Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications
Abstract
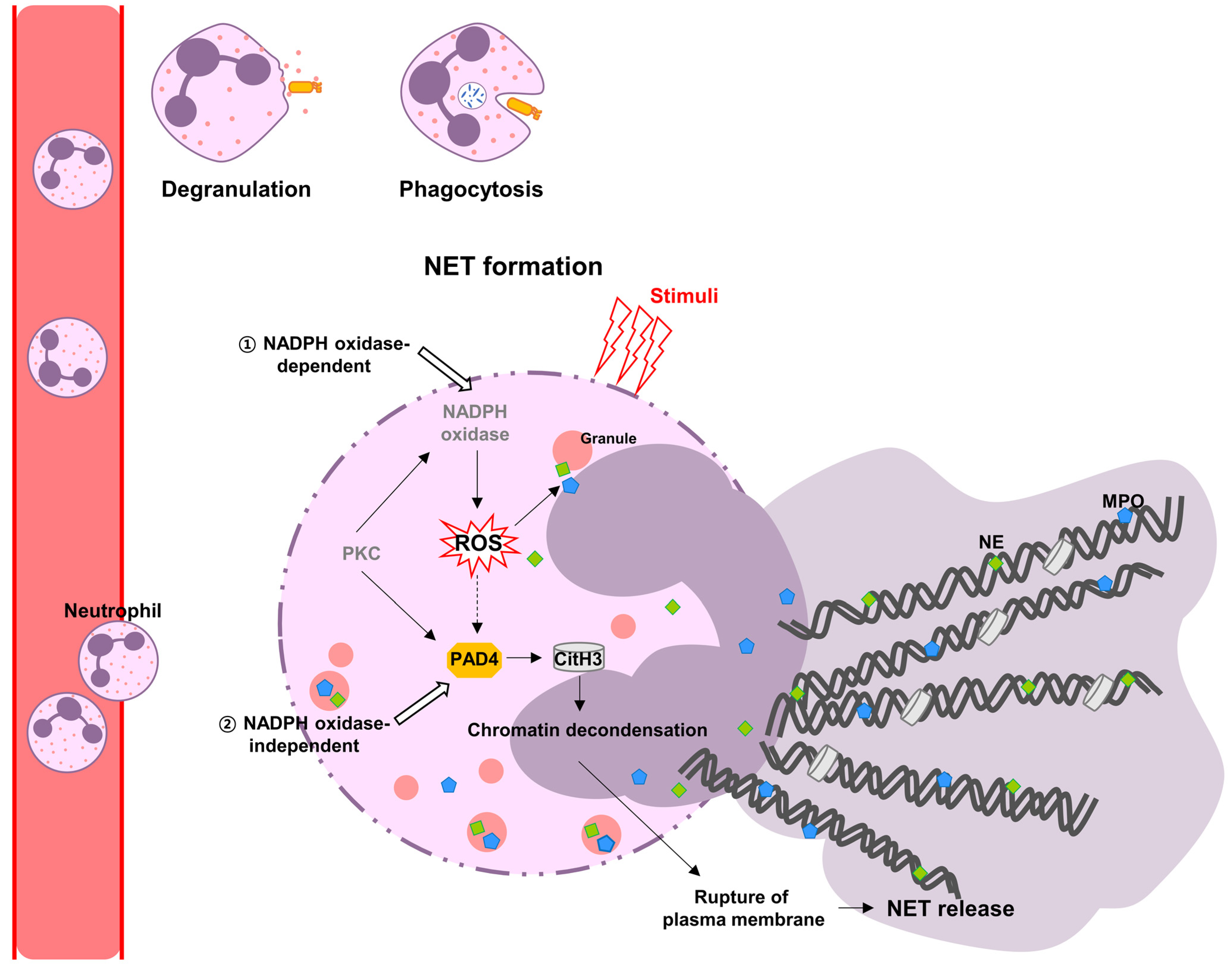
:1. Introduction
2. Neutrophil Extracellular Traps (NETs)
3. NETs in Airway Diseases
3.1. Asthma
3.2. Chronic Rhinosinusitis (CRS)
3.3. Cystic Fibrosis (CF)
3.4. Chronic Obstructive Pulmonary Disease (COPD)
3.5. Bronchiectasis
3.6. Coronavirus Disease 2019 (COVID-19)
4. Potential Therapeutic Targets of NETs
4.1. DNase
4.2. Neutrophil Protease Inhibitors
4.3. CXCR2 Antagonists
4.4. ROS Scavengers
4.5. Other Inhibitors
| Target Molecule | Inhibitor(s) | Clinical Trial | Disease(s) | Reference(s) |
|---|---|---|---|---|
| DNA | DNase I | FDA approved | Asthma | [23,92,159] |
| CF | [113,157,158] | |||
| Bronchiectasis | [162] | |||
| COVID-19 | [160,161] | |||
| Neutrophil elastase (NE) | AZD9668 | Phase IIa | CF | [172] |
| Phase IIb | COPD | [171,174] | ||
| Phase II | Bronchiectasis | [173] | ||
| GW311616A | NA | COPD | [170] | |
| Myeloperoxidase (MPO) | ABAH | NA | Asthma | [176] |
| CXCR2 | AZD5069 | Phase IIb | Asthma | [183,184] |
| Phase IIa [223] | COPD | [180] | ||
| Phase IIa | Bronchiectasis | [182] | ||
| Danirixin | Phase IIb | COPD | [187,188] | |
| CXCR1/2 | Reparixin | Phase II/III (COVID-19 pneumonia) [224] | COVID-19 | [67] |
| Reactive oxygen species (ROS) | N-acetyl-L-cysteine (NAC) | FDA approved | COPD | [196,197] |
| Bronchiectasis | [198] | |||
| Multiple pathways | Hydroxychloroquine (HCQ) | FDA approved | COVID-19 | [211] |
| Metformin | FDA approved | COVID-19 | [215,216,217] |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hidalgo, A.; Chilvers, E.R.; Summers, C.; Koenderman, L. The Neutrophil Life Cycle. Trends Immunol. 2019, 40, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Gouwy, M.; Proost, P. The chemokines CXCL8 and CXCL12: Molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol. Immunol. 2023, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef] [Green Version]
- Liew, P.X.; Kubes, P. The Neutrophil’s Role During Health and Disease. Physiol. Rev. 2019, 99, 1223–1248. [Google Scholar] [CrossRef]
- Mollinedo, F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol. 2019, 40, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glennon-Alty, L.; Hackett, A.P.; Chapman, E.A.; Wright, H.L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic. Biol. Med. 2018, 125, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Rivera, C.; Carlucci, P.M.; Moore, E.; Lingampalli, N.; Uchtenhagen, H.; James, E.; Liu, Y.; Bicker, K.L.; Wahamaa, H.; Hoffmann, V.; et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2017, 2, aag3358. [Google Scholar] [CrossRef] [Green Version]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2022, 1–15. [Google Scholar] [CrossRef]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Crisford, H.; Sapey, E.; Rogers, G.B.; Taylor, S.; Nagakumar, P.; Lokwani, R.; Simpson, J.L. Neutrophils in asthma: The good, the bad and the bacteria. Thorax 2021, 76, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.J.; Radic, M. Neutrophil extracellular traps: Double-edged swords of innate immunity. J. Immunol. 2012, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Steinberg, B.E.; Grinstein, S. Unconventional roles of the NADPH oxidase: Signaling, ion homeostasis, and cell death. Sci. STKE 2007, 2007, pe11. [Google Scholar] [CrossRef]
- Lu, T.; Kobayashi, S.D.; Quinn, M.T.; Deleo, F.R. A NET Outcome. Front. Immunol. 2012, 3, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [Green Version]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Keir, H.R.; Shoemark, A.; Dicker, A.J.; Perea, L.; Pollock, J.; Giam, Y.H.; Suarez-Cuartin, G.; Crichton, M.L.; Lonergan, M.; Oriano, M.; et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: An international, observational, multicohort study. Lancet Respir. Med. 2021, 9, 873–884. [Google Scholar] [CrossRef]
- Pham, D.L.; Ban, G.Y.; Kim, S.H.; Shin, Y.S.; Ye, Y.M.; Chwae, Y.J.; Park, H.S. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma. Clin. Exp. Allergy 2017, 47, 57–70. [Google Scholar] [CrossRef]
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz-Scroggins, M.E.; Dunican, E.M.; Charbit, A.R.; Raymond, W.; Looney, M.R.; Peters, M.C.; Gordon, E.D.; Woodruff, P.G.; Lefrancais, E.; Phillips, B.R.; et al. Extracellular DNA, Neutrophil Extracellular Traps, and Inflammasome Activation in Severe Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 1076–1085. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Douda, D.N.; Bruggemann, T.R.; Ricklefs, I.; Duvall, M.G.; Abdulnour, R.E.; Martinod, K.; Tavares, L.; Wang, X.; Cernadas, M.; et al. Neutrophil cytoplasts induce T(H)17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci. Immunol. 2018, 3, aao4747. [Google Scholar] [CrossRef] [Green Version]
- Fattahi, F.; Grailer, J.J.; Lu, H.; Dick, R.S.; Parlett, M.; Zetoune, F.S.; Nunez, G.; Ward, P.A. Selective Biological Responses of Phagocytes and Lungs to Purified Histones. J. Innate Immun. 2017, 9, 300–317. [Google Scholar] [CrossRef]
- Abrams, S.T.; Zhang, N.; Manson, J.; Liu, T.; Dart, C.; Baluwa, F.; Wang, S.S.; Brohi, K.; Kipar, A.; Yu, W.; et al. Circulating histones are mediators of trauma-associated lung injury. Am. J. Respir. Crit. Care Med. 2013, 187, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosmann, M.; Grailer, J.J.; Ruemmler, R.; Russkamp, N.F.; Zetoune, F.S.; Sarma, J.V.; Standiford, T.J.; Ward, P.A. Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. FASEB J. 2013, 27, 5010–5021. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.; Ito, T.; Kakuuchi, M.; Totoki, T.; Nagasato, T.; Yamamoto, M.; Maruyama, I. Recombinant Thrombomodulin Suppresses Histone-Induced Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 2535. [Google Scholar] [CrossRef]
- Huang, H.; Tohme, S.; Al-Khafaji, A.B.; Tai, S.; Loughran, P.; Chen, L.; Wang, S.; Kim, J.; Billiar, T.; Wang, Y.; et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015, 62, 600–614. [Google Scholar] [CrossRef] [Green Version]
- Massberg, S.; Grahl, L.; von Bruehl, M.L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010, 16, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, H.; Welin, A.; Michaelsson, E.; Osla, V.; Berg, S.; Christenson, K.; Sundqvist, M.; Dahlgren, C.; Karlsson, A.; Bylund, J. Neutrophil NET formation is regulated from the inside by myeloperoxidase-processed reactive oxygen species. Free Radic. Biol. Med. 2015, 89, 1024–1035. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Devaney, J.M.; Greene, C.M.; Taggart, C.C.; Carroll, T.P.; O’Neill, S.J.; McElvaney, N.G. Neutrophil elastase up-regulates interleukin-8 via toll-like receptor 4. FEBS Lett. 2003, 544, 129–132. [Google Scholar] [CrossRef] [Green Version]
- Pham, C.T. Neutrophil serine proteases: Specific regulators of inflammation. Nat. Rev. Immunol. 2006, 6, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Okeke, E.B.; Louttit, C.; Fry, C.; Najafabadi, A.H.; Han, K.; Nemzek, J.; Moon, J.J. Inhibition of neutrophil elastase prevents neutrophil extracellular trap formation and rescues mice from endotoxic shock. Biomaterials 2020, 238, 119836. [Google Scholar] [CrossRef]
- Alam, S.; Li, Z.; Janciauskiene, S.; Mahadeva, R. Oxidation of Z alpha1-antitrypsin by cigarette smoke induces polymerization: A novel mechanism of early-onset emphysema. Am. J. Respir. Cell Mol. Biol. 2011, 45, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Dunlea, D.M.; Fee, L.T.; McEnery, T.; McElvaney, N.G.; Reeves, E.P. The impact of alpha-1 antitrypsin augmentation therapy on neutrophil-driven respiratory disease in deficient individuals. J. Inflamm. Res. 2018, 11, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Modestino, L.; Poto, R.; Cristinziano, L.; Gentile, L.; Postiglione, L.; Spadaro, G.; Galdiero, M.R. Neutrophil extracellular traps and neutrophil-derived mediators as possible biomarkers in bronchial asthma. Clin. Exp. Med. 2022, 22, 285–300. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Tucker, S.L.; Sarr, D.; Rada, B. Neutrophil extracellular traps are present in the airways of ENaC-overexpressing mice with cystic fibrosis-like lung disease. BMC Immunol. 2021, 22, 7. [Google Scholar] [CrossRef]
- Granger, V.; Taille, C.; Roach, D.; Letuve, S.; Dupin, C.; Hamidi, F.; Noel, B.; Neukirch, C.; Aubier, M.; Pretolani, M.; et al. Circulating neutrophil and eosinophil extracellular traps are markers of severe asthma. Allergy 2020, 75, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Eisenblatter, M.; Voller, T.; Zenker, S.; Hermann, S.; van Lent, P.; Faust, A.; Geyer, C.; Petersen, B.; Roebrock, K.; et al. Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat. Commun. 2014, 5, 4593. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zuo, Y.; Yalavarthi, S.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Woodward, W.; Lezak, S.P.; Lugogo, N.L.; et al. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. J. Leukoc. Biol. 2021, 109, 67–72. [Google Scholar] [CrossRef]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, F.; Sun, Y.; Hong, H.; Wen, Y.; Lai, Y.; Xu, Z.; Luo, X.; Chen, Y.; Shi, J.; et al. LL-37 promotes neutrophil extracellular trap formation in chronic rhinosinusitis with nasal polyps. Clin. Exp. Allergy 2019, 49, 990–999. [Google Scholar] [CrossRef]
- Durr, U.H.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, A.; Berends, E.T.; Nerlich, A.; Molhoek, E.M.; Gallo, R.L.; Meerloo, T.; Nizet, V.; Naim, H.Y.; von Kockritz-Blickwede, M. The antimicrobial peptide LL-37 facilitates the formation of neutrophil extracellular traps. Biochem. J. 2014, 464, 3–11. [Google Scholar] [CrossRef]
- Persson, L.J.; Aanerud, M.; Hardie, J.A.; Miodini Nilsen, R.; Bakke, P.S.; Eagan, T.M.; Hiemstra, P.S. Antimicrobial peptide levels are linked to airway inflammation, bacterial colonisation and exacerbations in chronic obstructive pulmonary disease. Eur. Respir. J. 2017, 49, 1601328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lande, R.; Gregorio, J.; Facchinetti, V.; Chatterjee, B.; Wang, Y.H.; Homey, B.; Cao, W.; Wang, Y.H.; Su, B.; Nestle, F.O.; et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 2007, 449, 564–569. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakayama, H.; Sasaki, S.; Takahashi, K.; Iwabuchi, K. Mycobacterium avium-intracellulare complex promote release of pro-inflammatory enzymes matrix metalloproteinases by inducing neutrophil extracellular trap formation. Sci. Rep. 2022, 12, 5181. [Google Scholar] [CrossRef] [PubMed]
- Baraldo, S.; Bazzan, E.; Zanin, M.E.; Turato, G.; Garbisa, S.; Maestrelli, P.; Papi, A.; Miniati, M.; Fabbri, L.M.; Zuin, R.; et al. Matrix metalloproteinase-2 protein in lung periphery is related to COPD progression. Chest 2007, 132, 1733–1740. [Google Scholar] [CrossRef]
- Wells, J.M.; Parker, M.M.; Oster, R.A.; Bowler, R.P.; Dransfield, M.T.; Bhatt, S.P.; Cho, M.H.; Kim, V.; Curtis, J.L.; Martinez, F.J.; et al. Elevated circulating MMP-9 is linked to increased COPD exacerbation risk in SPIROMICS and COPD Gene. JCI Insight 2018, 3, 123614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.L.; Rogers, G.B.; Chen, A.C.; Burr, L.D.; McGuckin, M.A.; Serisier, D.J. Matrix metalloproteinases vary with airway microbiota composition and lung function in non-cystic fibrosis bronchiectasis. Ann. Am. Thorac. Soc. 2015, 12, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wysocka, J.; Sayegh, J.; Lee, Y.H.; Perlin, J.R.; Leonelli, L.; Sonbuchner, L.S.; McDonald, C.H.; Cook, R.G.; Dou, Y.; et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 2004, 306, 279–283. [Google Scholar] [CrossRef]
- Neeli, I.; Dwivedi, N.; Khan, S.; Radic, M. Regulation of extracellular chromatin release from neutrophils. J. Innate Immun. 2009, 1, 194–201. [Google Scholar] [CrossRef]
- Knight, J.S.; Subramanian, V.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Smith, C.K.; Hodgin, J.B.; Thompson, P.R.; Kaplan, M.J. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 2015, 74, 2199–2206. [Google Scholar] [CrossRef] [Green Version]
- Savchenko, A.S.; Borissoff, J.I.; Martinod, K.; De Meyer, S.F.; Gallant, M.; Erpenbeck, L.; Brill, A.; Wang, Y.; Wagner, D.D. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 2014, 123, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Ikari, J.; Anazawa, R.; Tanaka, N.; Katsumata, Y.; Shimada, A.; Suzuki, E.; Tatsumi, K. PAD4 Deficiency Improves Bleomycin-induced Neutrophil Extracellular Traps and Fibrosis in Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2020, 63, 806–818. [Google Scholar] [CrossRef]
- Lefrancais, E.; Mallavia, B.; Zhuo, H.; Calfee, C.S.; Looney, M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 2018, 3, e98178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.; Lopez-Villegas, E.O.; Sanchez-Garcia, F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef] [Green Version]
- Kenny, E.F.; Herzig, A.; Kruger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.V.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 2017, 6, 24437. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007, 176, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Giaglis, S.; Hasler, P.; Hahn, S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS ONE 2014, 9, e97088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, R.; Leunig, A.; Pekayvaz, K.; Popp, O.; Joppich, M.; Polewka, V.; Escaig, R.; Anjum, A.; Hoffknecht, M.L.; Gold, C.; et al. Self-sustaining IL-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. JCI Insight 2021, 6, 150862. [Google Scholar] [CrossRef]
- Hosoki, K.; Ying, S.; Corrigan, C.; Qi, H.; Kurosky, A.; Jennings, K.; Sun, Q.; Boldogh, I.; Sur, S. Analysis of a Panel of 48 Cytokines in BAL Fluids Specifically Identifies IL-8 Levels as the Only Cytokine that Distinguishes Controlled Asthma from Uncontrolled Asthma, and Correlates Inversely with FEV1. PLoS ONE 2015, 10, e0126035. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.G.; Simpson, J.L.; Saltos, N. Heterogeneity of airway inflammation in persistent asthma: Evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 2001, 119, 1329–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.J.; Quigley, K.; Cheng, X.; Suresh, A.; Tahir, S.; Ahmed-Jushuf, F.; Nawab, K.; Choy, K.; Walker, S.A.; Mathie, S.A.; et al. Lung Defense through IL-8 Carries a Cost of Chronic Lung Remodeling and Impaired Function. Am. J. Respir Cell Mol. Biol. 2018, 59, 557–571. [Google Scholar] [CrossRef]
- Qu, M.; Chen, Z.; Qiu, Z.; Nan, K.; Wang, Y.; Shi, Y.; Shao, Y.; Zhong, Z.; Zhu, S.; Guo, K.; et al. Neutrophil extracellular traps-triggered impaired autophagic flux via METTL3 underlies sepsis-associated acute lung injury. Cell Death Discov. 2022, 8, 375. [Google Scholar] [CrossRef]
- Vats, R.; Kaminski, T.W.; Brzoska, T.; Leech, J.A.; Tutuncuoglu, E.; Katoch, O.; Jonassaint, J.; Tejero, J.; Novelli, E.M.; Pradhan-Sundd, T.; et al. Liver-to-lung microembolic NETs promote gasdermin D-dependent inflammatory lung injury in sickle cell disease. Blood 2022, 140, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, M.; Juenemann, C.; Queisser, M.A.; Lochnit, G.; Barreto, G.; Galuska, S.P.; Lohmeyer, J.; Preissner, K.T. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: A predominant role of histones. PLoS ONE 2012, 7, e32366. [Google Scholar] [CrossRef] [PubMed]
- Keir, H.R.; Chalmers, J.D. Neutrophil extracellular traps in chronic lung disease: Implications for pathogenesis and therapy. Eur. Respir. Rev. 2022, 31, 210241. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.N.; Stein, R.T. Neutrophil Extracellular Traps in Pulmonary Diseases: Too Much of a Good Thing? Front. Immunol. 2016, 7, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, A.; Khan, M.A.; Bade, G.; Talwar, A. Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development. Biomedicines 2021, 9, 53. [Google Scholar] [CrossRef]
- Dwyer, M.; Shan, Q.; D’Ortona, S.; Maurer, R.; Mitchell, R.; Olesen, H.; Thiel, S.; Huebner, J.; Gadjeva, M. Cystic fibrosis sputum DNA has NETosis characteristics and neutrophil extracellular trap release is regulated by macrophage migration-inhibitory factor. J. Innate Immun. 2014, 6, 765–779. [Google Scholar] [CrossRef]
- Moore, W.C.; Bleecker, E.R. Asthma heterogeneity and severity-why is comprehensive phenotyping important? Lancet Respir Med. 2014, 2, 10–11. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A. Type 2 cytokines: Mechanisms and therapeutic strategies. Nat. Rev. Immunol. 2015, 15, 271–282. [Google Scholar] [CrossRef]
- Ray, A.; Kolls, J.K. Neutrophilic Inflammation in Asthma and Association with Disease Severity. Trends Immunol. 2017, 38, 942–954. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.; Pham, L.D.; Lee, D.H.; Ban, G.Y.; Lee, J.H.; Kim, S.H.; Park, H.S. Neutrophil Extracellular DNA Traps Induce Autoantigen Production by Airway Epithelial Cells. Mediators Inflamm. 2017, 2017, 5675029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jatakanon, A.; Uasuf, C.; Maziak, W.; Lim, S.; Chung, K.F.; Barnes, P.J. Neutrophilic inflammation in severe persistent asthma. Am. J. Respir Crit. Care Med. 1999, 160 Pt 1, 1532–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvestri, M.; Bontempelli, M.; Giacomelli, M.; Malerba, M.; Rossi, G.A.; Di Stefano, A.; Rossi, A.; Ricciardolo, F.L. High serum levels of tumour necrosis factor-alpha and interleukin-8 in severe asthma: Markers of systemic inflammation? Clin. Exp. Allergy 2006, 36, 1373–1381. [Google Scholar] [CrossRef]
- Bonnans, C.; Vachier, I.; Chavis, C.; Godard, P.; Bousquet, J.; Chanez, P. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am. J. Respir Crit. Care Med. 2002, 165, 1531–1535. [Google Scholar] [CrossRef]
- Baines, K.J.; Simpson, J.L.; Wood, L.G.; Scott, R.J.; Gibson, P.G. Systemic upregulation of neutrophil alpha-defensins and serine proteases in neutrophilic asthma. Thorax 2011, 66, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Cundall, M.; Sun, Y.; Miranda, C.; Trudeau, J.B.; Barnes, S.; Wenzel, S.E. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J. Allergy Clin. Immunol. 2003, 112, 1064–1071. [Google Scholar] [CrossRef]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef]
- Simpson, J.L.; Phipps, S.; Baines, K.J.; Oreo, K.M.; Gunawardhana, L.; Gibson, P.G. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur. Respir. J. 2014, 43, 1067–1076. [Google Scholar] [CrossRef]
- Lee, Y.G.; Hong, J.; Lee, P.H.; Lee, J.; Park, S.W.; Kim, D.; Jang, A.S. Serum Calprotectin Is a Potential Marker in Patients with Asthma. J. Korean Med. Sci. 2020, 35, e362. [Google Scholar] [CrossRef]
- Xia, M.; Xu, F.; Ni, H.; Wang, Q.; Zhang, R.; Lou, Y.; Zhou, J. Neutrophil activation and NETosis are the predominant drivers of airway inflammation in an OVA/CFA/LPS induced murine model. Respir Res. 2022, 23, 289. [Google Scholar] [CrossRef]
- Khalmuratova, R.; Shin, H.W. Crosstalk Between Mucosal Inflammation and Bone Metabolism in Chronic Rhinosinusitis. Clin. Exp. Otorhinolaryngol. 2021, 14, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.K.; Zhang, Y.L.; Wee, J.H.; Han, D.H.; Kim, H.J.; Rhee, C.S. Human Rhinovirus Infection Enhances the Th2 Environment in Allergic and Non-allergic Patients with Chronic Rhinosinusitis. Clin. Exp. Otorhinolaryngol. 2021, 14, 217–224. [Google Scholar] [CrossRef]
- Lee, H.S.; Volpe, S.J.; Chang, E.H. The Role of Viruses in the Inception of Chronic Rhinosinusitis. Clin. Exp. Otorhinolaryngol. 2022, 15, 310–318. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.W.; Basurrah, M.A.; Hwang, S.H. Clinical and Laboratory Features of Various Criteria of Eosinophilic Chronic Rhinosinusitis: A Systematic Review and Meta-Analysis. Clin. Exp. Otorhinolaryngol. 2022, 15, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Eun, K.M.; Kim, M.K.; Cho, D.; Han, S.A.; Han, S.Y.; Seo, Y.; Lee, D.H.; Cho, S.H.; Kim, D.W. Comparison Between Signature Cytokines of Nasal Tissues in Subtypes of Chronic Rhinosinusitis. Allergy Asthma Immunol. Res. 2019, 11, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zeng, M.; Liu, Z. Revisiting Asian chronic rhinosinusitis in the era of type 2 biologics. Clin. Exp. Allergy 2022, 52, 231–243. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Wen, W.; Liu, W.; Zhang, L.; Bai, J.; Fan, Y.; Xia, W.; Luo, Q.; Zheng, J.; Wang, H.; Li, Z.; et al. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J. Allergy Clin. Immunol. 2012, 129, 1522–1528.e5. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.Y.; Jiang, W.X.; Liao, B.; Zhai, G.T.; Wang, N.; Zhen, Z.; Ruan, J.W.; Long, X.B.; Wang, H.; et al. The activation and function of IL-36gamma in neutrophilic inflammation in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2018, 141, 1646–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, J.C.; Li, P.; Ely, K.A.; Shilts, M.H.; Wannemuehler, T.J.; Huang, L.C.; Sheng, Q.; Chowdhury, N.I.; Chandra, R.K.; Das, S.R.; et al. Chronic rhinosinusitis in elderly patients is associated with an exaggerated neutrophilic proinflammatory response to pathogenic bacteria. J. Allergy Clin. Immunol. 2019, 143, 990–1002.e6. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.Y.; Han, Y.E.; Kim, J.K.; Lim, H.S.; Eun, K.M.; Yang, S.K.; Kim, D.W. Elastase-Positive Neutrophils Are Associated with Refractoriness of Chronic Rhinosinusitis with Nasal Polyps in an Asian Population. Allergy Asthma Immunol. Res. 2020, 12, 42–55. [Google Scholar] [CrossRef]
- Cha, H.; Lim, H.S.; Park, J.A.; Jo, A.; Ryu, H.T.; Kim, D.W.; Kim, J.K.; Hong, S.N.; Shin, H.W.; Kim, D.W. Effects of Neutrophil and Eosinophil Extracellular Trap Formation on Refractoriness in Chronic Rhinosinusitis with Nasal Polyps. Allergy Asthma Immunol. Res. 2023, 15, 94–108. [Google Scholar] [CrossRef]
- Poposki, J.A.; Klingler, A.I.; Stevens, W.W.; Suh, L.A.; Tan, B.K.; Peters, A.T.; Abdala-Valencia, H.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; et al. Elevation of activated neutrophils in chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2022, 149, 1666–1674. [Google Scholar] [CrossRef]
- Hwang, J.W.; Kim, J.H.; Kim, H.J.; Choi, I.H.; Han, H.M.; Lee, K.J.; Kim, T.H.; Lee, S.H. Neutrophil extracellular traps in nasal secretions of patients with stable and exacerbated chronic rhinosinusitis and their contribution to induce chemokine secretion and strengthen the epithelial barrier. Clin. Exp. Allergy 2019, 49, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Van Crombruggen, K.; Vogl, T.; Perez-Novo, C.; Holtappels, G.; Bachert, C. Differential release and deposition of S100A8/A9 proteins in inflamed upper airway tissue. Eur. Respir. J. 2016, 47, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Delemarre, T.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Nauwynck, H.; Bachert, C.; Gevaert, E. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 179–188.e2. [Google Scholar] [CrossRef]
- Wang, X.; Sima, Y.; Zhao, Y.; Zhang, N.; Zheng, M.; Du, K.; Wang, M.; Wang, Y.; Hao, Y.; Li, Y.; et al. Endotypes of chronic rhinosinusitis based on inflammatory and remodeling factors. J. Allergy Clin. Immunol. 2022, 151, 458–468. [Google Scholar] [CrossRef]
- Kirchner, K.K.; Wagener, J.S.; Khan, T.Z.; Copenhaver, S.C.; Accurso, F.J. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 1996, 154, 1426–1429. [Google Scholar] [CrossRef]
- Marcos, V.; Zhou-Suckow, Z.; Onder Yildirim, A.; Bohla, A.; Hector, A.; Vitkov, L.; Krautgartner, W.D.; Stoiber, W.; Griese, M.; Eickelberg, O.; et al. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm. 2015, 2015, 408935. [Google Scholar] [CrossRef] [Green Version]
- Dubois, A.V.; Gauthier, A.; Brea, D.; Varaigne, F.; Diot, P.; Gauthier, F.; Attucci, S. Influence of DNA on the activities and inhibition of neutrophil serine proteases in cystic fibrosis sputum. Am. J. Respir. Cell Mol. Biol. 2012, 47, 80–86. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Staab, D.; Zychlinsky, A. Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS ONE 2011, 6, e28526. [Google Scholar] [CrossRef]
- Piva, T.C.; Luft, C.; Antunes, K.H.; Marostica, P.J.C.; Pinto, L.A.; Donadio, M.V.F. Extracellular DNA in sputum is associated with pulmonary function and hospitalization in patients with cystic fibrosis. Respir. Med. 2020, 172, 106144. [Google Scholar] [CrossRef]
- Dittrich, A.S.; Kuhbandner, I.; Gehrig, S.; Rickert-Zacharias, V.; Twigg, M.; Wege, S.; Taggart, C.C.; Herth, F.; Schultz, C.; Mall, M.A. Elastase activity on sputum neutrophils correlates with severity of lung disease in cystic fibrosis. Eur. Respir. J. 2018, 51, 1701910. [Google Scholar] [CrossRef] [Green Version]
- Rosenow, T.; Mok, L.C.; Turkovic, L.; Berry, L.J.; Sly, P.D.; Ranganathan, S.; Tiddens, H.; Stick, S.M. The cumulative effect of inflammation and infection on structural lung disease in early cystic fibrosis. Eur. Respir. J. 2019, 54, 1801771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Okamoto, K.; Rubin, B.K. Pulmonary function is negatively correlated with sputum inflammatory markers and cough clearability in subjects with cystic fibrosis but not those with chronic bronchitis. Chest 2006, 129, 1148–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratjen, F.; Hartog, C.M.; Paul, K.; Wermelt, J.; Braun, J. Matrix metalloproteases in BAL fluid of patients with cystic fibrosis and their modulation by treatment with dornase alpha. Thorax 2002, 57, 930–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaggar, A.; Hector, A.; Bratcher, P.E.; Mall, M.A.; Griese, M.; Hartl, D. The role of matrix metalloproteinases in cystic fibrosis lung disease. Eur. Respir. J. 2011, 38, 721–727. [Google Scholar] [CrossRef]
- Gray, R.D.; Imrie, M.; Boyd, A.C.; Porteous, D.; Innes, J.A.; Greening, A.P. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J. Cyst Fibros 2010, 9, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Reid, P.A.; McAllister, D.A.; Boyd, A.C.; Innes, J.A.; Porteous, D.; Greening, A.P.; Gray, R.D. Measurement of serum calprotectin in stable patients predicts exacerbation and lung function decline in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2015, 191, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Skopelja, S.; Hamilton, B.J.; Jones, J.D.; Yang, M.L.; Mamula, M.; Ashare, A.; Gifford, A.H.; Rigby, W.F. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight 2016, 1, e88912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, R.D.; Hardisty, G.; Regan, K.H.; Smith, M.; Robb, C.T.; Duffin, R.; Mackellar, A.; Felton, J.M.; Paemka, L.; McCullagh, B.N.; et al. Delayed neutrophil apoptosis enhances NET formation in cystic fibrosis. Thorax 2018, 73, 134–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutgers, S.R.; Timens, W.; Kaufmann, H.F.; van der Mark, T.W.; Koeter, G.H.; Postma, D.S. Comparison of induced sputum with bronchial wash, bronchoalveolar lavage and bronchial biopsies in COPD. Eur. Respir. J. 2000, 15, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Pesci, A.; Majori, M.; Cuomo, A.; Borciani, N.; Bertacco, S.; Cacciani, G.; Gabrielli, M. Neutrophils infiltrating bronchial epithelium in chronic obstructive pulmonary disease. Respir. Med. 1998, 92, 863–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Culpitt, S.V.; Maziak, W.; Loukidis, S.; Nightingale, J.A.; Matthews, J.L.; Barnes, P.J. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999, 160 Pt 1, 1635–1639. [Google Scholar] [CrossRef]
- Parr, D.G.; White, A.J.; Bayley, D.L.; Guest, P.J.; Stockley, R.A. Inflammation in sputum relates to progression of disease in subjects with COPD: A prospective descriptive study. Respir. Res. 2006, 7, 136. [Google Scholar] [CrossRef] [Green Version]
- Baines, K.J.; Simpson, J.L.; Gibson, P.G. Innate immune responses are increased in chronic obstructive pulmonary disease. PLoS ONE 2011, 6, e18426. [Google Scholar] [CrossRef]
- Doe, C.; Bafadhel, M.; Siddiqui, S.; Desai, D.; Mistry, V.; Rugman, P.; McCormick, M.; Woods, J.; May, R.; Sleeman, M.A.; et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest 2010, 138, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Roos, A.B.; Sanden, C.; Mori, M.; Bjermer, L.; Stampfli, M.R.; Erjefalt, J.S. IL-17A Is Elevated in End-Stage Chronic Obstructive Pulmonary Disease and Contributes to Cigarette Smoke-induced Lymphoid Neogenesis. Am. J. Respir. Crit. Care Med. 2015, 191, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.; Marwitz, S.; Holz, O.; Kirsten, A.; Bahmer, T.; Waschki, B.; Magnussen, H.; Rabe, K.F.; Goldmann, T.; Uddin, M.; et al. Neutrophil extracellular trap formation and extracellular DNA in sputum of stable COPD patients. Respir. Med. 2015, 109, 1360–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabcanovic-Musija, F.; Obermayer, A.; Stoiber, W.; Krautgartner, W.D.; Steinbacher, P.; Winterberg, N.; Bathke, A.C.; Klappacher, M.; Studnicka, M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015, 16, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermayer, A.; Stoiber, W.; Krautgartner, W.D.; Klappacher, M.; Kofler, B.; Steinbacher, P.; Vitkov, L.; Grabcanovic-Musija, F.; Studnicka, M. New aspects on the structure of neutrophil extracellular traps from chronic obstructive pulmonary disease and in vitro generation. PLoS ONE 2014, 9, e97784. [Google Scholar] [CrossRef]
- Wilkinson, T.M.; Patel, I.S.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1090–1095. [Google Scholar] [CrossRef]
- Keatings, V.M.; Collins, P.D.; Scott, D.M.; Barnes, P.J. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 1996, 153, 530–534. [Google Scholar] [CrossRef]
- Thulborn, S.J.; Mistry, V.; Brightling, C.E.; Moffitt, K.L.; Ribeiro, D.; Bafadhel, M. Neutrophil elastase as a biomarker for bacterial infection in COPD. Respir. Res. 2019, 20, 170. [Google Scholar] [CrossRef] [Green Version]
- Shoemark, A.; Cant, E.; Carreto, L.; Smith, A.; Oriano, M.; Keir, H.R.; Perea, L.; Canto, E.; Terranova, L.; Vidal, S.; et al. A point-of-care neutrophil elastase activity assay identifies bronchiectasis severity, airway infection and risk of exacerbation. Eur. Respir. J. 2019, 53, 1900303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loukides, S.; Bouros, D.; Papatheodorou, G.; Lachanis, S.; Panagou, P.; Siafakas, N.M. Exhaled H(2)O(2) in steady-state bronchiectasis: Relationship with cellular composition in induced sputum, spirometry, and extent and severity of disease. Chest 2002, 121, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedi, P.; Davidson, D.J.; McHugh, B.J.; Rossi, A.G.; Hill, A.T. Blood Neutrophils Are Reprogrammed in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2018, 198, 880–890. [Google Scholar] [CrossRef]
- Angrill, J.; Agusti, C.; De Celis, R.; Filella, X.; Rano, A.; Elena, M.; De La Bellacasa, J.P.; Xaubet, A.; Torres, A. Bronchial inflammation and colonization in patients with clinically stable bronchiectasis. Am. J. Respir. Crit. Care Med. 2001, 164, 1628–1632. [Google Scholar] [CrossRef]
- Sly, P.D.; Gangell, C.L.; Chen, L.; Ware, R.S.; Ranganathan, S.; Mott, L.S.; Murray, C.P.; Stick, S.M.; Investigators, A.C. Risk factors for bronchiectasis in children with cystic fibrosis. N. Engl. J. Med. 2013, 368, 1963–1970. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, J.D.; Moffitt, K.L.; Suarez-Cuartin, G.; Sibila, O.; Finch, S.; Furrie, E.; Dicker, A.; Wrobel, K.; Elborn, J.S.; Walker, B.; et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2017, 195, 1384–1393. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.L.; Grissell, T.V.; Douwes, J.; Scott, R.J.; Boyle, M.J.; Gibson, P.G. Innate immune activation in neutrophilic asthma and bronchiectasis. Thorax 2007, 62, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibila, O.; Perea, L.; Canto, E.; Shoemark, A.; Cassidy, D.; Smith, A.H.; Suarez-Cuartin, G.; Rodrigo-Troyano, A.; Keir, H.R.; Oriano, M.; et al. Antimicrobial peptides, disease severity and exacerbations in bronchiectasis. Thorax 2019, 74, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garratt, L.W.; Sutanto, E.N.; Ling, K.M.; Looi, K.; Iosifidis, T.; Martinovich, K.M.; Shaw, N.C.; Kicic-Starcevich, E.; Knight, D.A.; Ranganathan, S.; et al. Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis. Eur. Respir. J. 2015, 46, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Vaninov, N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Bassler, K.; Schlickeiser, S.; Zhang, B.W.; Kramer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440. [Google Scholar] [CrossRef] [PubMed]
- Parackova, Z.; Zentsova, I.; Bloomfield, M.; Vrabcova, P.; Smetanova, J.; Klocperk, A.; Meseznikov, G.; Casas Mendez, L.F.; Vymazal, T.; Sediva, A. Disharmonic Inflammatory Signatures in COVID-19: Augmented Neutrophils’ but Impaired Monocytes’ and Dendritic Cells’ Responsiveness. Cells 2020, 9, 2206. [Google Scholar] [CrossRef]
- Veras, F.P.; Pontelli, M.C.; Silva, C.M.; Toller-Kawahisa, J.E.; de Lima, M.; Nascimento, D.C.; Schneider, A.H.; Caetite, D.; Tavares, L.A.; Paiva, I.M.; et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 2020, 217, e20201129. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Sumby, P.; Barbian, K.D.; Gardner, D.J.; Whitney, A.R.; Welty, D.M.; Long, R.D.; Bailey, J.R.; Parnell, M.J.; Hoe, N.P.; Adams, G.G.; et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc. Natl. Acad. Sci. USA 2005, 102, 1679–1684. [Google Scholar] [CrossRef] [Green Version]
- Hosseinnejad, A.; Ludwig, N.; Wienkamp, A.K.; Rimal, R.; Bleilevens, C.; Rossaint, R.; Rossaint, J.; Singh, S. DNase I functional microgels for neutrophil extracellular trap disruption. Biomater. Sci. 2021, 10, 85–99. [Google Scholar] [CrossRef]
- Sawicki, G.S.; Chou, W.; Raimundo, K.; Trzaskoma, B.; Konstan, M.W. Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, H.J.; Borowitz, D.S.; Christiansen, D.H.; Morris, E.M.; Nash, M.L.; Ramsey, B.W.; Rosenstein, B.J.; Smith, A.L.; Wohl, M.E. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N. Engl. J. Med. 1994, 331, 637–642. [Google Scholar] [CrossRef]
- Durward, A.; Forte, V.; Shemie, S.D. Resolution of mucus plugging and atelectasis after intratracheal rhDNase therapy in a mechanically ventilated child with refractory status asthmaticus. Crit. Care Med. 2000, 28, 560–562. [Google Scholar] [CrossRef]
- Park, H.H.; Park, W.; Lee, Y.Y.; Kim, H.; Seo, H.S.; Choi, D.W.; Kwon, H.K.; Na, D.H.; Kim, T.H.; Choy, Y.B.; et al. Bioinspired DNase-I-Coated Melanin-Like Nanospheres for Modulation of Infection-Associated NETosis Dysregulation. Adv. Sci. 2020, 7, 2001940. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Park, H.H.; Park, W.; Kim, H.; Jang, J.G.; Hong, K.S.; Lee, J.Y.; Seo, H.S.; Na, D.H.; Kim, T.H.; et al. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials 2021, 267, 120389. [Google Scholar] [CrossRef]
- O’Donnell, A.E.; Barker, A.F.; Ilowite, J.S.; Fick, R.B. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998, 113, 1329–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrig, S.; Duerr, J.; Weitnauer, M.; Wagner, C.J.; Graeber, S.Y.; Schatterny, J.; Hirtz, S.; Belaaouaj, A.; Dalpke, A.H.; Schultz, C.; et al. Lack of neutrophil elastase reduces inflammation, mucus hypersecretion, and emphysema, but not mucus obstruction, in mice with cystic fibrosis-like lung disease. Am. J. Respir. Crit. Care Med. 2014, 189, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Parr, D.G.; Guest, P.G.; Reynolds, J.H.; Dowson, L.J.; Stockley, R.A. Prevalence and impact of bronchiectasis in alpha1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 2007, 176, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, N.G. Alpha-1 Antitrypsin Therapy in Cystic Fibrosis and the Lung Disease Associated with Alpha-1 Antitrypsin Deficiency. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 2), S191–S196. [Google Scholar]
- Vogelmeier, C.; Gillissen, A.; Buhl, R. Use of secretory leukoprotease inhibitor to augment lung antineutrophil elastase activity. Chest 1996, 110 (Suppl. 6), 261S–266S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zani, M.L.; Tanga, A.; Saidi, A.; Serrano, H.; Dallet-Choisy, S.; Baranger, K.; Moreau, T. SLPI and trappin-2 as therapeutic agents to target airway serine proteases in inflammatory lung diseases: Current and future directions. Biochem. Soc. Trans. 2011, 39, 1441–1446. [Google Scholar] [CrossRef]
- Raundhal, M.; Morse, C.; Khare, A.; Oriss, T.B.; Milosevic, J.; Trudeau, J.; Huff, R.; Pilewski, J.; Holguin, F.; Kolls, J.; et al. High IFN-gamma and low SLPI mark severe asthma in mice and humans. J. Clin. Investig. 2015, 125, 3037–3050. [Google Scholar] [CrossRef] [Green Version]
- Cambier, S.; Metzemaekers, M.; de Carvalho, A.C.; Nooyens, A.; Jacobs, C.; Vanderbeke, L.; Malengier-Devlies, B.; Gouwy, M.; Heylen, E.; Meersseman, P.; et al. Atypical response to bacterial coinfection and persistent neutrophilic bronchoalveolar inflammation distinguish critical COVID-19 from influenza. JCI Insight 2022, 7, e155055. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Y.; Li, X.; Wang, R.; Zeng, Z.; Cheng, M.; Gao, L.; Xu, D.; Wen, F.; Wang, T.; et al. Inhibition of neutrophil elastase prevents cigarette smoke exposure-induced formation of neutrophil extracellular traps and improves lung function in a mouse model of chronic obstructive pulmonary disease. Int. Immunopharmacol. 2023, 114, 109537. [Google Scholar] [CrossRef]
- Vogelmeier, C.; Aquino, T.O.; O’Brien, C.D.; Perrett, J.; Gunawardena, K.A. A randomised, placebo-controlled, dose-finding study of AZD9668, an oral inhibitor of neutrophil elastase, in patients with chronic obstructive pulmonary disease treated with tiotropium. COPD 2012, 9, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Perrett, J.; Forsman-Semb, K.; Marks-Konczalik, J.; Gunawardena, K.; Entwistle, N. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur. Respir. J. 2012, 40, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Stockley, R.; De Soyza, A.; Gunawardena, K.; Perrett, J.; Forsman-Semb, K.; Entwistle, N.; Snell, N. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir. Med. 2013, 107, 524–533. [Google Scholar] [CrossRef] [Green Version]
- Kuna, P.; Jenkins, M.; O’Brien, C.D.; Fahy, W.A. AZD9668, a neutrophil elastase inhibitor, plus ongoing budesonide/formoterol in patients with COPD. Respir. Med. 2012, 106, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, H.; Azuma, M.; Umeno, A.; Shimizu, M.; Murotomi, K.; Yoshida, Y.; Nishioka, Y.; Tsuneyama, K. Singlet oxygen -derived nerve growth factor exacerbates airway hyperresponsiveness in a mouse model of asthma with mixed inflammation. Allergol. Int. 2022, 71, 395–404. [Google Scholar] [CrossRef]
- Zheng, W.; Warner, R.; Ruggeri, R.; Su, C.; Cortes, C.; Skoura, A.; Ward, J.; Ahn, K.; Kalgutkar, A.; Sun, D.; et al. PF-1355, a mechanism-based myeloperoxidase inhibitor, prevents immune complex vasculitis and anti-glomerular basement membrane glomerulonephritis. J. Pharmacol. Exp. Ther. 2015, 353, 288–298. [Google Scholar] [CrossRef] [Green Version]
- Antonelou, M.; Michaelsson, E.; Evans, R.D.R.; Wang, C.J.; Henderson, S.R.; Walker, L.S.K.; Unwin, R.J.; Salama, A.D.; Investigators, R.-I. Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN. J. Am. Soc. Nephrol. 2020, 31, 350–364. [Google Scholar] [CrossRef]
- Chaikijurajai, T.; Tang, W.H.W. Myeloperoxidase: A potential therapeutic target for coronary artery disease. Expert Opin. Ther. Targets 2020, 24, 695–705. [Google Scholar] [CrossRef]
- Pedersen, F.; Waschki, B.; Marwitz, S.; Goldmann, T.; Kirsten, A.; Malmgren, A.; Rabe, K.F.; Uddin, M.; Watz, H. Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Eur. Respir. J. 2018, 51, 1700970. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.; Watz, H.; Malmgren, A.; Pedersen, F. NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma. Front. Immunol. 2019, 10, 47. [Google Scholar] [CrossRef]
- De Soyza, A.; Pavord, I.; Elborn, J.S.; Smith, D.; Wray, H.; Puu, M.; Larsson, B.; Stockley, R. A randomised, placebo-controlled study of the CXCR2 antagonist AZD5069 in bronchiectasis. Eur. Respir. J. 2015, 46, 1021–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watz, H.; Uddin, M.; Pedersen, F.; Kirsten, A.; Goldmann, T.; Stellmacher, F.; Groth, E.; Larsson, B.; Bottcher, G.; Malmgren, A.; et al. Effects of the CXCR2 antagonist AZD5069 on lung neutrophil recruitment in asthma. Pulm. Pharmacol. Ther. 2017, 45, 121–123. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef]
- Moss, R.B.; Mistry, S.J.; Konstan, M.W.; Pilewski, J.M.; Kerem, E.; Tal-Singer, R.; Lazaar, A.L.; Investigators, C.F. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J. Cyst Fibros 2013, 12, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Busch-Petersen, J.; Carpenter, D.C.; Burman, M.; Foley, J.; Hunsberger, G.E.; Kilian, D.J.; Salmon, M.; Mayer, R.J.; Yonchuk, J.G.; Tal-Singer, R. Danirixin: A Reversible and Selective Antagonist of the CXC Chemokine Receptor 2. J. Pharmacol. Exp. Ther 2017, 362, 338–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazaar, A.L.; Miller, B.E.; Donald, A.C.; Keeley, T.; Ambery, C.; Russell, J.; Watz, H.; Tal-Singer, R.; For, I. CXCR2 antagonist for patients with chronic obstructive pulmonary disease with chronic mucus hypersecretion: A phase 2b trial. Respir. Res. 2020, 21, 149. [Google Scholar] [CrossRef]
- Keir, H.R.; Richardson, H.; Fillmore, C.; Shoemark, A.; Lazaar, A.L.; Miller, B.E.; Tal-Singer, R.; Chalmers, J.D.; Mohan, D. CXCL-8-dependent and -independent neutrophil activation in COPD: Experiences from a pilot study of the CXCR2 antagonist danirixin. ERJ Open Res. 2020, 6, 23120541. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Allegretti, M.; Ley, K. Therapeutic inhibition of CXCR2 by Reparixin attenuates acute lung injury in mice. Br. J. Pharmacol 2008, 155, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostafin, M.; Pruchniak, M.P.; Ciepiela, O.; Reznick, A.Z.; Demkow, U. Different procedures of diphenyleneiodonium chloride addition affect neutrophil extracellular trap formation. Anal. Biochem. 2016, 509, 60–66. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Han, Z.; Wang, J.; Zhang, X.; Wang, Y.; Liu, Q.; Yang, Z. Neutrophil extracellular traps promote cadmium chloride-induced lung injury in mice. Environ. Pollut. 2019, 254 Pt A, 113021. [Google Scholar] [CrossRef]
- Bonilla, M.C.; Quiros, O.N.; Wendt, M.; Hennig-Pauka, I.; Morgelin, M.; von Kockritz-Blickwede, M.; de Buhr, N. New Insights into Neutrophil Extracellular Trap (NETs) Formation from Porcine Neutrophils in Response to Bacterial Infections. Int J. Mol. Sci. 2022, 23, 8953. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.S.; Antunes, G.L.; Kaiber, D.B.; da Costa, M.S.; Marques, E.P.; Ferreira, F.S.; Gassen, R.B.; Breda, R.V.; Wyse, A.T.S.; Pitrez, P.; et al. Reactive oxygen species are involved in eosinophil extracellular traps release and in airway inflammation in asthma. J. Cell Physiol 2019, 234, 23633–23646. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Sim, M.S.; Lee, D.H.; Kim, C.; Choi, Y.; Park, H.S.; Chung, I.Y. Lysophosphatidylserine induces eosinophil extracellular trap formation and degranulation: Implications in severe asthma. Allergy 2020, 75, 3159–3170. [Google Scholar] [CrossRef] [PubMed]
- Zawrotniak, M.; Kozik, A.; Rapala-Kozik, M. Selected mucolytic, anti-inflammatory and cardiovascular drugs change the ability of neutrophils to form extracellular traps (NETs). Acta Biochim. Pol. 2015, 62, 465–473. [Google Scholar] [CrossRef]
- Rogliani, P.; Matera, M.G.; Page, C.; Puxeddu, E.; Cazzola, M.; Calzetta, L. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: A comparative analysis across erdosteine, carbocysteine, and N-acetylcysteine. Respir. Res. 2019, 20, 104. [Google Scholar] [CrossRef]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Eur. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Qi, Q.; Ailiyaer, Y.; Liu, R.; Zhang, Y.; Li, C.; Liu, M.; Wang, X.; Jing, L.; Li, Y. Effect of N-acetylcysteine on exacerbations of bronchiectasis (BENE): A randomized controlled trial. Respir. Res. 2019, 20, 73. [Google Scholar] [CrossRef]
- Lewis, H.D.; Liddle, J.; Coote, J.E.; Atkinson, S.J.; Barker, M.D.; Bax, B.D.; Bicker, K.L.; Bingham, R.P.; Campbell, M.; Chen, Y.H.; et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 2015, 11, 189–191. [Google Scholar] [CrossRef]
- Biron, B.M.; Chung, C.S.; O’Brien, X.M.; Chen, Y.; Reichner, J.S.; Ayala, A. Cl-Amidine Prevents Histone 3 Citrullination and Neutrophil Extracellular Trap Formation, and Improves Survival in a Murine Sepsis Model. J. Innate Immun. 2017, 9, 22–32. [Google Scholar] [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014, 114, 947–956. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Mei, Y.; Dong, W.; Wang, J.; Huang, F.; Wu, J. Evaluation of protein arginine deiminase-4 inhibitor in TNBS- induced colitis in mice. Int. Immunopharmacol. 2020, 84, 106583. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Kamide, Y.; Ueki, S.; Miyabe, Y.; Konno, Y.; Oka, N.; Takeuchi, H.; Koyota, S.; Hirokawa, M.; Yamada, T.; et al. Eosinophil ETosis-Mediated Release of Galectin-10 in Eosinophilic Granulomatosis with Polyangiitis. Arthritis Rheumatol. 2021, 73, 1683–1693. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Y.; Li, J.; Huang, J.; Zhang, L.; Feng, J.; Li, J.; Xia, Q.; Zhao, Q.; Huang, L.; et al. Eosinophil extracellular traps drive asthma progression through neuro-immune signals. Nat. Cell Biol. 2021, 23, 1060–1072. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franze, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J. Crohns. Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Winslow, S.; Odqvist, L.; Diver, S.; Riise, R.; Abdillahi, S.; Wingren, C.; Lindmark, H.; Wellner, A.; Lundin, S.; Yrlid, L.; et al. Multi-omics links IL-6 trans-signalling with neutrophil extracellular trap formation and Haemophilus infection in COPD. Eur. Respir. J. 2021, 58, 2003312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, Q.; Wang, F.; Guo, X.; Liu, T.; Zhao, Y.; Gu, B.; Chen, H.; Li, Y. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin. Immunol. 2020, 216, 108461. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Dorner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Mazetto, B.M.; Hounkpe, B.W.; Saraiva, S.; Bizzacchi, J.M.A.; De Paula, E.V.; Orsi, F.A. Hydroxychloroquine Therapy and Netosis Regulators Expression in Patients with Primary Antiphospholipid Syndrome. Blood 2018, 132, 5049. [Google Scholar] [CrossRef]
- Jung, H.; Bobba, R.; Su, J.; Shariati-Sarabi, Z.; Gladman, D.D.; Urowitz, M.; Lou, W.; Fortin, P.R. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis. Rheum. 2010, 62, 863–868. [Google Scholar] [CrossRef]
- Skendros, P.; Mitsios, A.; Chrysanthopoulou, A.; Mastellos, D.C.; Metallidis, S.; Rafailidis, P.; Ntinopoulou, M.; Sertaridou, E.; Tsironidou, V.; Tsigalou, C.; et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J. Clin. Investig. 2020, 130, 6151–6157. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.; Jaramillo, A.M.; Ferrer, A.; de Zegher, F. High neutrophil count in girls and women with hyperinsulinaemic hyperandrogenism: Normalization with metformin and flutamide overcomes the aggravation by oral contraception. Hum. Reprod. 2005, 20, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.; Savinko, T.; Wong, A.K.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef] [Green Version]
- Menegazzo, L.; Scattolini, V.; Cappellari, R.; Bonora, B.M.; Albiero, M.; Bortolozzi, M.; Romanato, F.; Ceolotto, G.; Vigili de Kreutzeberg, S.; Avogaro, A.; et al. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol. 2018, 55, 593–601. [Google Scholar] [CrossRef]
- Usman, A.; Bliden, K.P.; Cho, A.; Walia, N.; Jerjian, C.; Singh, A.; Kundan, P.; Duhan, S.; Tantry, U.S.; Gurbel, P.A. Metformin use in patients hospitalized with COVID-19: Lower inflammation, oxidative stress, and thrombotic risk markers and better clinical outcomes. J. Thromb. Thrombolysis 2022, 53, 363–371. [Google Scholar] [CrossRef]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with COVID-19. medRxiv 2020. [Google Scholar]
- Oh, T.K.; Song, I.A. Metformin use and risk of COVID-19 among patients with type II diabetes mellitus: An NHIS-COVID-19 database cohort study. Acta Diabetol. 2021, 58, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, G.D.; Choi, D.W.; Shin, D.U.; Eom, J.E.; Kim, S.Y.; Chai, O.H.; Kim, H.J.; Lee, S.Y.; Shin, H.S. Epilobiumpyrricholophum Extract Suppresses Porcine Pancreatic Elastase and Cigarette Smoke Extract-Induced Inflammatory response in a Chronic Obstructive Pulmonary Disease Model. Foods 2021, 10, 2929. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, D.U.; Eom, J.E.; Jung, S.Y.; Song, H.J.; Lim, K.M.; Kim, G.D.; Yun, S.I.; Kim, M.Y.; Shin, H.S.; et al. Artemisia gmelinii Attenuates Lung Inflammation by Suppressing the NF-kappaB/MAPK Pathway. Antioxidants 2022, 11, 568. [Google Scholar] [CrossRef]
- Li, H.; Qiao, C.; Zhao, L.; Jing, Q.; Xue, D.; Zhang, Y. Epigallocatechin-3-gallate reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis. J. Leukoc. Biol. 2022, 112, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.A.; Gandhi, A.A.; Dai, L.; Weiner, J.; Estes, S.K.; Yalavarthi, S.; Gockman, K.; Sun, D.; Knight, J.S. Antineutrophil properties of natural gingerols in models of lupus. JCI Insight 2021, 6, 138385. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, D.; Luo, J.; Jie, J.; Liu, H.; Peng, L.; Bai, X.; Li, D. Zingerone Inhibits the Neutrophil Extracellular Trap Formation and Protects against Sepsis via Nrf2-Mediated ROS Inhibition. Oxid Med. Cell Longev. 2022, 2022, 3990607. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, A.M.; Forster, K.; Radeczky, E.; Linnhoff, A.; Balint, B.; Watz, H.; Wray, H.; Salkeld, L.; Cullberg, M.; Larsson, B. The safety and tolerability of oral AZD5069, a selective CXCR2 antagonist, in patients with moderate-to-severe COPD. Pulm. Pharmacol. Ther. 2015, 31, 36–41. [Google Scholar] [CrossRef]
- Landoni, G.; Piemonti, L.; Monforte, A.D.; Grossi, P.; Zangrillo, A.; Bucci, E.; Allegretti, M.; Goisis, G.; Gavioli, E.M.; Patel, N.; et al. A Multicenter Phase 2 Randomized Controlled Study on the Efficacy and Safety of Reparixin in the Treatment of Hospitalized Patients with COVID-19 Pneumonia. Infect. Dis. Ther. 2022, 11, 1559–1574. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, A.; Kim, D.W. Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications. Int. J. Mol. Sci. 2023, 24, 5034. https://doi.org/10.3390/ijms24055034
Jo A, Kim DW. Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications. International Journal of Molecular Sciences. 2023; 24(5):5034. https://doi.org/10.3390/ijms24055034
Chicago/Turabian StyleJo, Ara, and Dae Woo Kim. 2023. "Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications" International Journal of Molecular Sciences 24, no. 5: 5034. https://doi.org/10.3390/ijms24055034
APA StyleJo, A., & Kim, D. W. (2023). Neutrophil Extracellular Traps in Airway Diseases: Pathological Roles and Therapeutic Implications. International Journal of Molecular Sciences, 24(5), 5034. https://doi.org/10.3390/ijms24055034





