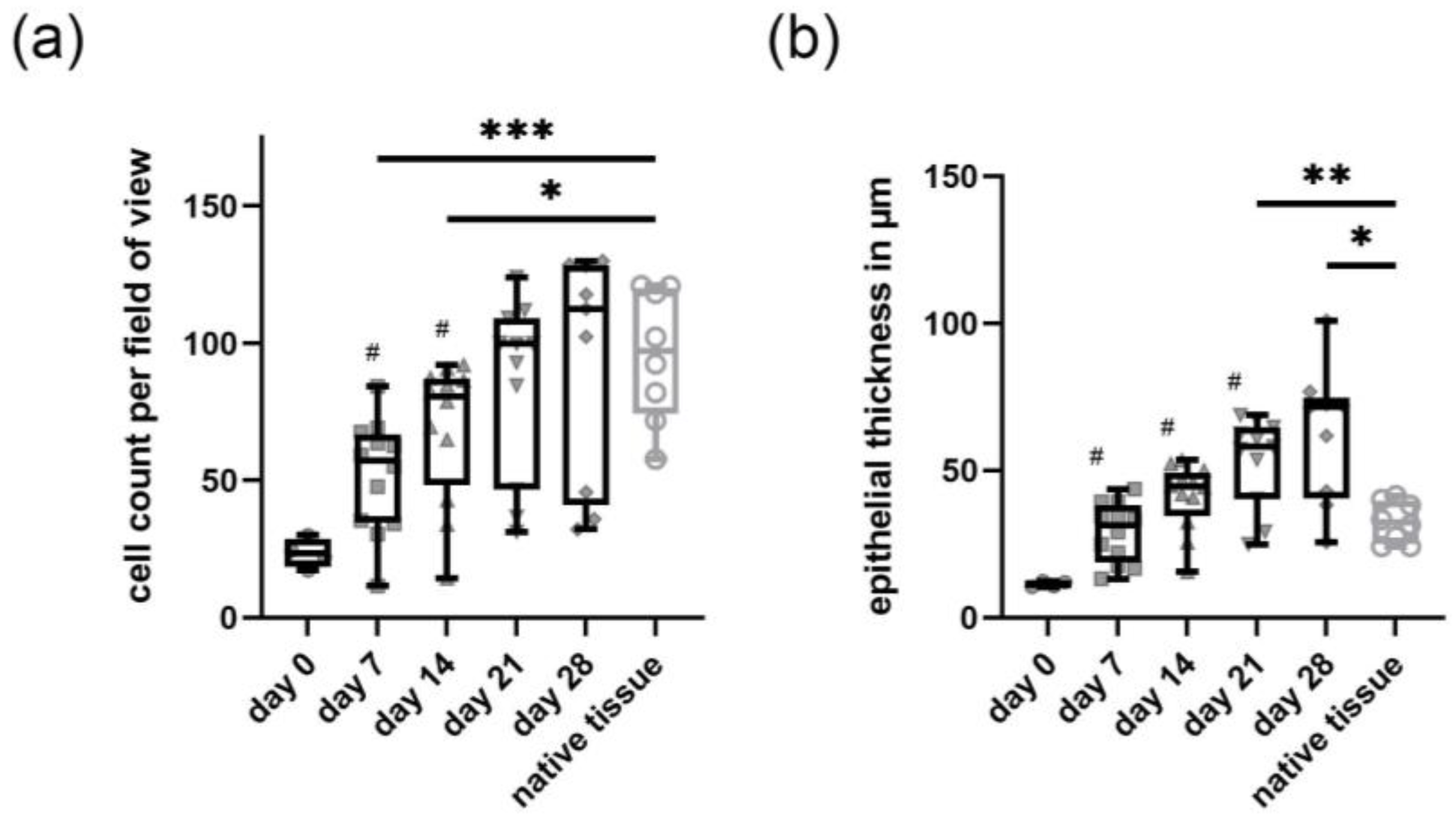
2.1. In Vitro Epithelium Formation of Primary Canine Tracheal Epithelial Cells
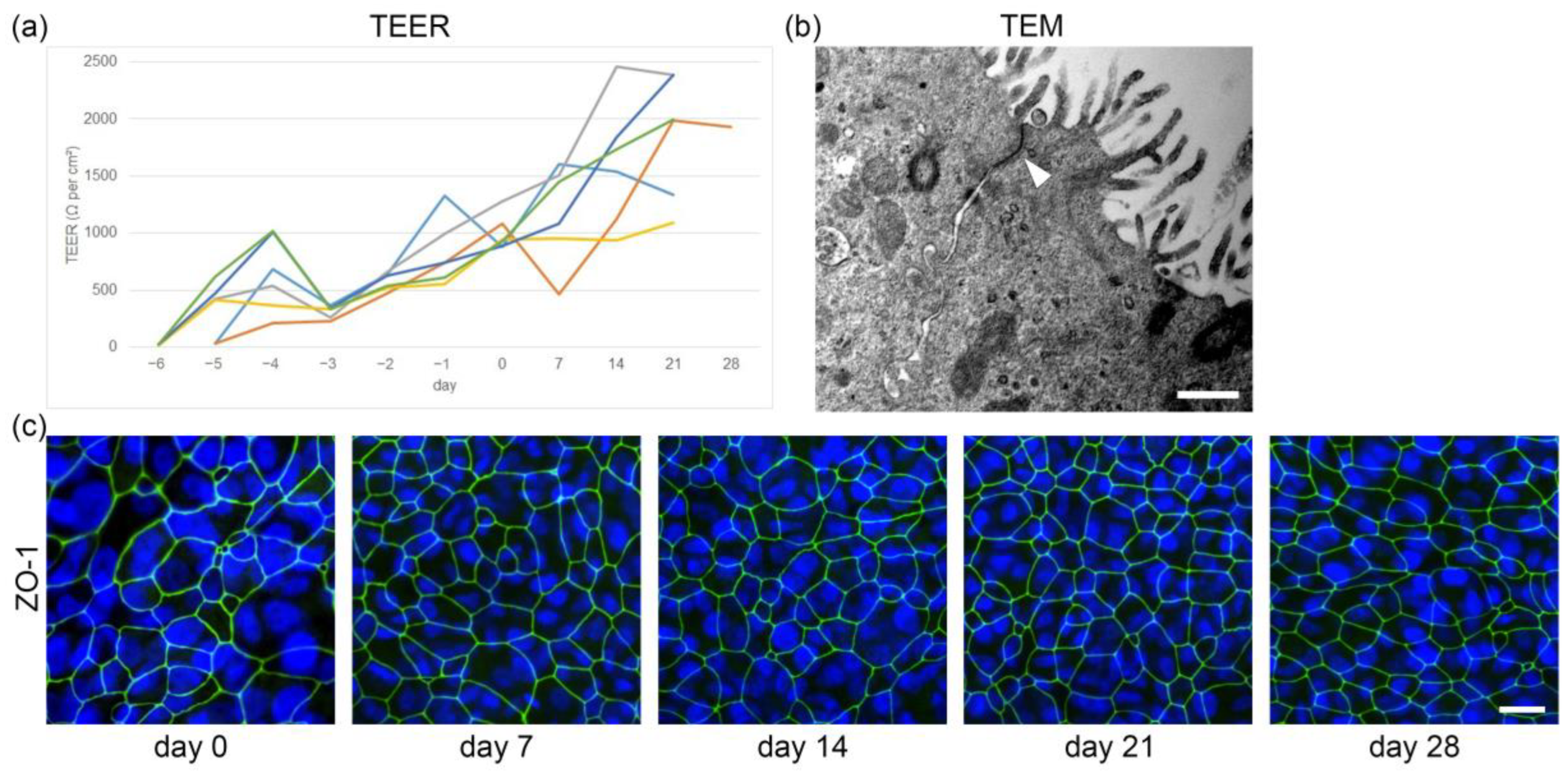
2.2. Development of Barrier Function in Primary Canine Tracheal Epithelial Cells
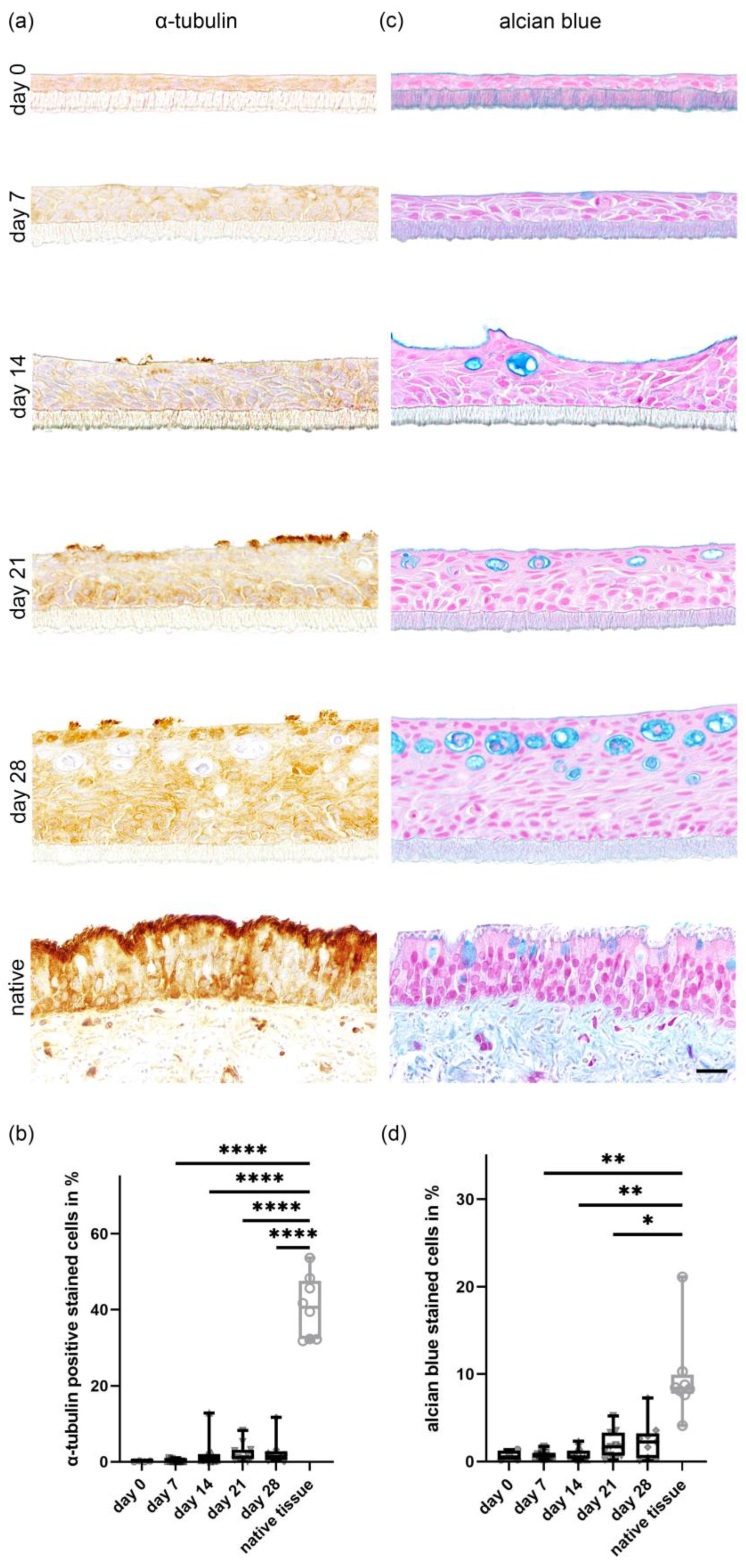
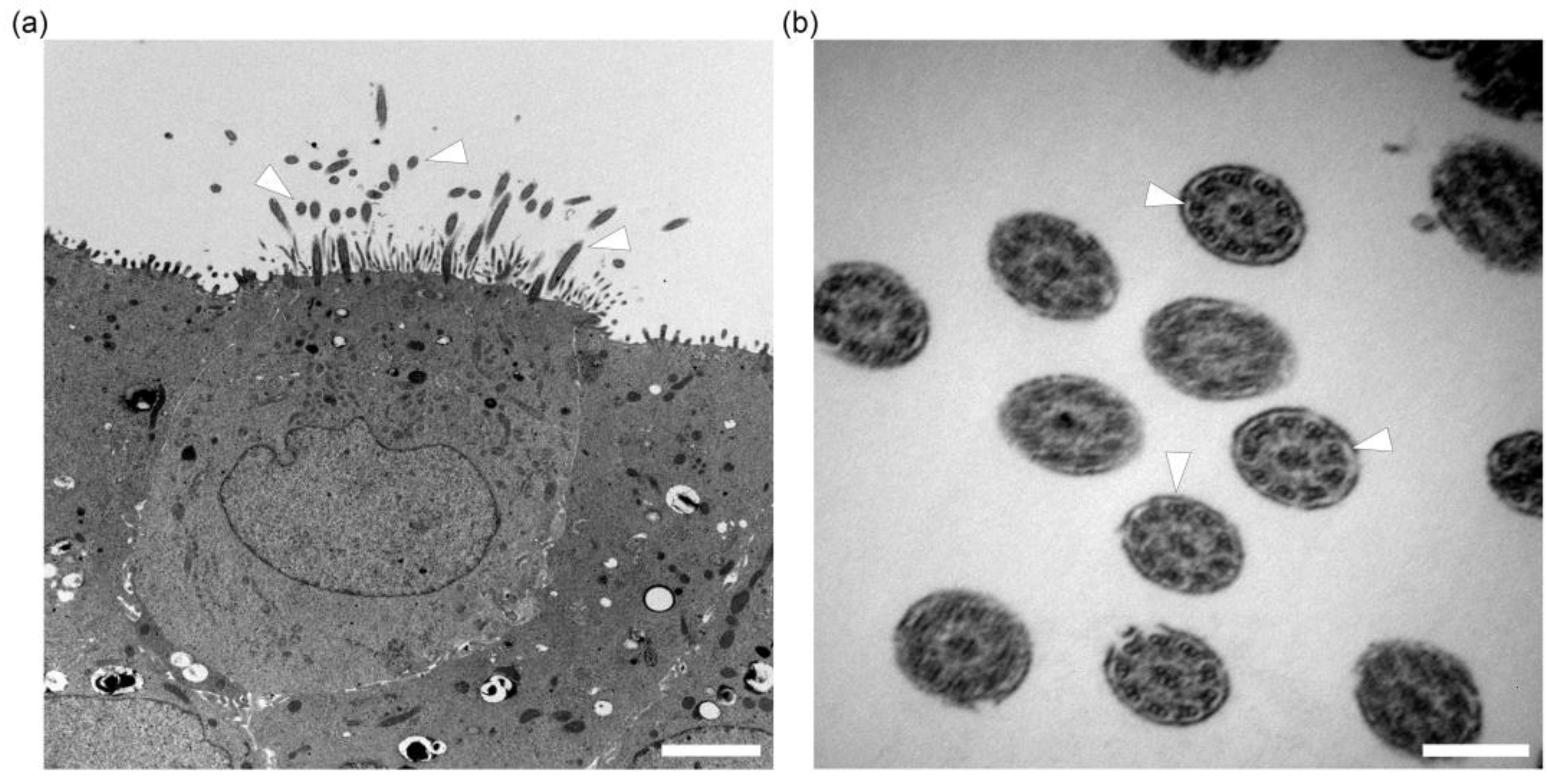
2.3. In Vitro Ciliogenesis in Primary Canine Tracheal Epithelial Cells
2.4. In Vitro Differentiation of Goblet Cells Derived from Primary Tracheal Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Collection of Samples
4.2. Isolation and Maintenance of Canine Tracheal Epithelial Cell Cultures
4.3. Establishment of ALI Cultures
4.4. TEER Measurements
4.5. Histochemistry (H&E and Alcian Blue)
4.6. Immunohistochemistry and Immunofluorescence
4.7. Histological Evaluation of ALI Cultures
4.8. Scanning Electron Microscopy
4.9. Transmission Electron Microscopy
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weitnauer, M.; Mijošek, V.; Dalpke, A.H. Control of local immunity by airway epithelial cells. Mucosal Immunol. 2016, 9, 287–298. [Google Scholar] [CrossRef]
- Opitz, B.; van Laak, V.; Eitel, J.; Suttorp, N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am. J. Respir. Crit. Care Med. 2010, 181, 1294–1309. [Google Scholar] [CrossRef]
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018, 560, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, K.; Morimoto, M. Mammalian tracheal development and reconstruction: Insights from in vivo and in vitro studies. Development 2021, 148, dev198192. [Google Scholar] [CrossRef] [PubMed]
- Deprez, M.; Zaragosi, L.-E.; Truchi, M.; Becavin, C.; García, S.R.; Arguel, M.-J.; Plaisant, M.; Magnone, V.; Lebrigand, K.; Abelanet, S.; et al. A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med. 2020, 202, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Go, M.; Takano, K.-I.; Kurose, M.; Ohkuni, T.; Koizumi, J.-I.; Kamekura, R.; Ogasawara, N.; Masaki, T.; Fuchimoto, J.; et al. Regulation of tight junctions in upper airway epithelium. BioMed Res. Int. 2013, 2013, 947072. [Google Scholar] [CrossRef]
- Houtmeyers, E.; Gosselink, R.; Gayan-Ramirez, G.; Decramer, M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999, 13, 1177–1188. [Google Scholar] [CrossRef]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L.M. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.S.; Abdelwhab, E.M. Evidence for SARS-CoV-2 infection of animal hosts. Pathogens 2020, 9, 529. [Google Scholar] [CrossRef]
- Beineke, A.; Puff, C.; Seehusen, F.; Baumgärtner, W. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunopathol. 2009, 127, 1–18. [Google Scholar] [CrossRef]
- da Fontoura Budaszewski, R.; von Messling, V. Morbillivirus experimental animal models: Measles virus pathogenesis insights from canine distemper virus. Viruses 2016, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Durchfeld, B.; Baumgärtner, W.; Krakowka, S. Intranasal infection of ferrets (Mustela putorius furo) with canine parainfluenza virus. J. Vet. Med. B 1991, 38, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.G.; Cornwell, H.J.; Thompson, H.; Stewart, M. Canine herpesvirus respiratory infection. Vet. Rec. 1970, 87, 108–109. [Google Scholar] [CrossRef]
- Schulz, B.S.; Kurz, S.; Weber, K.; Balzer, H.J.; Hartmann, K. Detection of respiratory viruses and Bordetella bronchiseptica in dogs with acute respiratory tract infections. Vet. J. 2014, 201, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Day, M.J.; Carey, S.; Clercx, C.; Kohn, B.; MarsilIo, F.; Thiry, E.; Freyburger, L.; Schulz, B.; Walker, D.J. Aetiology of canine infectious respiratory disease complex and prevalence of its pathogens in Europe. J. Comp. Pathol. 2020, 176, 86–108. [Google Scholar] [CrossRef]
- Ostrowski, L.E.; Nettesheim, P. Inhibition of ciliated cell differentiation by fluid submersion. Exp. Lung Res. 1995, 21, 957–970. [Google Scholar] [CrossRef]
- Gray, T.E.; Guzman, K.; Davis, C.W.; Abdullah, L.H.; Nettesheim, P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am. J. Resp. Cell Mol. 1996, 14, 104–112. [Google Scholar] [CrossRef]
- Cozens, D.; Sutherland, E.; Marchesi, F.; Taylor, G.; Berry, C.C.; Davies, R.L. Temporal differentiation of bovine airway epithelial cells grown at an air-liquid interface. Sci. Rep. 2018, 8, 14893. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Han, F.; Liang, J.; Yang, J.; Shi, J.; Xue, J.; Yang, L.; Li, Y.; Luo, M.; Wang, Y.; et al. A species-specific activation of Toll-like receptor signaling in bovine and sheep bronchial epithelial cells triggered by Mycobacterial infections. Mol. Immunol. 2016, 71, 23–33. [Google Scholar] [CrossRef]
- Prytherch, Z.; Job, C.; Marshall, H.; Oreffo, V.; Foster, M.; Bérubé, K. Tissue-specific stem cell differentiation in an in vitro airway model. Macromol. Biosci. 2011, 11, 1467–1477. [Google Scholar] [CrossRef]
- Clark, A.B.; Randell, S.H.; Nettesheim, P.; Gray, T.E.; Bagnell, B.; Ostrowski, L.E. Regulation of ciliated cell differentiation in cultures of rat tracheal epithelial cells. Am. J. Respir. Cell Mol. Biol. 1995, 12, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Runft, S.; Färber, I.; Krüger, J.; Krüger, N.; Armando, F.; Rocha, C.; Pöhlmann, S.; Burigk, L.; Leitzen, E.; Ciurkiewicz, M. Alternatives to animal models and their application in the discovery of species susceptibility to SARS-CoV-2 and other respiratory infectious pathogens: A review. Vet. Pathol. 2022, 59, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Färber, I.; Krüger, J.; Rocha, C.; Armando, F.; von Köckritz-Blickwede, M.; Pöhlmann, S.; Braun, A.; Baumgärtner, W.; Runft, S.; Krüger, N. Investigations on SARS-CoV-2 susceptibility of domestic and wild animals using primary cell culture models derived from the upper and lower respiratory tract. Viruses 2022, 14, 828. [Google Scholar] [CrossRef]
- Gultom, M.; Licheri, M.; Laloli, L.; Wider, M.; Strässle, M.; V’kovski, P.; Steiner, S.; Kratzel, A.; Thao, T.T.N.; Probst, L.; et al. Susceptibility of well-differentiated airway epithelial cell cultures from domestic and wild animals to severe acute respiratory syndrome coronavirus 2. Emerg. Infect. Dis. 2021, 27, 1811–1820. [Google Scholar] [CrossRef]
- Shin, D.L.; Chludzinski, E.; Wu, N.H.; Peng, J.Y.; Ciurkiewicz, M.; Sawatsky, B.; Pfaller, C.K.; Baechlein, C.; von Messling, V.; Haas, L.; et al. Overcoming the barrier of the respiratory epithelium during canine distemper virus infection. mBio 2022, 13, e0304321. [Google Scholar] [CrossRef] [PubMed]
- Anderton, T.L.; Maskell, D.J.; Preston, A. Ciliostasis is a key early event during colonization of canine tracheal tissue by Bordetella bronchiseptica. Microbiology 2004, 150, 2843–2855. [Google Scholar] [CrossRef]
- Johnson, L.G.; Dickman, K.G.; Moore, K.L.; Mandel, L.J.; Boucher, R.C. Enhanced Na+ transport in an air-liquid interface culture system. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1993, 264, L560–L565. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Plasschaert, L.W.; Žilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef]
- Abraham, G.; Zizzadoro, C.; Kacza, J.; Ellenberger, C.; Abs, V.; Franke, J.; Schoon, H.A.; Seeger, J.; Tesfaigzi, Y.; Ungemach, F.R. Growth and differentiation of primary and passaged equine bronchial epithelial cells under conventional and air-liquid-interface culture conditions. BMC Vet. Res. 2011, 7, 26. [Google Scholar] [CrossRef]
- Ziegler, P.; Tian, Y.; Bai, Y.; Abrahamsson, S.; Bäckerholm, A.; Reznik, A.S.; Green, A.; Moore, J.A.; Lee, S.E.; Myerburg, M.M.; et al. A primary nasopharyngeal three-dimensional air-liquid interface cell culture model of the pseudostratified epithelium reveals differential donor- and cell type-specific susceptibility to Epstein-Barr virus infection. PLoS Pathog. 2021, 17, e1009041. [Google Scholar] [CrossRef]
- Khoufache, K.; Cabaret, O.; Farrugia, C.; Rivollet, D.; Alliot, A.; Allaire, E.; Cordonnier, C.; Bretagne, S.; Botterel, F. Primary in vitro culture of porcine tracheal epithelial cells in an air-liquid interface as a model to study airway epithelium and Aspergillus fumigatus interactions. Med. Mycol. 2010, 48, 1049–1055. [Google Scholar] [CrossRef]
- Peeters, D.; Day, M.J.; Farnir, F.; Moore, P.; Clercx, C. Distribution of leucocyte subsets in the canine respiratory tract. J. Comp. Pathol. 2005, 132, 261–272. [Google Scholar] [CrossRef]
- Schwerer, M.J.; Kraft, K.; Baczako, K.; Maier, H. Cytokeratin expression and epithelial differentiation in Warthin’s tumour and its metaplastic (infarcted) variant. Histopathology 2001, 39, 347–352. [Google Scholar] [CrossRef]
- Evans, M.J.; Moller, P.C. Biology of airway basal cells. Exp. Lung Res. 1991, 17, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Van Winkle, L.S.; Fanucchi, M.V.; Plopper, C.G. Cellular and molecular characteristics of basal cells in airway epithelium. Exp. Lung Res. 2001, 27, 401–415. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, H.; Huang, H.; Li, H.; Saqi, A.; Qiang, L.; Que, J. Distinct stem/progenitor cells proliferate to regenerate the trachea, intrapulmonary airways and alveoli in COVID-19 patients. Cell Res. 2020, 30, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Ruysseveldt, E.; Martens, K.; Steelant, B. Airway basal cells, protectors of epithelial walls in health and respiratory diseases. Front. Allergy 2021, 2, 787128. [Google Scholar] [CrossRef]
- Amatngalim, G.D.; van Wijck, Y.; de Mooij-Eijk, Y.; Verhoosel, R.M.; Harder, J.; Lekkerkerker, A.N.; Janssen, R.A.; Hiemstra, P.S. Basal cells contribute to innate immunity of the airway epithelium through production of the antimicrobial protein RNase 7. J. Immunol. 2015, 194, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef]
- Krunkosky, T.M.; Jordan, J.L.; Chambers, E.; Krause, D.C. Mycoplasma pneumoniae host–pathogen studies in an air–liquid culture of differentiated human airway epithelial cells. Microb. Pathog. 2007, 42, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Tong, J.; Fu, Y.; Müller, S.; Weldearegay, Y.B.; Becher, P.; Valentin-Weigand, P.; Meens, J.; Herrler, G. Infection of bovine well-differentiated airway epithelial cells by Pasteurella multocida: Actions and counteractions in the bacteria–host interactions. Vet. Res. 2020, 51, 140. [Google Scholar] [CrossRef] [PubMed]
- Balder, R.; Krunkosky, T.M.; Nguyen, C.Q.; Feezel, L.; Lafontaine, E.R. Hag mediates adherence of Moraxella catarrhalis to ciliated human airway cells. Infect. Immun. 2009, 77, 4597–4608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Peeples, M.E.; Boucher, R.C.; Collins, P.L.; Pickles, R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002, 76, 5654–5666. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.-D. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 2004, 101, 4620–4624. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Brito, F.; Benaoudia, S.; Royston, L.; Cagno, V.; Fernandes-Rocha, M.; Piuz, I.; Zdobnov, E.; Huang, S.; Constant, S.; et al. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J. Allergy Clin. Immunol. 2018, 141, 2074–2084. [Google Scholar] [CrossRef]
- Davis, A.S.; Chertow, D.S.; Moyer, J.E.; Suzich, J.; Sandouk, A.; Dorward, D.W.; Logun, C.; Shelhamer, J.H.; Taubenberger, J.K. Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. J. Histochem. Cytochem. 2015, 63, 312–328. [Google Scholar] [CrossRef]
- O’Boyle, N.; Sutherland, E.; Berry, C.C.; Davies, R.L. Optimisation of growth conditions for ovine airway epithelial cell differentiation at an air-liquid interface. PLoS ONE 2018, 13, e0193998. [Google Scholar] [CrossRef]
- Cozens, D.; Grahame, E.; Sutherland, E.; Taylor, G.; Berry, C.C.; Davies, R.L. Development and optimization of a differentiated airway epithelial cell model of the bovine respiratory tract. Sci. Rep. 2018, 8, 853. [Google Scholar] [CrossRef]
- Bateman, A.C.; Karasin, A.I.; Olsen, C.W. Differentiated swine airway epithelial cell cultures for the investigation of influenza A virus infection and replication. Influenza Other Resp. 2013, 7, 139–150. [Google Scholar] [CrossRef]
- Luengen, A.E.; Kniebs, C.; Buhl, E.M.; Cornelissen, C.G.; Schmitz-Rode, T.; Jockenhoevel, S.; Thiebes, A.L. Choosing the right differentiation medium to develop mucociliary phenotype of primary nasal epithelial cells in vitro. Sci. Rep. 2020, 10, 6963. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Feinstein, T.N.; Oro, A.; Schwartz, M.; Lee, A.D.; Lo, C.W. Rapid ex-vivo ciliogenesis and dose-dependent effect of notch inhibition on ciliogenesis of respiratory epithelia. Biomolecules 2020, 10, 1182. [Google Scholar] [CrossRef]
- Cortez, V.; Schultz-Cherry, S. The role of goblet cells in viral pathogenesis. FEBS J. 2021, 288, 7060–7072. [Google Scholar] [CrossRef]
- Rogers, D.F. The airway goblet cell. Int. J. Biochem. Cell Biol. 2003, 35, 1–6. [Google Scholar] [CrossRef]
- Kim, V.; Criner, G.J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, H.S.; Case, L.M.; Wesselkamper, S.C.; Borchers, M.T.; Martin, L.D.; Shertzer, H.G.; Nadel, J.A.; Leikauf, G.D. Metalloproteinases mediate Mucin 5AC Expression by Epidermal Growth Factor Receptor Activation. Am. J. Respir. Crit. Care Med. 2005, 171, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, K.; Jung, B.; Shim, J.J.; Burgel, P.-R.; Dao-Pick, T.; Ueki, I.F.; Protin, U.; Kroschel, P.; Nadel, J.A. Activation of epidermal growth factor receptors is responsible for mucin synthesis induced by cigarette smoke. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2001, 280, L165–L172. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.J.; Morton, J.D.; Kajiwara, N.; Agapov, E.; Holtzman, M.J. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Investig. 2002, 110, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, M.J.; Tyner, J.W.; Kim, E.Y.; Lo, M.S.; Patel, A.C.; Shornick, L.P.; Agapov, E.; Zhang, Y. Acute and chronic airway responses to viral infection: Implications for asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 132–140. [Google Scholar] [CrossRef]
- Burgel, P.-R.; Nadel, J.A. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 2004, 59, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- O’Boyle, N.; Sutherland, E.; Berry, C.C.; Davies, R.L. Temporal dynamics of ovine airway epithelial cell differentiation at an air-liquid interface. PLoS ONE 2017, 12, e0181583. [Google Scholar] [CrossRef]
- Meng, F.; Wu, N.-H.; Seitz, M.; Herrler, G.; Valentin-Weigand, P. Efficient suilysin-mediated invasion and apoptosis in porcine respiratory epithelial cells after streptococcal infection under air-liquid interface conditions. Sci. Rep. 2016, 6, 26748. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Humle, N.; Hede, E.; Moos, T.; Burkhart, A.; Thomsen, L.B. The blood-brain barrier studied in vitro across species. PLoS ONE 2021, 16, e0236770. [Google Scholar] [CrossRef]
- Wu, N.-H.; Yang, W.; Beineke, A.; Dijkman, R.; Matrosovich, M.; Baumgärtner, W.; Thiel, V.; Valentin-Weigand, P.; Meng, F.; Herrler, G. The differentiated airway epithelium infected by influenza viruses maintains the barrier function despite a dramatic loss of ciliated cells. Sci. Rep. 2016, 6, 39668. [Google Scholar] [CrossRef]
- Allnoch, L.; Beythien, G.; Leitzen, E.; Becker, K.; Kaup, F.-J.; Stanelle-Bertram, S.; Schaumburg, B.; Mounogou Kouassi, N.; Beck, S.; Zickler, M.; et al. Vascular inflammation is associated with loss of aquaporin 1 expression on endothelial cells and increased fluid leakage in SARS-CoV-2 infected golden syrian hamsters. Viruses 2021, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Mulisch, M.; Welsch, U. Romeis-Mikroskopische Technik; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Ciurkiewicz, M.; Armando, F.; Schreiner, T.; de Buhr, N.; Pilchová, V.; Krupp-Buzimikic, V.; Gabriel, G.; von Köckritz-Blickwede, M.; Baumgärtner, W.; Schulz, C.; et al. Ferrets are valuable models for SARS-CoV-2 research. Vet. Pathol. 2022, 59, 661–672. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runft, S.; Färber, I.; Krüger, J.; Schöne, K.; Lehmbecker, A.; Baumgärtner, W. In Vitro Characteristics of Canine Primary Tracheal Epithelial Cells Maintained at an Air–Liquid Interface Compared to In Vivo Morphology. Int. J. Mol. Sci. 2023, 24, 4987. https://doi.org/10.3390/ijms24054987
Runft S, Färber I, Krüger J, Schöne K, Lehmbecker A, Baumgärtner W. In Vitro Characteristics of Canine Primary Tracheal Epithelial Cells Maintained at an Air–Liquid Interface Compared to In Vivo Morphology. International Journal of Molecular Sciences. 2023; 24(5):4987. https://doi.org/10.3390/ijms24054987
Chicago/Turabian StyleRunft, Sandra, Iris Färber, Johannes Krüger, Kerstin Schöne, Annika Lehmbecker, and Wolfgang Baumgärtner. 2023. "In Vitro Characteristics of Canine Primary Tracheal Epithelial Cells Maintained at an Air–Liquid Interface Compared to In Vivo Morphology" International Journal of Molecular Sciences 24, no. 5: 4987. https://doi.org/10.3390/ijms24054987
APA StyleRunft, S., Färber, I., Krüger, J., Schöne, K., Lehmbecker, A., & Baumgärtner, W. (2023). In Vitro Characteristics of Canine Primary Tracheal Epithelial Cells Maintained at an Air–Liquid Interface Compared to In Vivo Morphology. International Journal of Molecular Sciences, 24(5), 4987. https://doi.org/10.3390/ijms24054987





