Abstract
Heading date (HD) is an important trait for wide adaptability and yield stability in wheat. The Vernalization 1 (VRN1) gene is a key regulatory factor controlling HD in wheat. The identification of allelic variations in VRN1 is crucial for wheat improvement as climate change becomes more of a threat to agriculture. In this study, we identified an EMS-induced late-heading wheat mutant je0155 and crossed it with wide-type (WT) Jing411 to construct an F2 population of 344 individuals. Through Bulk Segregant Analysis (BSA) of early and late-heading plants, we identified a Quantitative Trait Locus (QTL) for HD on chromosome 5A. Further genetic linkage analysis limited the QTL to a physical region of 0.8 Mb. Cloning and sequencing revealed three copies of VRN-A1 in the WT and mutant lines; one copy contained a missense mutation of C changed to T in exon 4 and another copy contained a mutation in intron 5. Genotype and phenotype analysis of the segregation population validated that the mutations in VRN-A1 contributed to the late HD phenotype in the mutant. Expression analysis of C- or T-type alleles in exon 4 of the WT and mutant lines indicated that this mutation led to lower expression of VRN-A1, which resulted in the late-heading of je0155. This study provides valuable information for the genetic regulation of HD and many important resources for HD refinement in wheat breeding programs.
1. Introduction
Wheat (Triticum aestivum L.) is the most widely grown staple crop worldwide. It contains a large and complex allohexaploid genome (2n = 6x = 42, AABBDD genome) [1]. Wheat cultivars are classified into winter and spring types based on whether it requires cold temperatures to promote flowering, and therefore can be well adaptable to different geographical conditions [2]. Heading date is an important factor that determines the ability of wheat to adapt to a wide range of different ecological environments, thus affecting global grain yield [3,4,5]. The identification of genes or alleles affecting heading time would facilitate the optimization of the heading stage by providing a target for marker-assisted selection in wheat breeding.
The main factors affecting heading time in wheat have been identified as the vernalization requirement and photoperiod [6,7]. Winter wheat can only transition from vegetative growth to reproductive growth after a long period of low-temperature induction [8,9]. VRN1, VRN2, and VRN3 are the main regulatory factors affecting the vernalization time of wheat [10,11,12,13]. VRN1, encoding a MADS-box family protein, is the central genetic element in regulating vernalization. VRN-A1, VRN-B1, and VRN-D1, the three homologous genes of VRN1, are located on chromosomes 5A, 5B, and 5D, respectively [14,15,16]. The VRN2 locus includes two tandem repeat genes, ZCCT1 and ZCCT2, which encode the zinc finger and CCT domain-containing proteins [9]. Nucleotide deletion or mutation in the CCT domain was found to accelerate spring growth in diploid and tetraploid wheat varieties [17]. VRN3 is an orthologous gene of the Arabidopsis flowering factor FLOWER LOCUS T (FT), and the mutation of the intron region of this gene in barley led to early flowering [18,19]. VRN1 and VRN3 are flowering promotion factors, and long-term low temperatures can induce the expression of VRN1 [8,10,20]. After vernalization, VRN1 blocks the expression of the flowering inhibitor VRN2 and promotes the expression of VRN3 by binding with its promoter, thus inducing the flowering of winter wheat [19,21]. Photoperiod (Ppd) genes contain Ppd-A1 (2A), Ppd-B1 (2B), and Ppd-D1 (2D), and out of these Ppd-D1 has the largest impact on heading time in wheat [22,23,24].
The effects of vernalization genes VRN1, VRN2, and VRN3 on plant heading time have been extensively studied. An insertion in the promoter region of recessive VRN-A1 led to a later heading phenotype, whereas dominant allelic variants VRN-A1a, VRN-A1b, and VRN-A1c resulted in early heading due to a large fragment deletion of the promoter and first intron [25,26,27]. Additionally, 20–32 bp small deletions in the promoter region of VRN-A1 and the variation of CArG box in VRN1 led to early heading [11,28,29]. The allelic variations of Vrn-B1 mainly occurred in intron1, and the Vrn-B1b allele caused spring growth habit in wheat [5,25,30]. Alleles of Vrn-D1 are mainly deletions or insertions in intron1 or the promoter region, and Vrn-D1b led to delayed heading time compared with Vrn-D1a [25,31]. Vrn-D1b has a 4235-bp deletion in intron1, accompanied by a single nucleotide mutation (G-A) in the promoter region [28]. The dominant VRN3 allele carried a retroelement insertion in the promoter region, which resulted in the early flowering phenotype in wheat [18].
The copy number variation in plants is of great significance in the genetic regulation of plant growth and development [32,33,34,35]. It regulates many important agronomic traits such as plant height, stress resistance, and flowering time [31,33,36]. Of particular note, the copy number variation of VRN-A1 and Ppd-B1 is central to the regulation of wheat flowering time [31,37]. Exon 4 and exon 7 in VRN-A1 contain a C/T double peak, suggesting that at least two copies were present. Further studies indicated that they were associated with flowering time [37,38]. The RILs with three copies of VRN-A1 required a longer period of cold exposure to induce the transition to flowering than that of two copies [33]. The sequencing of 205 winter wheat varieties in China revealed that 22.9% of them contained one copy of VRN-A1, and the remaining 77.1% contained at least two copies [31].
In this study, we used gene mapping to identify a missense mutation in one copy of VRN-A1, which causes the late heading of wheat mutant je0155. Further investigation of VRN gene expression suggested that this mutation caused a lower transcription level of VRN-A1, which altered VRN2 and VRN3 expression and led to late heading in je0155. The identification of mutations in different copies of VRN-A1 offers important insights for improving HD in wheat breeding.
2. Results
2.1. A Single Recessive Gene Controlled Late Heading Phenotype of a Wheat Mutant
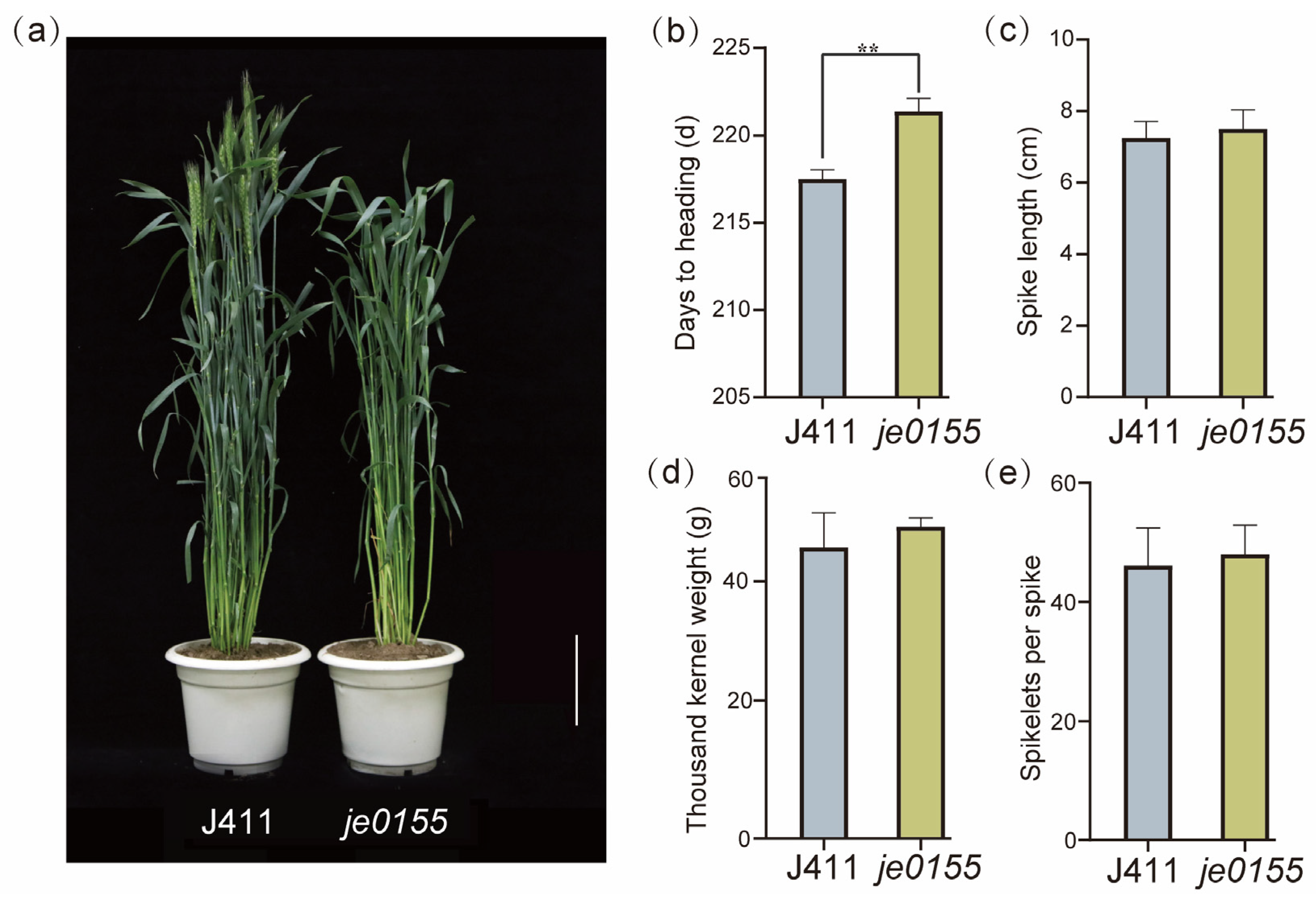
To explore and enrich the genetic regulation of heading date in wheat, we identified a late heading mutant je0155 from our Jing411-derived mutant library. As shown in Figure 1a, the heading date of je0155 was much later than that of wild-type Jing411 (J411). Further investigation suggested that the heading time of je0155 was 3–4 days later than that of J411, presenting a statistically significant difference (Figure 1b). There appeared to be no difference in other agronomic traits such as spike length, thousand kernel weight, and spikelets number (Figure 1c–e). To map the genetic factors of heading date, we constructed an F2 population of 344 individuals derived from a cross between WT and mutant lines. The phenotypic analysis of F2:3 lines suggested that the ratio of early/semi-early heading and late heading lines fits a Mendelian model of 3:1 (Table S1), indicating that late heading is controlled by a single recessive gene.
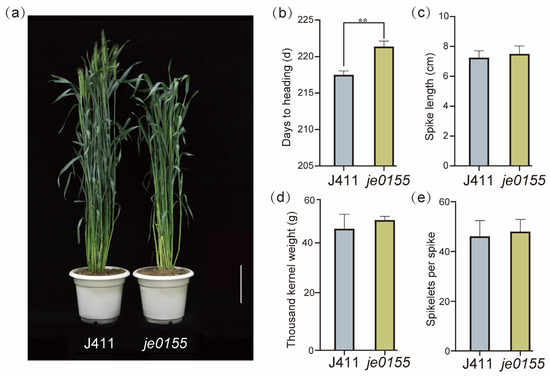
Figure 1.
Comparison of phenotype and yield-related traits between WT and mutant: (a) Phenotypic comparison between WT (Jing411) and late heading mutant je0155. Plants were grown under field conditions and the picture was taken when the WT was heading. Bars = 10 cm; (b) Days to heading; (c) Spike length; (d) Thousand kernel weight; (e) Spikelets per spike. Values are averaged from five replicates. ** indicates significant differences by t-test at p < 0.01.
2.2. A Primary Gene Affecting Heading Date Was Mapped on the Long Arm of Chromosome 5A
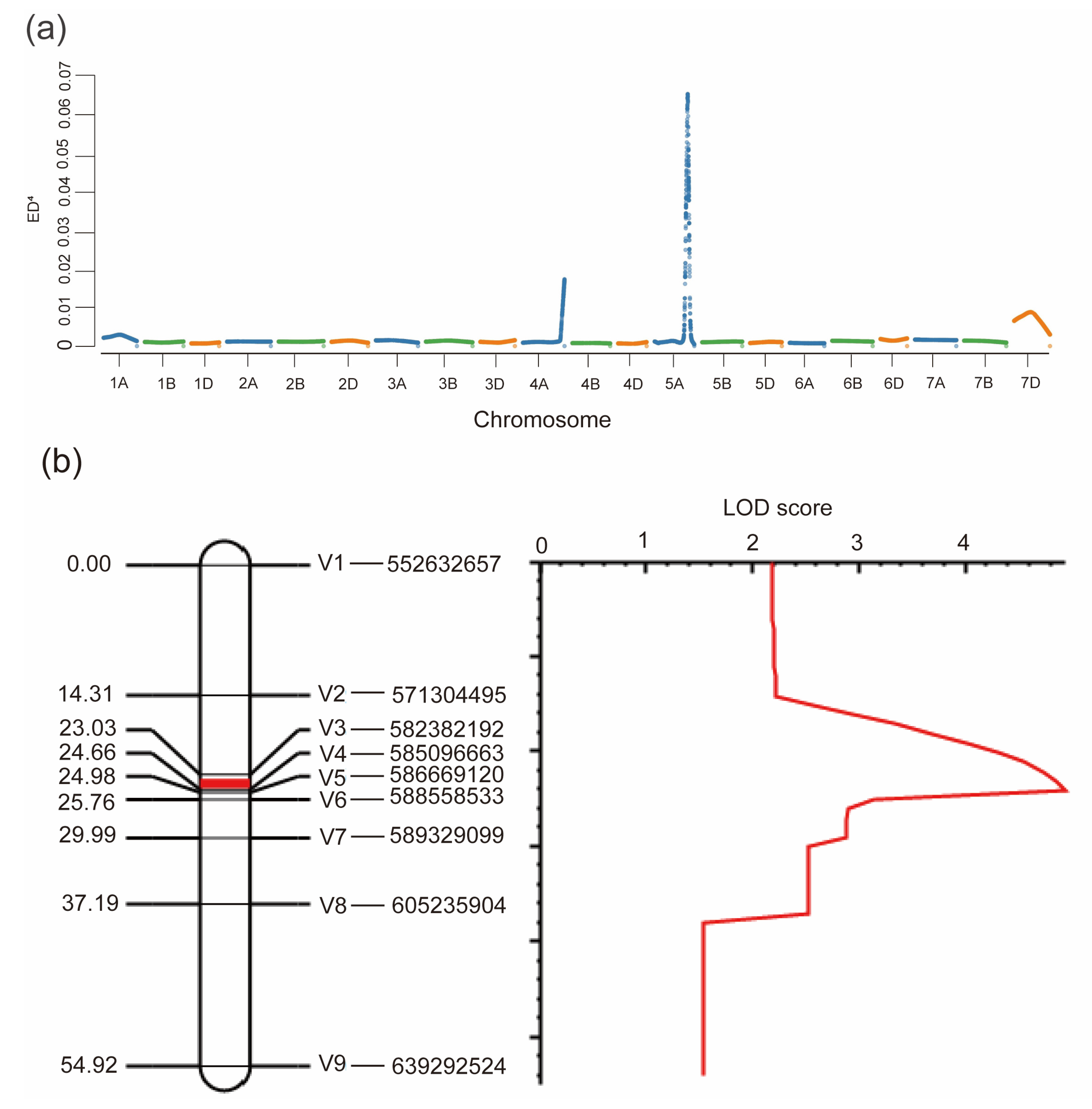
We performed Bulked Segregant Analysis (BSA) to map the gene for HD in the F2 population. Based on the F2:3 phenotype data, three early and three late heading bulks with roughly 30 individuals each, were used for BSA. Finally, a total of 22,705 SNPs were identified and used to perform further analysis. The Euclidean Distance (ED) value was calculated based on the reads and genotypes of bulks and their parents. The results detected a high peak region from 550 Mb to 650 Mb on chromosome 5A, and this region is speculated to be the gene locus associated with HD (Figure 2a). To validate the BSA-based mapping region, we successfully developed nine genome-specific KASP markers and genotyped the whole F2 population (Figure S1). We used QTL Icimapping analysis to create a linkage map covering this region on chromosome 5A. Based on the phenotypes of the F2:3 lines and the linkage map, we identified a major QTL with a LOD score of 4.9 and flanked by markers V3 and V4 with a genetic distance of 1.26 cM corresponding to a physical interval of 0.8 Mb according to the IWGSC RefSeq v2.1 (Figure 2b).
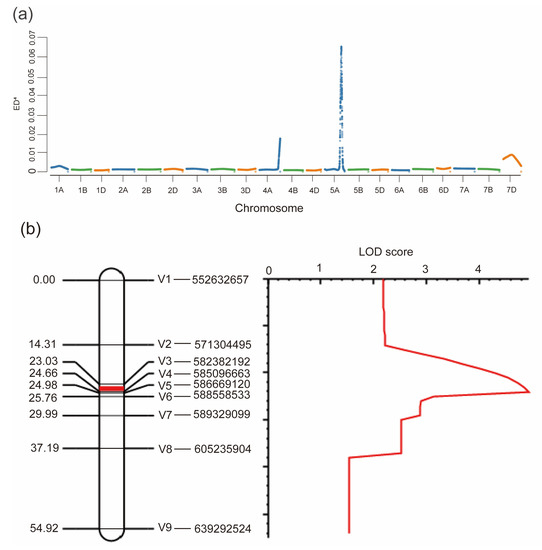
Figure 2.
Mapping of the HD gene by BSA and genetic linkage analysis: (a) Distribution of ED4 values across all chromosomes. Higher ED4 values indicate a higher association effect with HD; (b) QTL analysis of HD on chromosome 5A. The logarithm of odds (LOD) threshold was set to 3.0, and a QTL located on a 1.63 cM of genetic linkage map between markers V3 and V4 was identified.
2.3. Mutations in Different Copies of VRN-A1 in je0155
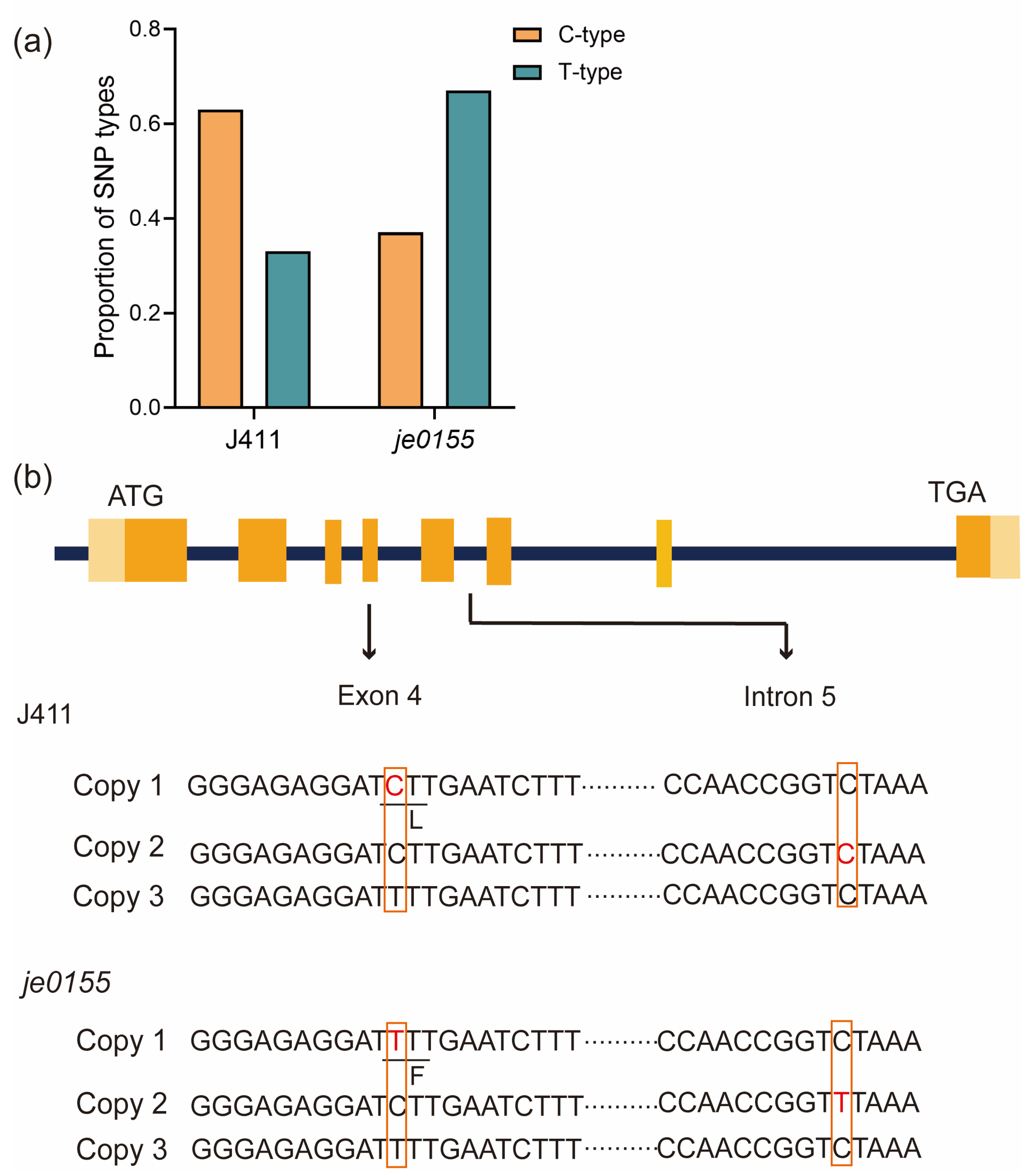
Since the vernalization gene VRN-A1, a key regulator of wheat heading and flowering [10], is located in the mapped region, we speculated that the VRN-A1 would be the putative causal gene for heading date variation in je0155. After PCR amplification by genome-specific primers, we randomly selected clones of VRN-A1 from WT and mutant lines for sequencing. Sanger sequencing of the individual clones suggested that two types of bases (C or T type) at the 10,606th position in exon 4 were present in WT and je0155. The amounts of C- and T-type clones in WT were 46 and 26, respectively, with a ratio of 2:1, while the mutant fit a ratio of 1:2 with 26 and 50 clones (Figure 3a and Table S2). This indicates that WT and je0155 have three copies of VRN-A1, and the mutant has a C to T mutation at the 10,606th position in exon 4 in one of its copies (Figure 3b). This mutation results in an amino acid change from Leu (L) to Phe (F) in the K domain of VRN-A1 protein, indicating that this missense variant is responsible for heading date variation in the mutant. Intriguingly, another copy of the C-type of exon 4 displayed a base mutation from C to T at the 11,141st position of Intron-5 in je0155. Taken together, these two mutations in two copies of VRN-A1 may contribute to the late heading phenotype in the mutant.
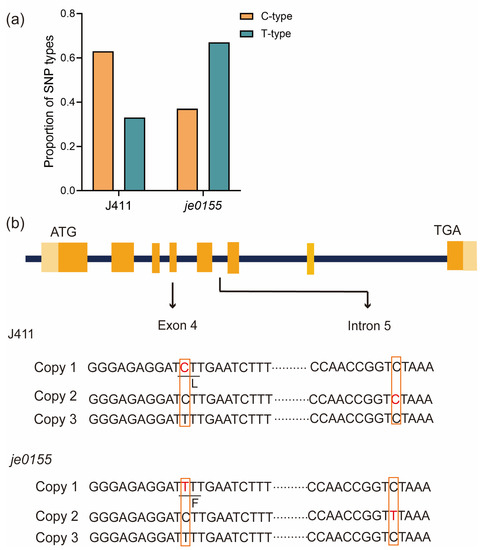
Figure 3.
Copy number and mutation analysis of VRN-A1 in je0155: (a) The ratio of C- and T-type alleles of exon 4 in WT and je0155; (b) Gene structure and sequence of three copies of VRN-A1 with mutations in exon 4 and intron 5 of different copies of VRN-A1 in je0155. The light orange boxes indicate the 5′ or 3′ UTR region, while the orange boxes indicate the exon regions. The red fonts represent the mutation site in J411 and je0155.
2.4. Mutation in VRN-A1 Affected Heading Time in the Population
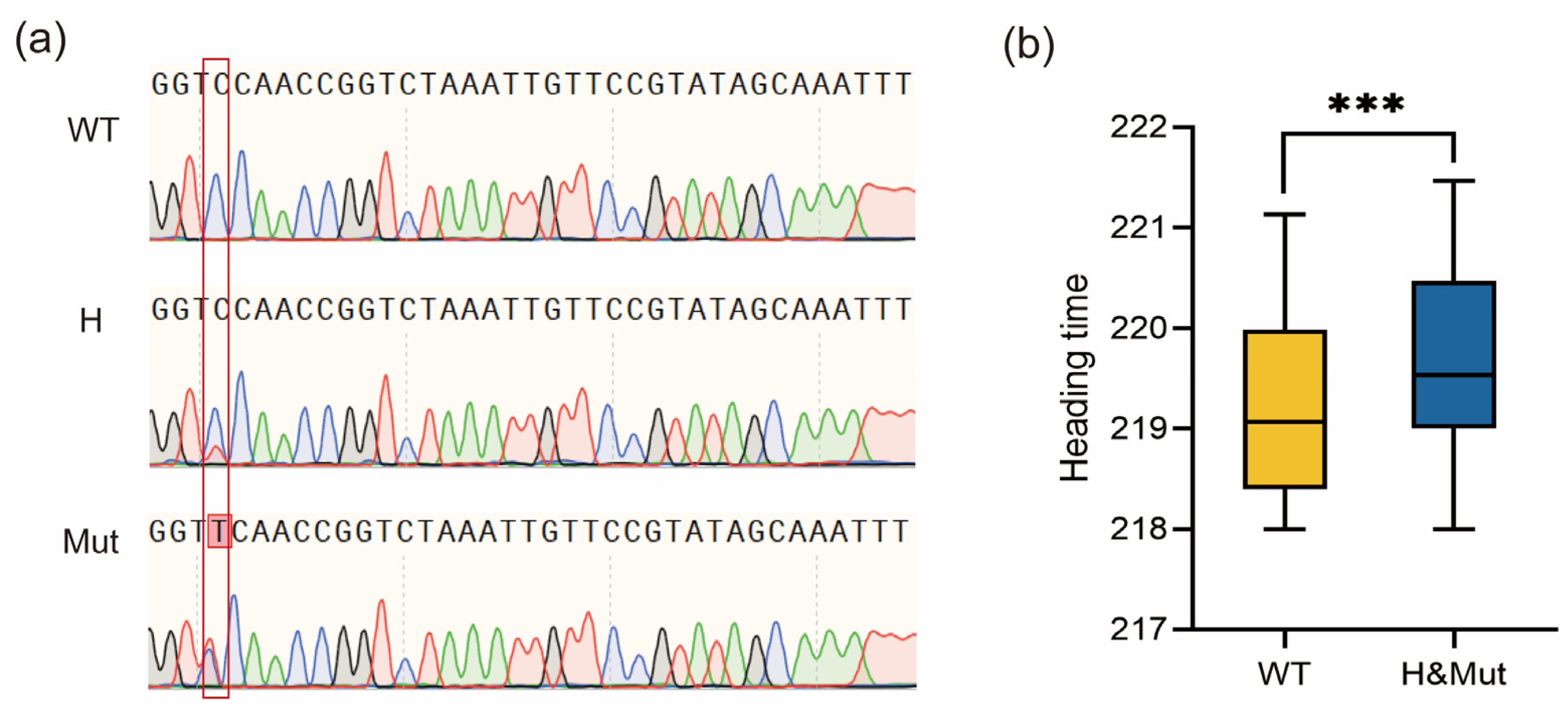
To verify the effects of VRN-A1 mutations on heading date, we developed a molecular marker for the intron 5 mutation site through PCR sequencing. Due to containing three copies of the VRN-A1 gene, the WT showed a single peak of C at the mutation site, while the mutant showed overlapping peaks of C and T, which is consistent with the peaks of heterozygous plants (Figure 4a). Using this marker, we analyzed the genotypes of the F2 population. By combining genotypic and phenotypic data from this population, we determined that the heading date of plants with mutated and heterozygous genotypes was significantly later than that of WT plants (Figure 4b). Due to the allelic variation in different copies of VRN-A1, we could not use molecular markers to distinguish the mutation in exon 4 between WT and je0155. However, since different copies of VRN-A1 are tightly linked, the genotypes detected by markers of intron 5 also reflect genotypes in exon 4. These results indicate that the mutations in VRN-A1 putatively contributed to the later heading phenotype in je0155.
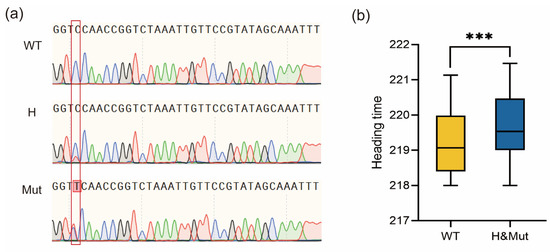
Figure 4.
Development of PCR sequencing markers and the phenotypic analysis of the F2:3 population: (a) Sequences of different genotypes of mutations in intron 5 from the F2 population detected by Sanger sequencing markers. The red box represents different peaks detected by Sanger sequencing in the population; (b) Heading time comparison of F2:3 lines genotyped by PCR sequencing markers. WT, H, and Mut indicate homozygous WT, heterozygous, and homozygous mutant genotypes, respectively. *** indicates significant differences at 0.001 level by t-test.
2.5. Expression Pattern of VRN1, VRN2, and VRN3 Genes
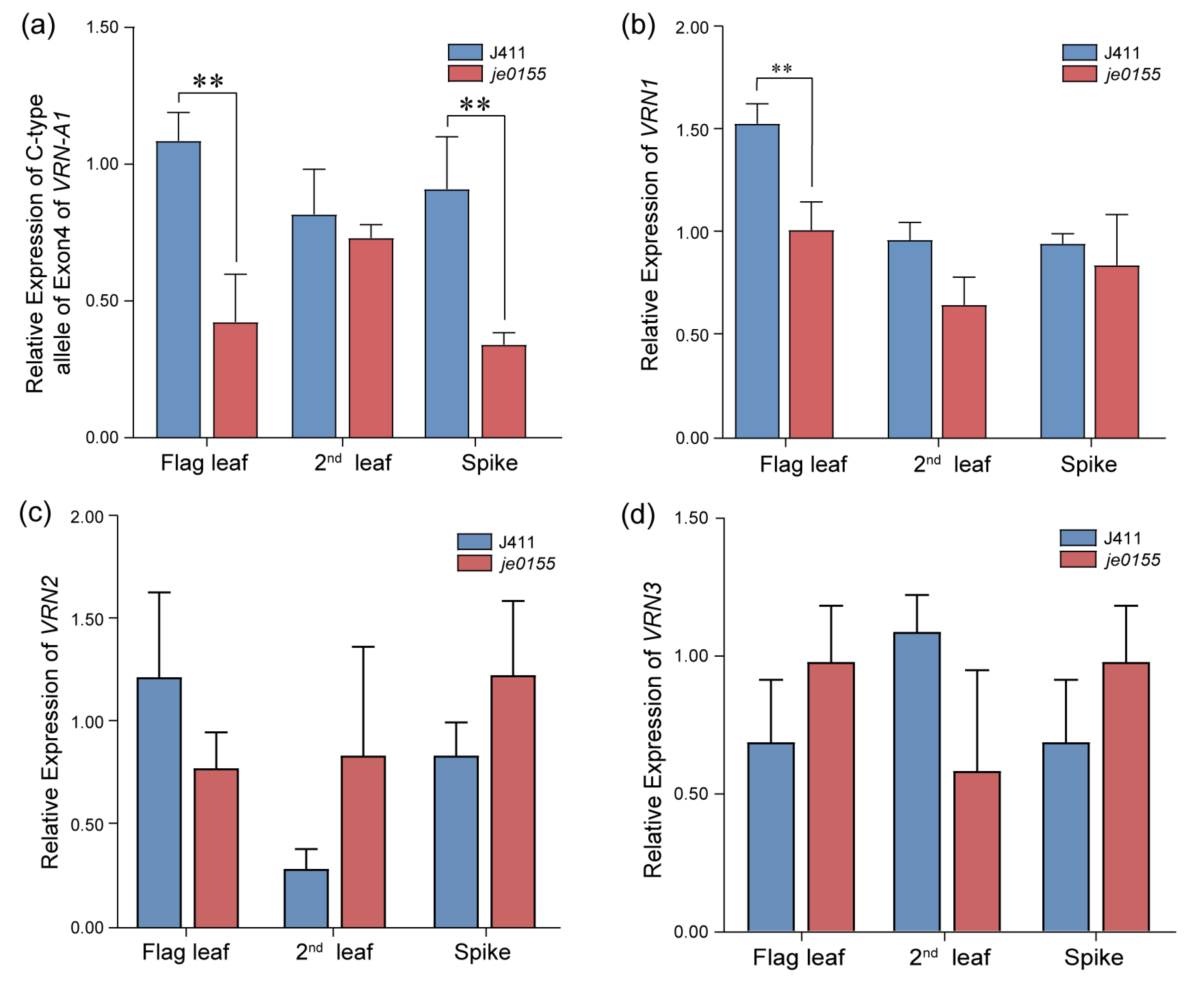
To analyze expression changes of vernalization genes between WT and mutant lines, we conducted an RT-qPCR for the C- and T-type alleles of exon 4 of VRN-A1 and the VRN genes in the flag leaf, the second leaf, and the spikes at the early heading stage. In the flag leaf and spike of je0155, the expression level of the C-type allele of exon 4 of VRN-A1 was significantly decreased compared with that of WT (Figure 5a). There was no significant difference between the expression levels of the VRN-A1 T-type allele in the WT and mutant lines (Figure S2). Additionally, the expression level of VRN1 was significantly decreased in the flag leaf in je0155 compared to WT (Figure 5b). In contrast, the expression of VRN-A1 was similar between the spikes of the WT and mutant lines. Generally, the expression of VRN2 and VRN3 genes showed no significant difference between WT and mutant in different tissues. Notably, the expression of VRN2 was slightly increased, while that of VRN3 was decreased in the second leaf of je0155 compared to WT (Figure 5c,d).
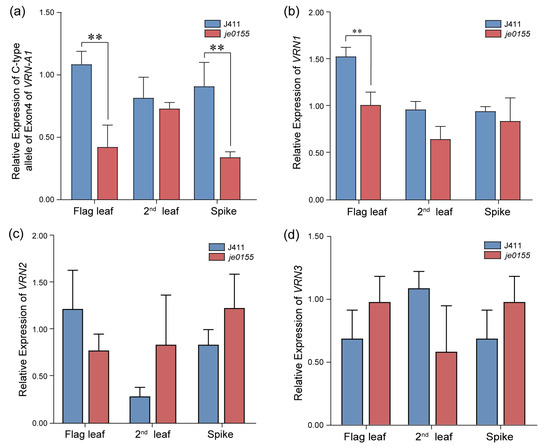
Figure 5.
Real-time qPCR of vernalization genes in WT and je0155. Values are means ± SD from five biological replicates. Second leaf indicates the second leaf below the spike: (a) Expression of C-type allele of exon 4 of VRN-A1 in the flag leaf, the second leaf, and the young spike of WT and je0155; (b–d) Relative expression of VRN1 (b), VRN2 (c), and VRN3 (d). ** represents a significant difference at 0.01 level by t-test.
3. Discussion
The heading date is an important factor in determining the ability of different wheat varieties to adapt to their local climate conditions and environmental stresses [39,40]. The time of heading directly influences grain yield in wheat, making it an important characteristic to consider when breeding new varieties [41]. The development of novel genetic resources and the identification of the key genes associated with heading date are needed to provide important cues for the modification of HD in wheat breeding. In this study, we identified new germplasm, EMS-induced mutant je0155, with HD variations of 3–4 days. By combining BSA with genetic mapping, a stable QTL was identified on a physical interval of 0.8 Mb on chromosome 5A (Figure 2a,b), and this region contained the VRN-A1 gene. Further gene cloning and sequencing revealed that the VRN-A1 gene has three copies in both WT and mutant lines. These results are consistent with a previous study that found a high percentage of Chinese wheat varieties contained three copies of VRN-A1 [32]. It has been reported that the distinct copies of VRN-A1 showed either a C or T in exon 4 [37] and these different alleles are central to the regulation of flowering time [42]. In our study, one copy of the C-type of exon 4 in WT was mutated into a T-type in je0155, which resulted in a conserved amino acid change from Leucine to phenylalanine. A previous study suggested that the T-type allele of VRN-A1 results in a deleterious substitution and that the new encoded protein is less active than that of the C-type. The expression of the C-type allele was also found to affect flowering time more strongly than that of the T-type allele [42]. It is reasonable to speculate that one copy of the C-type allele changed to a T-type in the mutant, resulting in the delayed HD phenotype. Similarly, the high frequency of the C-type allele in the Buster-like cultivar resulted in an earlier flowering phenotype, while the high frequency of the T-type allele in the Charger-like cultivar triggered a later flowering phenotype [42]. Therefore, we have determined that the mutation of C to T in exon 4 is likely responsible for late HD in je0155.
However, we could not exclude the possibility that the mutation in intron 5 of another copy of the C-type allele may have also contributed to a postponed HD in the mutant. Previous studies have suggested that both the large fragment deletion in the first intron of VRN-A1 and the C to A mutation in the GArG box of the Vrn-D1b promoter resulted in delayed heading [28,29,30]. Moreover, a mutation in the non-coding region of VRN3 was found to cause HD variations in an array of winter wheat varieties in China [43]. In our study, genotype and phenotype analysis of the segregation population suggested that the mutation in VRN-A1 contributed to HD variations (Figure 3 and Figure 4). Since both identified mutations in the duplicates of VRN-A1 were tightly linked, we could not conclude which mutation contributed to delayed HD.
The mutation in exon 4 may affect the expression of VRN-A1, thereby influencing the HD in je0155. Transcript level analysis suggested that the expression of the C-type allele in the flag leaf and young spikes of the mutant was around half of that in WT, while the expression of the T-type allele showed no significant difference between the WT and mutant (Figure S2). Consistently, the early flowering wheat cultivar Buster expressed a higher amount of C-type alleles than the later flowering wheat cultivar Charger [42]. It is well documented that as the central regulator of flowering, VRN-1 represses the expression of VRN2 while increasing VRN3 expression to promote flowering after vernalization in wheat [11,18]. Accordingly, the lower expression of C-type VRN-A1 resulted in a relatively higher expression of VRN2 and a slightly lower expression of VRN3 (Figure 5), which led to late heading in the mutant.
In conclusion, this study mapped the gene responsible for HD variation in the wheat mutant je0155 to a 0.8 Mb region of chromosome 5A by BSA and genetic linkage analysis. Through gene cloning and sequencing analysis, we found that a missense mutation in exon 4 of one copy of VRN-A1 contributes to late heading in the mutant. This mutation caused a lower expression of VRN-A1, resulting in an increase in VRN2 and a slight decrease in VRN3 transcription, thereby leading to the late heading phenotype in the mutant. The allelic mutations of different copies of VRN-A1 identified in this study provide a novel genetic resource for the modification of HD in wheat breeding. By developing our understanding of the genetic regulation of HD, we hope to contribute to the creation of more adaptable wheat varieties.
4. Materials and Methods
4.1. Plant Materials and Phenotypic Analysis
The late heading mutant (je0155) was obtained by using Ethyl methane sulfonate (EMS) to mutagenize the wheat variety Jing411 (J411). The mutant je0155 was crossed with J411 to produce an F2 population. For genotype detection, 344 F2 plants were sown to create F2:3 lines, each of which consisted of 30 plants in two rows. The F2:3 population (344 lines) and parent lines were sown at the Changping station of the Institution of Crop Science of the Chinese Academy of Agriculture Sciences (Beijing, China). The heading time of each plant was recorded when two-thirds of the spikes had emerged. At least 15 plants from each line were used for phenotypic investigation.
4.2. Genomic DNA Extraction
Fresh leaf samples at the seedling stage were processed for DNA extraction. First, the leaves were frozen and ground to a uniform powder using a Vibration Mill Type MM301 (Restsch GmbH, Germany) and completely mixed with 600 μL DNA extraction buffer. Samples were incubated at 65 °C for 1 h, then mixed with 200 μL 5 mol∙L−1 KAc and centrifuged. A 300 μL aliquot of the supernatant was collected from each sample, and the resulting DNA was precipitated by isopropanol and washed twice with 70% ethanol. A NanoDrop ND-2000 spectrophotometer (Thermo Scientific) was used to measure the quality and quantity of DNA, and the final concentration was normalized to 60 ng∙μL−1.
4.3. Bulked Segregant Analysis (BSA)
Based on the heading time data of the F2:3 families, the 93 early-heading plants and 75 late-heading plants were selected to construct three early-heading mixed pools and three late-heading mixed pools. A total of 500 ng of DNA was taken from each sample and mixed into the corresponding pool. Simultaneously, 8 individual plants of J411 and je0155 were selected to construct the parental pool. In this study, the Illumina HiSeq X high-throughput sequencing platform was used to sequence the whole exome of six progenitor mixed pools and two parent pools. Exome capture sequencing and analysis of the original sequencing data (Tcuni Technologies, Chengdu, China) were conducted as previously reported [44]. After sequencing, the raw data was filtered and all low-quality reads were removed, resulting in a total of 170.01 Gb of clean data. The Burrows–Wheeler Aligner (BWA) software was used to align the filtered data to the Chinese Spring IWGSC v1.1 reference genome. After the data was aligned, SAMtools was used to convert the matched files into BAM files, Biobambam2 software was used to remove duplicate reads generated by PCR, and BCFtools was used to detect SNPs to obtain VCF files. After analysis, 536,272 SNPs were obtained. We then used Euclidean Distance (ED) algorithm to calculate the relative allelic frequencies between two bulks, which reflects the correlation between markers and traits of interest [45]. To eliminate background noise, the ED value was fitted using a Loess curve, and the region with a high peak of ED was speculated as a candidate region.
4.4. Development of Kompetitive Allele Specific PCR (KASP) Markers
Based on BSA data, the SNPs between two parent lines on candidate chromosomal region were selected and converted to Kompetitive Allele Specific PCR (KASP) markers by using the online primer design pipeline PolyMarker (http://polymarker.tgac.ac.uk/) (accessed on 29 July 2021). The parent plants were used to detect the specificity of KASP primers. The PCR reaction was performed using a CFX 96 Real-Time System (Bio Rad, Hercules, CA, USA). A 5μL reaction system consisted of 2.5 μL KASP master mixture (LGC Genomics, Middlesex, UK), 2.4 μL 60 ng∙μL−1 DNA, 0.04 μL 50 mM Mg2+, and 0.06 μL primer mix (primer A (100 μM):primer B (100 μM):primer R (100 μM):ddH2O = 12:12:30:46). A PCR amplification procedure was conducted for: 95 °C for 15 min, followed by 10 cycles of touch-down (95 °C for 20 s; touch-down at 65 °C initially and decreasing by 0.5 °C per cycle for 30 s), then followed by 30 additional cycles of annealing (95 °C for 10 s; 57 °C for 60 s). All KASP primers were described in Table S3.
4.5. Construction of a Genetic Linkage Map
Based on the genotypes detected by KASP markers in the whole F2 population, a linkage map was constructed using the MAP function of IciMapping version 4.2. Recombination frequency was converted into centimorgan (cM) distances with the Kosambi map function. The estimates of recombination fraction pairwise between all markers are listed in Table S4. Based on the linkage map and heading date from F2:3 lines, QTL mapping was conducted using the inclusive composite interval mapping (ICIM) analysis. A value of phenotypic variance (PVE) explained by an individual QTL was determined using ICIM. Significant QTLs were identified by permutation tests with 1000 times at an error threshold of 0.05 and a logarithm of odds (LOD) threshold of 3.0.
4.6. Identification of VRN-A1
Four pairs of primers were designed to amplify the whole length of VRN-A1. To sequence VRN-A1, the whole gene was divided into two segments prior to amplification. PCRs were performed using 2× Phanta Flash Master Mix Dye Plus (Vazyme) with the following procedure: 98 °C for 30 s, followed by 35 cycles of annealing (98 °C for 10 s, 60–65 °C for 5 s and 72 °C for 1 min 30 s), and 72 °C for 1 min. Then, the PCR product was cloned using the pEASY®-Blunt zero-cloning vector (Transgen) at 37 °C, 1:7 for 30 min. After transformation into receptive cells, the universal primer M13 was used for detection, and the positive clones were cultured overnight. All primers used are described in Table S3.
4.7. Quantitative Real-Time PCR Analysis
To quantify the relative expression of VRN-A1 genes, the spikes, flag leaves, and the second leaves of J411 and je0155 were sampled at the early heading stage. Total RNA from each part was isolated using a TransZol Up Plus RNA Kit (TransGen). By using DNase I (Takara) and an RNA purification kit (Tiangen), all DNA contamination in the extracted RNA was eliminated. A TransScript First-Strand cDNA Synthesis SuperMix kit (TransGen) was used to synthesize the first strand cDNA from the extracted RNA. A qRT–PCR was conducted using the ChamQ Universal SYBR qPCR Master Mix (Vazyme) and CFX 96 Real-TimeSystem (Bio-Rad, Hercules, CA, USA). This experiment was performed with five independent biological replicates and three technical repeats. For expression analysis of VRN1, VRN2, and VRN3 genes, primers described in previous studies were used [42,46]. The internal control gene ACTIN was used to normalize the expression data. Relative expression levels were determined using the 2−ΔΔCT method.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24055008/s1.
Author Contributions
L.L. supervised the study and designed the research. Q.X. and H.X. performed most of the experiments and data analysis. C.Z. and H.G. helped for phenotypes analysis. L.Z. and Y.X. assisted in the data analysis. J.G., S.Z. and Y.D. participated in wheat planting and data analysis. Q.X. and H.X. wrote the draft manuscript. L.L. and L.X. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Research and Development Program of China (Grant No. 2022YFD1200700), the National Natural Science Foundation of China (Grant No. 32172040), the China Agriculture Research System of MOF and MARA (Grant No. CARS-03), and the Agricultural Science and Technology Innovation Program (Grant No. CAAS-ZDRW202109).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Xueyong Zhang (Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing, China) for helpful suggestion on this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The International Wheat Genome Sequencing Consortium (IWGSC); Appels, R.; Eversole, K.; Feuillet, C.; Keller, B.; Rogers, J.; Stein, N.; Pozniak, C.J.; Stein, N.; Choulet, F.; et al. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, eaar7191. [Google Scholar] [CrossRef]
- Luo, X.; He, Y. Experiencing winter for spring flowering: A molecular epigenetic perspective on vernalization. J. Integr. Plant Biol. 2019, 62, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, V.; Gomez-Ariza, J.; Cerise, M.; Fornara, F. The Importance of Being on Time: Regulatory Networks Controlling Photoperiodic Flowering in Cereals. Front. Plant Sci. 2017, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; Donnell, C.O.; Ktenioudaki, A. Global food security—Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20. [Google Scholar] [CrossRef]
- Golovnina, K.A.; Kondratenko, E.Y.; Blinov, A.G.; Goncharov, N.P. Molecular characterization of vernalization loci VRN1 in wild and cultivated wheats. BMC Plant Biol. 2010, 10, 168. [Google Scholar] [CrossRef]
- Whittal, A.; Kaviani, M.; Graf, R.; Humphreys, G.; Navabi, A. Allelic variation of vernalization and photoperiod response genes in a diverse set of North American high latitude winter wheat genotypes. PLoS ONE 2018, 13, e0203068. [Google Scholar] [CrossRef]
- Dragovich, A.Y.; Fisenko, A.V.; Yankovskaya, A.A. Vernalization (VRN) and Photoperiod (PPD) Genes in Spring Hexaploid Wheat Landraces. Russ. J. Genet. 2021, 57, 329–340. [Google Scholar] [CrossRef]
- Deng, W.; Casao, M.C.; Wang, P.; Sato, K.; Hayes, P.M.; Finnegan, E.J.; Trevaskis, B. Direct links between the vernalization response and other key traits of cereal crops. Nat. Commun. 2015, 6, 5882. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, G.; Zhuang, M.; Yin, J.; Wang, X. Molecular cloning and functional characterization of TaIRI9 gene in wheat (Triticum aestivum L.). Gene 2021, 791, 145694. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The Wheat VRN2 Gene Is a Flowering Repressor Down-Regulated by Vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Wigge, P.A.; Kim, M.C.; Jaeger, K.E.; Busch, W.; Schmid, M.; Lohmann, J.U.; Weigel, D. Integration of Spatial and Temporal Information during Floral Induction in Arabidopsis. Science 2005, 309, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Amasino, R.M. Vernalization and epigenetics: How plants remember winter. Curr. Opin. Plant Biol. 2004, 7, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kabbaj, H.; El Hassouni, K.; Maccaferri, M.; Sanchez-Garcia, M.; Tuberosa, R.; Bassi, F. Genomic Regions Associated with the Control of Flowering Time in Durum Wheat. Plants 2020, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Nitcher, R.; Pearce, S.; Tranquilli, G.; Zhang, X.; Dubcovsky, J. Effect of the Hope FT-B1 Allele on Wheat Heading Time and Yield Components. J. Hered. 2014, 105, 666–675. [Google Scholar] [CrossRef]
- Båga, M.; Chodaparambil, S.V.; Limin, A.E.; Pecar, M.; Fowler, D.B.; Chibbar, R.N. Identification of quantitative trait loci and associated candidate genes for low-temperature tolerance in cold-hardy winter wheat. Funct. Integr. Genom. 2006, 7, 53–68. [Google Scholar] [CrossRef]
- Kippes, N.; Chen, A.; Zhang, X.; Lukaszewski, A.J.; Dubcovsky, J. Development and characterization of a spring hexaploid wheat line with no functional VRN2 genes. Theor. Appl. Genet. 2016, 129, 1417–1428. [Google Scholar] [CrossRef]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef]
- Jin, F.; Wei, L. The expression patterns of three VRN genes in common wheat (Triticum aestivum L.) in response to vernalization. Cereal Res. Commun. 2016, 44, 1–12. [Google Scholar] [CrossRef]
- Chen, A.; Dubcovsky, J. Wheat TILLING Mutants Show That the Vernalization Gene VRN1 Down-Regulates the Flowering Repressor VRN2 in Leaves but Is Not Essential for Flowering. PLOS Genet. 2012, 8, e1003134. [Google Scholar] [CrossRef]
- Li, C.; Distelfeld, A.; Comis, A.; Dubcovsky, J. Wheat flowering repressor VRN2 and promoter CO2 compete for interactions with NUCLEAR FACTOR-Y complexes. Plant J. 2011, 67, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Dunford, R.P.; Yano, M.; Kurata, N.; Sasaki, T.; Huestis, G.; Rocheford, T.; Laurie, D.A. Comparative Mapping of the Barley Ppd-H1 Photoperiod Response Gene Region, Which Lies Close to a Junction between Two Rice Linkage Segments. Genetics 2002, 161, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Beales, J.; Turner, A.; Griffiths, S.; Snape, J.W.; Laurie, D.A. A Pseudo-Response Regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Arjona, J.; Villegas, D.; Ammar, K.; Dreisigacker, S.; Alfaro, C.; Royo, C. The Effect of Photoperiod Genes and Flowering Time on Yield and Yield Stability in Durum Wheat. Plants 2020, 9, 1723. [Google Scholar] [CrossRef]
- Fu, D.; Szűcs, P.; Yan, L.; Helguera, M.; Skinner, J.S.; Von Zitzewitz, J.; Hayes, P.M.; Dubcovsky, J. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol. Genet. Genom. 2005, 274, 442–443. [Google Scholar] [CrossRef]
- Zhang, X.K.; Xiao, Y.G.; Zhang, Y.; Xia, X.C.; Dubcovsky, J.; He, Z.H. Allelic Variation at the Vernalization Genes Vrn-A1, Vrn-B1, Vrn-D1, and Vrn-B3 in Chinese Wheat Cultivars and Their Association with Growth Habit. Crop. Sci. 2008, 48, 458–470. [Google Scholar] [CrossRef]
- Milec, Z.; Tomková, L.; Sumíková, T.; Pánková, K. A new multiplex PCR test for the determination of Vrn-B1 alleles in bread wheat (Triticum aestivum L.). Mol. Breed. 2011, 30, 317–323. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wu, S.; Yang, J.; Liu, H.; Zhou, Y. A single nucleotide polymorphism at the Vrn-D1 promoter region in common wheat is associated with vernalization response. Theor. Appl. Genet. 2012, 125, 1697–1704. [Google Scholar] [CrossRef]
- Muterko, A.; Kalendar, R.; Salina, E. Novel alleles of the VERNALIZATION1 genes in wheat are associated with modulation of DNA curvature and flexibility in the promoter region. BMC Plant Biol. 2016, 16, 9. [Google Scholar] [CrossRef]
- Santra, D.K.; Santra, M.; Allan, R.E.; Campbell, K.G.; Kidwell, K.K. Genetic and Molecular Characterization of Vernalization Genes Vrn-A1, Vrn-B1, and Vrn-D1 in Spring Wheat Germplasm from the Pacific Northwest Region of the U.S.A. Plant Breed. 2009, 128, 576–584. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, M.; Wang, S.; Chen, F.; Cui, D. Allelic variation at the vernalization and photoperiod sensitivity loci in Chinese winter wheat cultivars (Triticum aestivum L.). Front. Plant Sci. 2015, 6, 470. [Google Scholar] [CrossRef] [PubMed]
- Würschum, T.; Boeven, P.H.G.; Langer, S.M.; Longin, C.F.H.; Leiser, W.L. Multiply to conquer: Copy number variations at Ppd-B1 and Vrn-A1 facilitate global adaptation in wheat. BMC Genet. 2015, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guedira, M.; Xiong, M.; Hao, Y.F.; Johnson, J.; Harrison, S.; Marshall, D.; Brown-Guedira, G. Heading Date QTL in Winter Wheat (Triticum aestivum L.) Coincide with Major Developmental Genes VERNALIZATION1 and PHOTOPERIOD1. PLoS ONE 2016, 11, e0154242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Islam, S.; Zhao, Y.; Anwar, M.; Alhabbar, Z.; She, M.; Yang, R.; Juhasz, A.; Tang, G.; Chen, J.; et al. Non-escaping frost tolerant QTL linked genetic loci at reproductive stage in six wheat DH populations. Crop. J. 2021, 10, 147–165. [Google Scholar] [CrossRef]
- Gonzalez, E.; Kulkarni, H.; Bolivar, H.; Mangano, A.; Sanchez, R.; Catano, G.; Nibbs, R.J.; Freedman, B.I.; Quinones, M.P.; Bamshad, M.J.; et al. The Influence of CCL3L1 Gene-Containing Segmental Duplications on HIV-1/AIDS Susceptibility. Science 2005, 307, 1434–1440. [Google Scholar] [CrossRef]
- Eagles, H.A.; Cane, K.; Trevaskis, B. Veery wheats carry an allele of Vrn-A1 that has implications for freezing tolerance in winter wheats. Plant Breed. 2011, 130, 413–418. [Google Scholar] [CrossRef]
- Díaz, A.; Zikhali, M.; Turner, A.S.; Isaac, P.; Laurie, D.A. Copy Number Variation Affecting the Photoperiod-B1 and Vernalization-A1 Genes Is Associated with Altered Flowering Time in Wheat (Triticum aestivum). PLoS ONE 2012, 7, e33234. [Google Scholar] [CrossRef]
- Muterko, A.; Salina, E. Origin and Distribution of the VRN-A1 Exon 4 and Exon 7 Haplotypes in Domesticated Wheat Species. Agronomy 2018, 8, 156. [Google Scholar] [CrossRef]
- Huijser, P.; Schmid, M. The control of developmental phase transitions in plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, G.; Zhang, L.; Xia, C.; Zhao, T.; Jia, J.; Liu, X.; Kong, X. Fine Mapping of a Novel Heading Date Gene, TaHdm605, in Hexaploid Wheat. Front. Plant Sci. 2018, 9, 1059. [Google Scholar] [CrossRef]
- Hill, C.B.; Li, C. Genetic Architecture of Flowering Phenology in Cereals and Opportunities for Crop Improvement. Front. Plant Sci. 2016, 7, 1906. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.E.; Karsai, I.; Kiss, T.; Adamski, N.M.; Liu, Z.; Ding, Y.; Allard, V.; Boden, S.A.; Griffiths, S. VERNALIZATION1 controls developmental responses of winter wheat under high ambient temperatures. Development 2019, 146, dev172684. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gao, M.; Zhang, J.; Zuo, A.; Shang, X.; Cui, D. Molecular characterization of vernalization and response genes in bread wheat from the Yellow and Huai Valley of China. BMC Plant Biol. 2013, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhou, C.; Fu, M.; Guo, H.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Li, Y.; et al. Cloning and functional characterization of Rht8, a “Green Revolution” replacement gene in wheat. Mol. Plant 2022, 15, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.T.; Demarest, B.L.; Bisgrove, B.W.; Gorsi, B.; Su, Y.-C.; Yost, H.J. MMAPPR: Mutation Mapping Analysis Pipeline for Pooled RNA-seq. Genome Res. 2013, 23, 687–697. [Google Scholar] [CrossRef]
- Xu, S.; Dong, Q.; Deng, M.; Lin, D.; Xiao, J.; Cheng, P.; Xing, L.; Niu, Y.; Gao, C.; Zhang, W.; et al. The vernalization-induced long non-coding RNA VAS functions with the transcription factor TaRF2b to promote TaVRN1 expression for flowering in hexaploid wheat. Mol. Plant 2021, 14, 1525–1538. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).