Effects of Terahertz Radiation on the Aggregation of Alzheimer’s Aβ42 Peptide
Abstract
1. Introduction
2. Results and Discussion
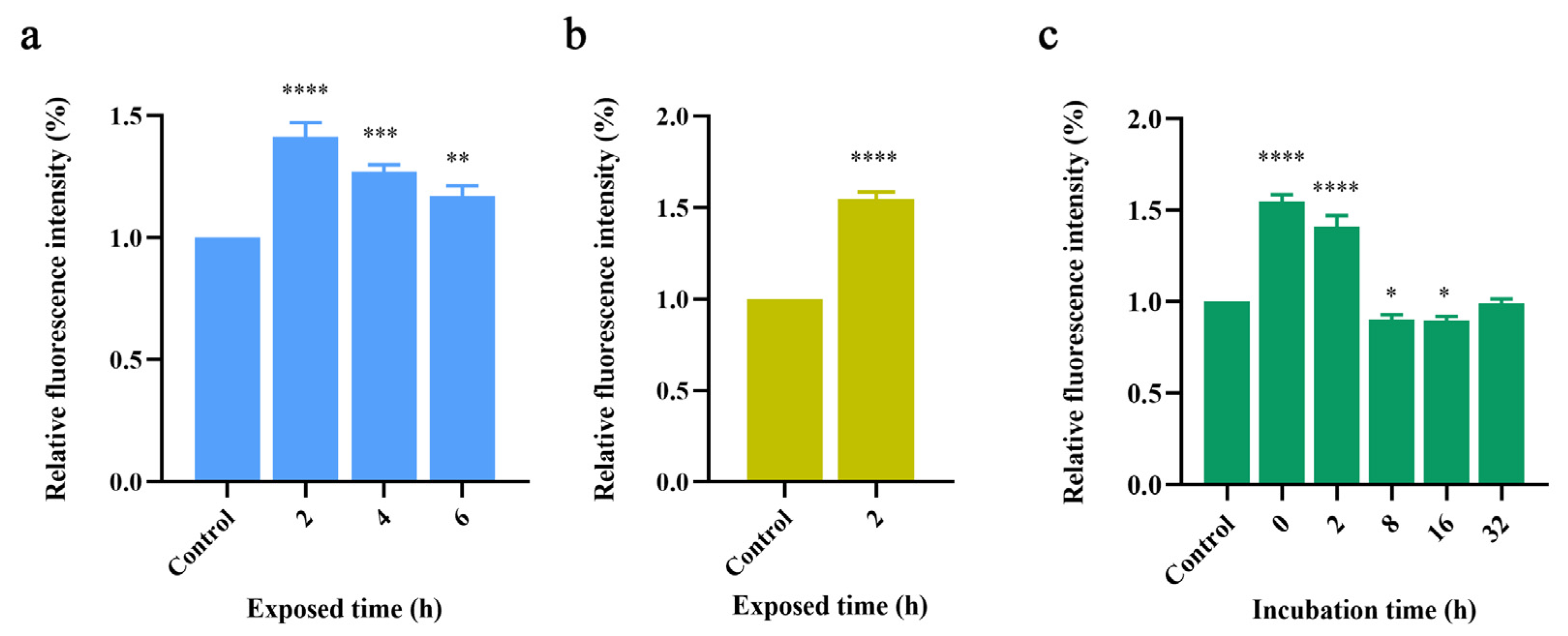
2.1. Effect of Terahertz Radiation on Various Aβ42 Forms
2.1.1. Thioflavin-T Fluorescence Experiments
2.1.2. Morphological Analysis of Aβ42 Aggregates by Electron Microscopy
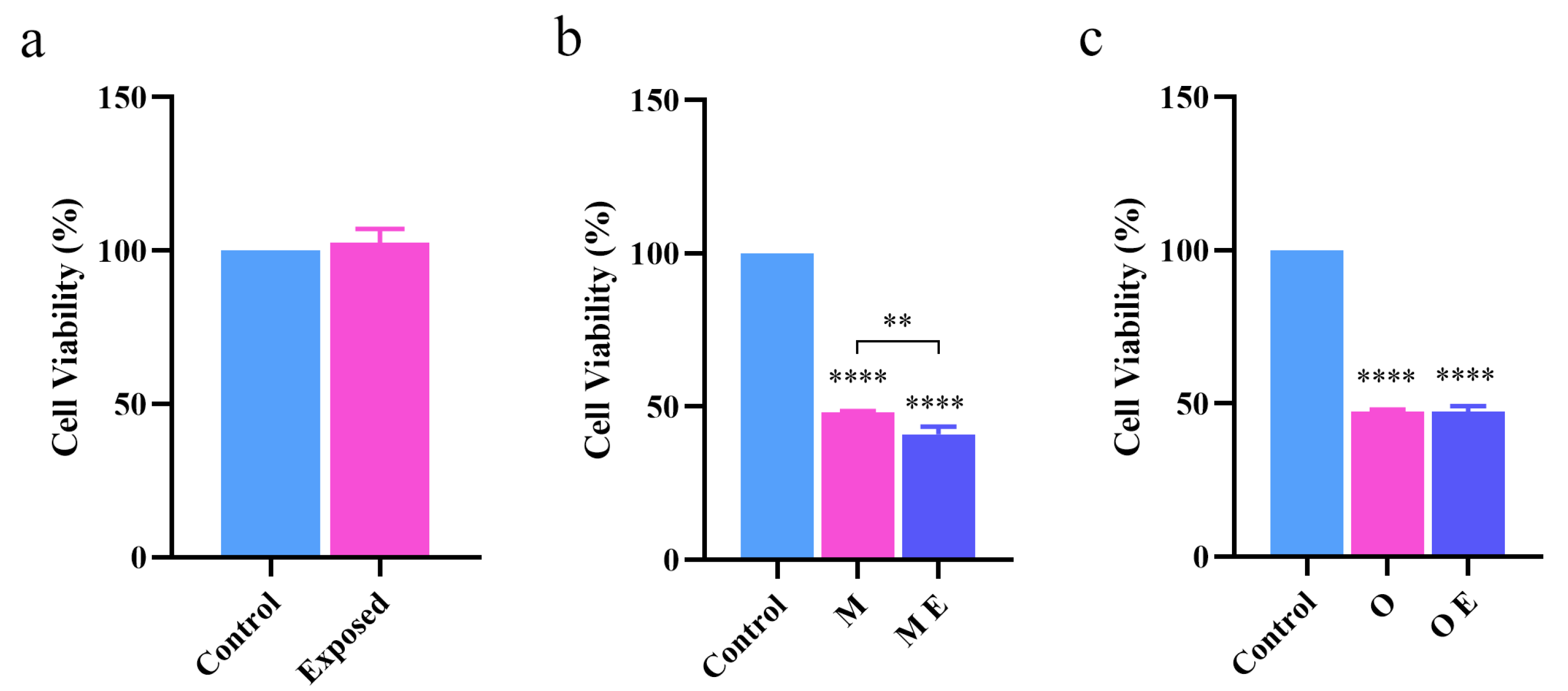
2.2. Effect of Terahertz Radiation on Cytotoxicity Caused by Aβ42 Aggregation
2.2.1. MTT Assay
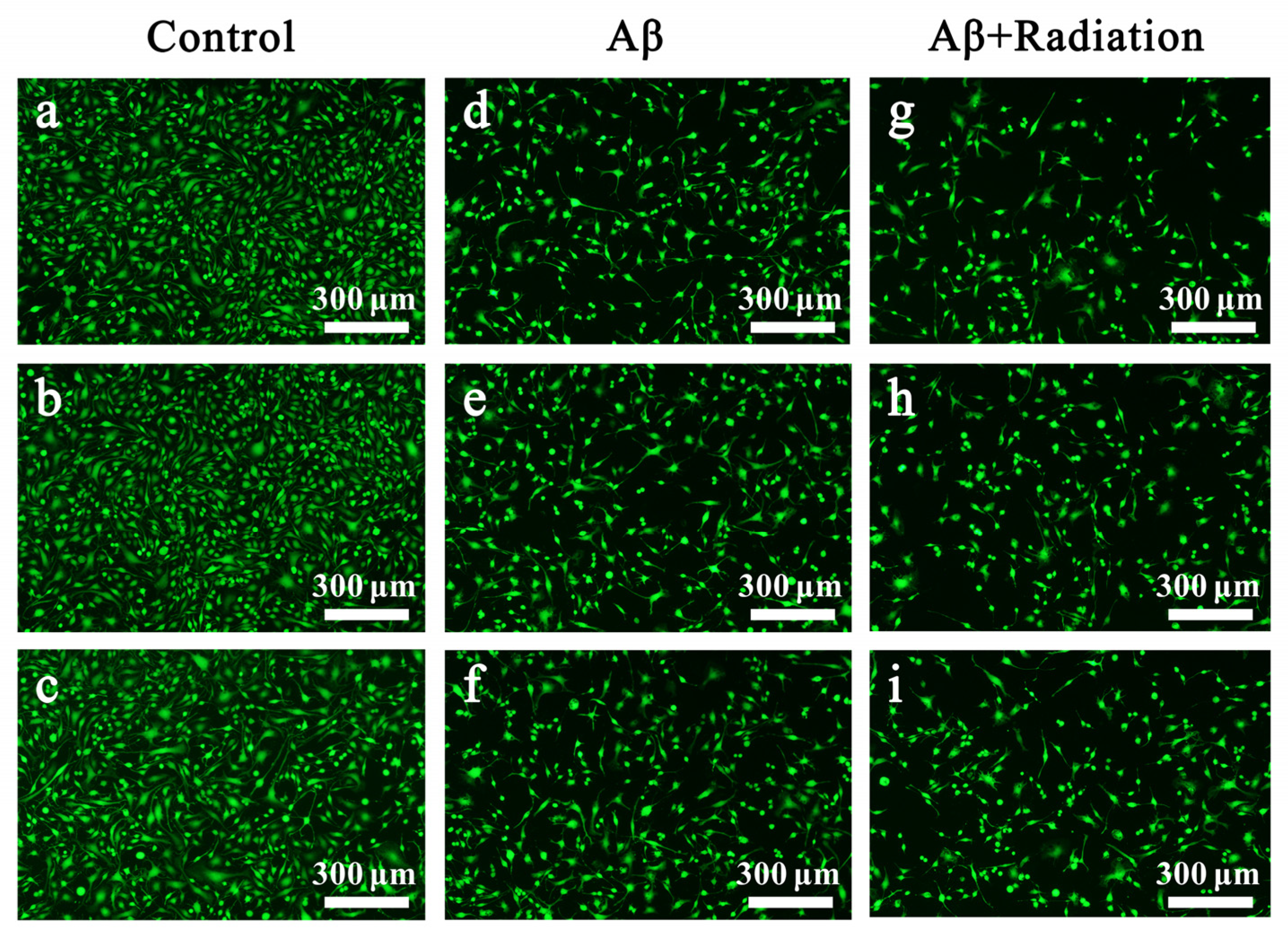
2.2.2. Fluorescent Staining of Live Cells
2.3. Mechanism of the Effect of Terahertz Radiation on the Aggregation of Aβ42
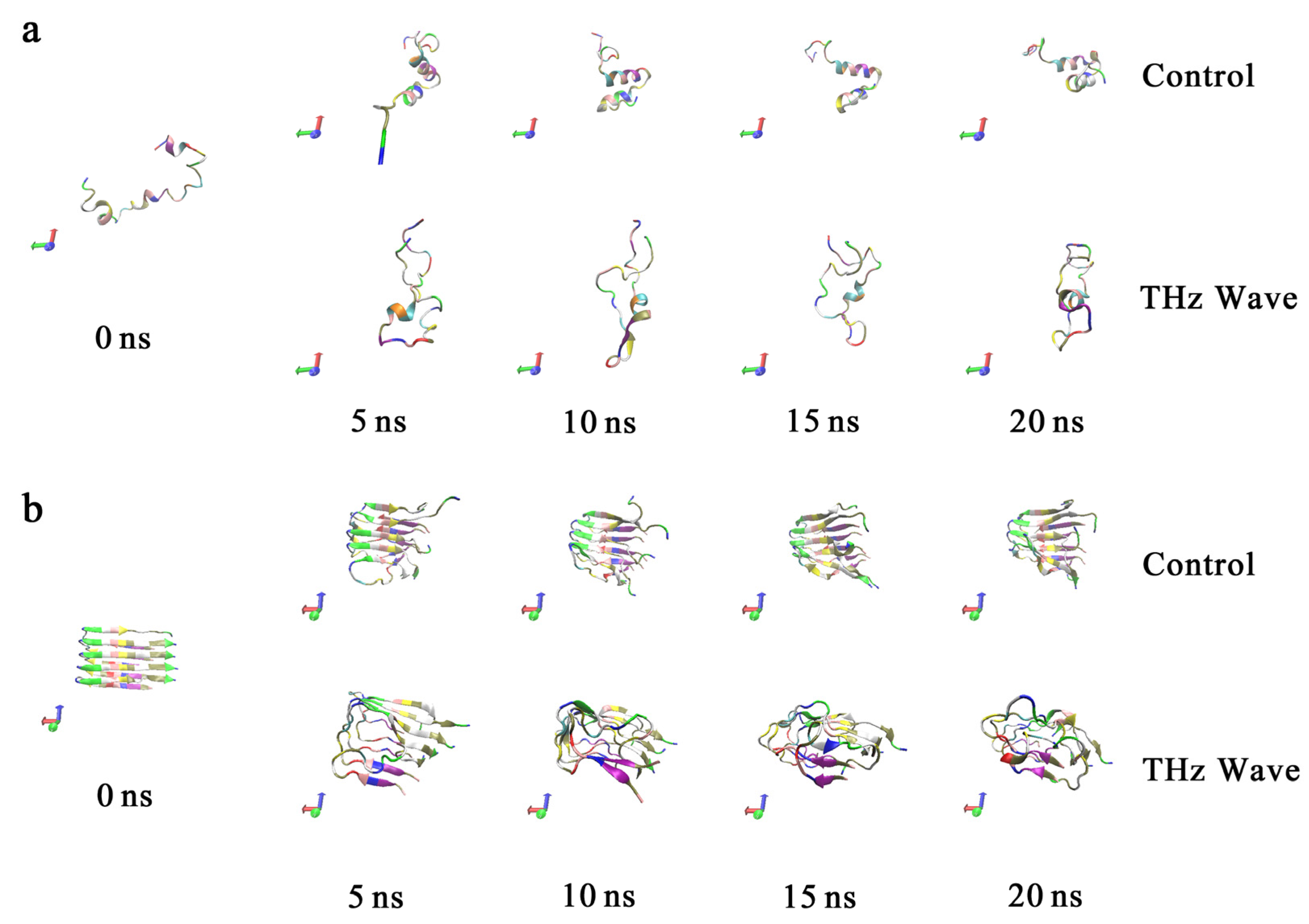
2.3.1. Analysis of the Molecular Trajectories of Aβ42 under the Action of Terahertz Radiation
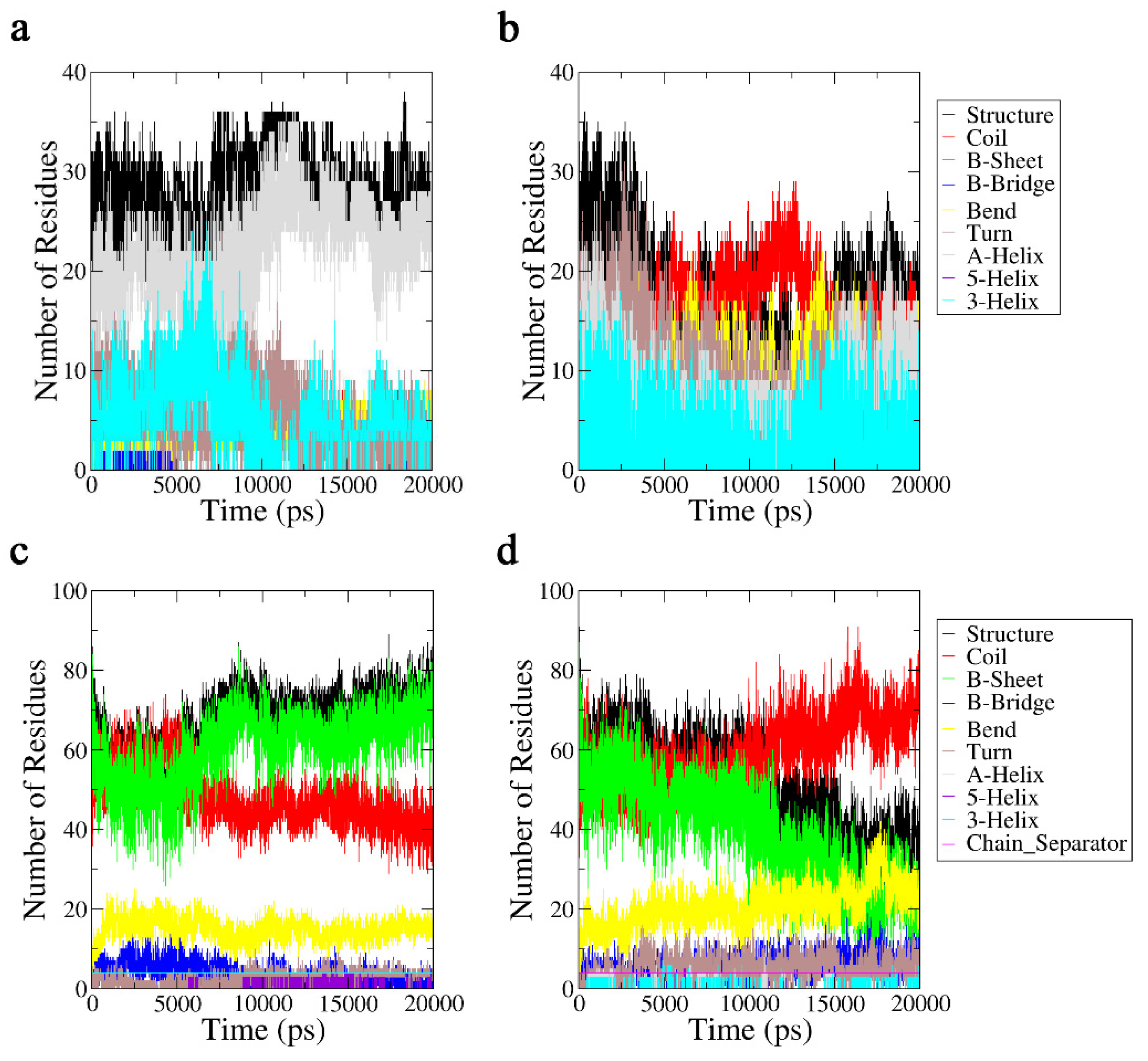
2.3.2. Secondary Structure Analysis
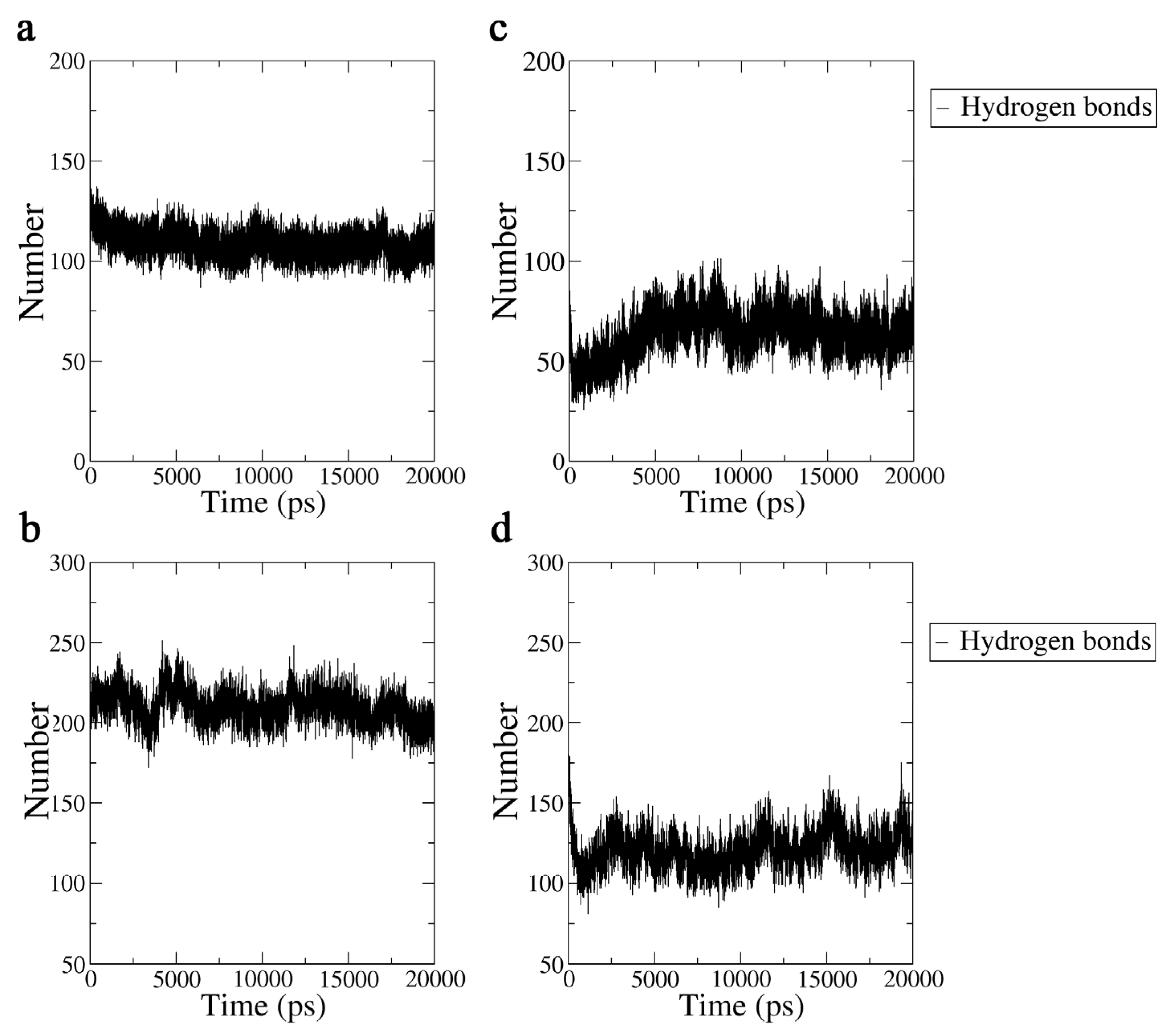
2.3.3. Hydrogen Bond Analysis
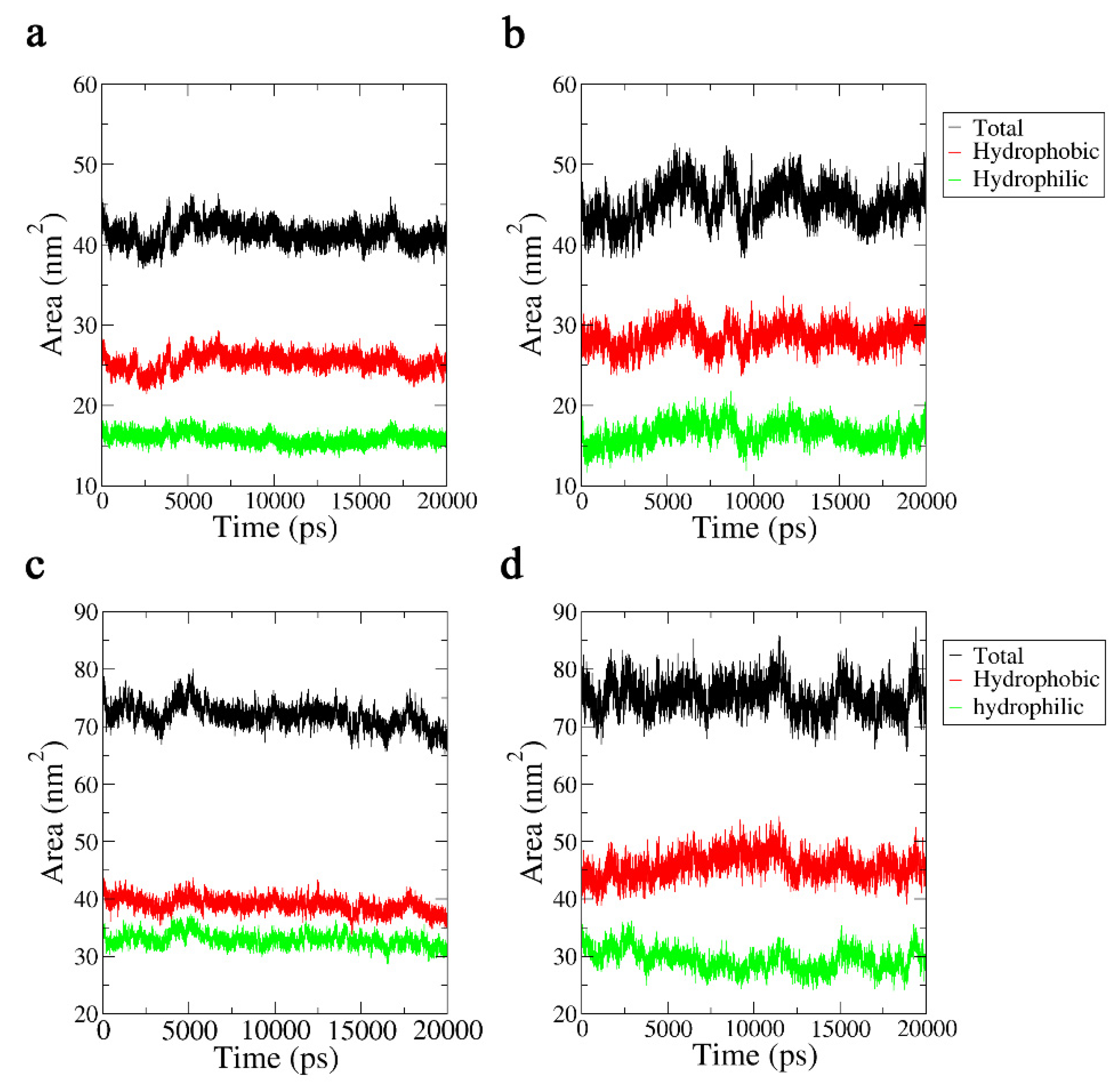
2.3.4. Solvent-Accessible Surface Area Analysis
3. Materials and Methods
3.1. Samples and Reagents
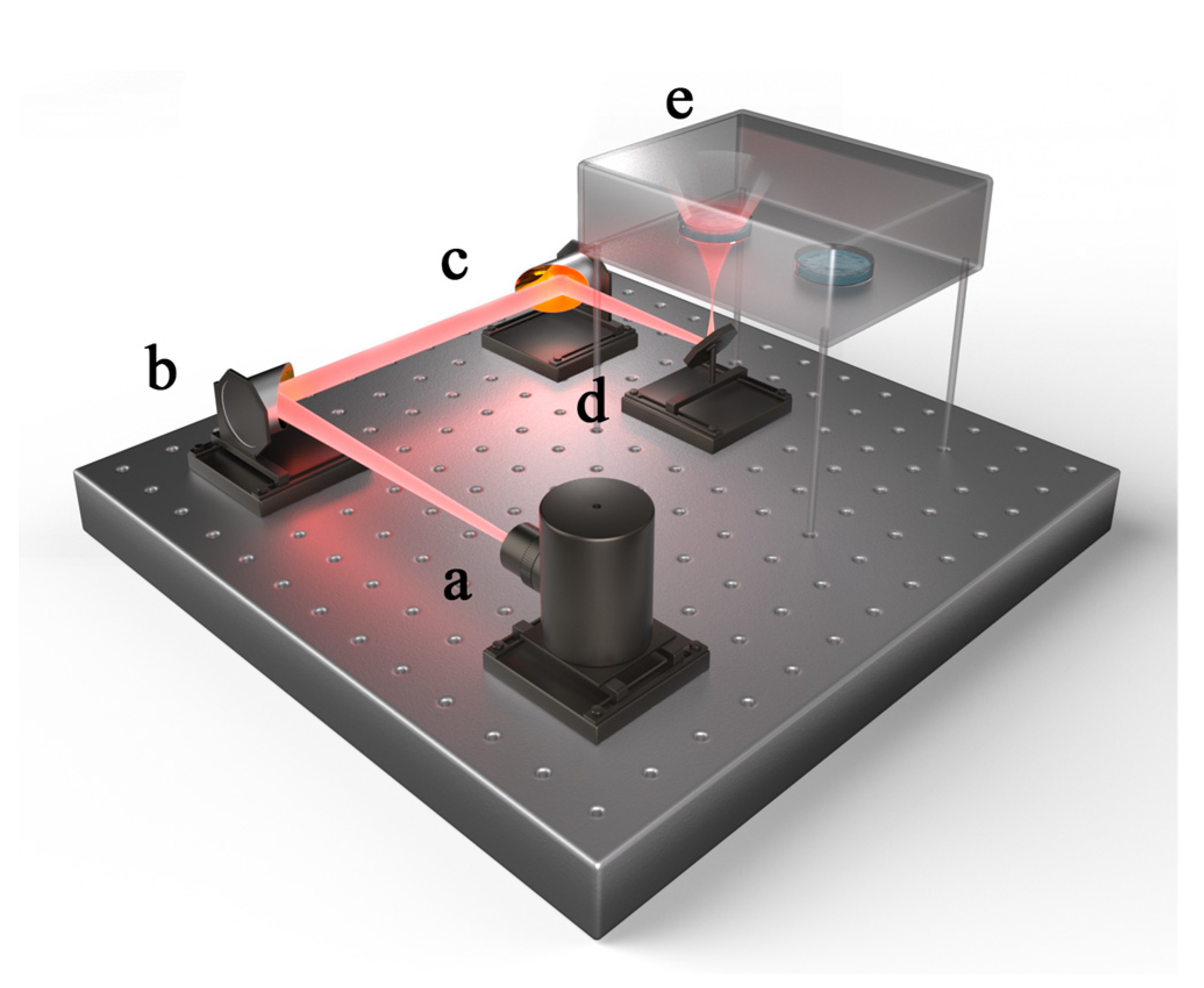
3.2. Radiation Method
3.3. Thioflavin T Fluorescence Test
3.4. Transmission Electron Microscopy
3.5. Cell Culture and Toxicity Assay
3.6. Live-Cell Fluorescence Experiments
3.7. Molecular Dynamics Simulations
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Global Status Report on the Public Health Response to Dementia. Available online: https://www.who.int/publications/i/item/9789240033245 (accessed on 15 November 2022).
- Small, D.H.; Mok, S.S.; Bornstein, J.C. Alzheimer’s Disease and Aβ Toxicity: From Top to Bottom. Nat. Rev. Neurosci. 2001, 2, 595–598. [Google Scholar] [CrossRef]
- Larson, M.E.; Lesné, S.E. Soluble Aβ Oligomer Production and Toxicity. J. Neurochem. 2012, 120, 125–139. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Klein, W.L. The Aβ Oligomer Hypothesis for Synapse Failure and Memory Loss in Alzheimer’s Disease. Neurobiol. Learn. Mem. 2011, 96, 529–543. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, L.; Liu, J.; Yu, J. The Essential Role of Soluble Aβ Oligomers in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 1905–1924. [Google Scholar] [CrossRef]
- Johansen, C. Exposure to Electromagnetic Fields and Risk of Central Nervous System Disease in Utility Workers. Epidemiology 2000, 11, 539–543. [Google Scholar] [CrossRef]
- Park, R.M.; Schulte, P.A.; Bowman, J.D.; Walker, J.T.; Bondy, S.C.; Yost, M.G.; Touchstone, J.A.; Dosemeci, M. Potential Occupational Risks for Neurodegenerative Diseases. Am. J. Ind. Med. 2005, 48, 63–77. [Google Scholar] [CrossRef]
- Harmanci, H.; Emre, M.; Gurvit, H.; Bilgic, B.; Hanagasi, H.; Gurol, E.; Sahin, H.; Tinaz, S. Risk Factors for Alzheimer Disease: A Population-Based Case-Control Study in Istanbul, Turkey. Alzheimer Dis. Assoc. Disord. 2003, 17, 139–145. [Google Scholar] [CrossRef]
- Park, J.; Kwon, J.H.; Kim, N.; Song, K. Effects of 1950 MHz Radiofrequency Electromagnetic Fields on Aβ Processing in Human Neuroblastoma and Mouse Hippocampal Neuronal Cells. J. Radiat. Res. 2018, 59, 18–26. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.Y.; Kim, H.J.; Kim, J.C.; Lee, J.S.; Kim, N.; Park, M.J. Effects of Combined Radiofrequency Field Exposure on Amyloid-Beta–Induced Cytotoxicity in HT22 Mouse Hippocampal Neurones. J. Radiat. Res. 2016, 57, 620–626. [Google Scholar] [CrossRef]
- Arendash, G.W. Review of the Evidence That Transcranial Electromagnetic Treatment Will Be a Safe and Effective Therapeutic Against Alzheimer’s Disease. J. Alzheimers Dis. 2016, 53, 753–771. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, Z.H.; Zhao, X.Y.; Zhang, H.; Zhang, T.Y.; Yu, Y. Review of Terahertz Time Domain and Frequency Domain Spectroscopy Spectrosc. Spectr. Anal. 2018, 38, 2688–2699. [Google Scholar] [CrossRef]
- Peng, X.Y.; Zhou, H. Biological effects of terahertz waves. Acta Phys. Sin. 2021, 70, 76–89. [Google Scholar] [CrossRef]
- Govorun, V.M.; Tretiakov, V.E.; Tulyakov, N.N.; Fleurov, V.B.; Demin, A.I.; Volkov, A.Y.; Batanov, V.A.; Kapitanov, A.B. Far-Infrared Radiation Effect on the Structure and Properties of Proteins. Int. J. Infrared Millim. Waves 1991, 12, 1469–1474. [Google Scholar] [CrossRef]
- Homenko, A.; Kapilevich, B.; Kornstein, R.; Firer, M.A. Effects of 100 GHz Radiation on Alkaline Phosphatase Activity and Antigen–Antibody Interaction. Bioelectromagnetics 2009, 30, 167–175. [Google Scholar] [CrossRef]
- Zhang, X.-X.; He, M.-X.; Chen, Y.; Li, C.; Zhao, J.-W.; Wang, P.-F.; Peng, X. Non-Thermal Effects of 0.1 THz Radiation on Intestinal Alkaline Phosphatase Activity and Conformation*. Chin. Phys. B 2019, 28, 128702. [Google Scholar] [CrossRef]
- Amicis, A.D.; Sanctis, S.D.; Cristofaro, S.D.; Franchini, V.; Lista, F.; Regalbuto, E.; Giovenale, E.; Gallerano, G.P.; Nenzi, P.; Bei, R.; et al. Biological Effects of in Vitro THz Radiation Exposure in Human Foetal Fibroblasts. Mutat. Res. Toxicol. Environ. Mutagen. 2015, 793, 150–160. [Google Scholar] [CrossRef]
- Alexandrov, B.S.; Gelev, V.; Bishop, A.R.; Usheva, A.; Rasmussen, K.Ø. DNA Breathing Dynamics in the Presence of a Terahertz Field. Phys. Lett. A 2010, 374, 1214–1217. [Google Scholar] [CrossRef]
- Cohen, F.E.; Sternberg, M.J.E. On the Prediction of Protein Structure: The Significance of the Root-Mean-Square Deviation. J. Mol. Biol. 1980, 138, 321–333. [Google Scholar] [CrossRef]
- Kabsch, W.; Sander, C. Dictionary of Protein Secondary Structure: Pattern Recognition of Hydrogen-Bonded and Geometrical Features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef]
- Lee, B.; Richards, F.M. The Interpretation of Protein Structures: Estimation of Static Accessibility. J. Mol. Biol. 1971, 55, 379–400, IN3–IN4. [Google Scholar] [CrossRef]
- Santoro, A.; Grimaldi, M.; Buonocore, M.; Stillitano, I.; D’Ursi, A.M. Exploring the Early Stages of the Amyloid Aβ(1–42) Peptide Aggregation Process: An NMR Study. Pharmaceuticals 2021, 14, 732. [Google Scholar] [CrossRef]
- Lührs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Döbeli, H.; Schubert, D.; Riek, R. 3D Structure of Alzheimer’s Amyloid-β(1–42) Fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 17342–17347. [Google Scholar] [CrossRef]
- Bekker, H.; Berendsen, H.J.C.; Dijkstra, E.J.; Achterop, S.; Vondrumen, R.; Vanderspoel, D.; Sijbers, A.; Keegstra, H.; Renardus, M.K.R. Gromacs—A Parallel Computer for Molecular-Dynamics Simulations. In Proceedings of the 4th International Conference on Computational Physics (PC 92), Prague, Czech Republic, 24–28 August 1992; World Scientific Publishing: Singapore, 1993; pp. 252–256. [Google Scholar]
- Berendsen, H.J.C.; van der Spoel, D.; van Drunen, R. GROMACS: A Message-Passing Parallel Molecular Dynamics Implementation. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Lindahl, E.; Hess, B.; van der Spoel, D. GROMACS 3.0: A Package for Molecular Simulation and Trajectory Analysis. Mol. Model. Annu. 2001, 7, 306–317. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A High-Throughput and Highly Parallel Open Source Molecular Simulation Toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; van Gunsteren, W.F. Definition and Testing of the GROMOS Force-Field Versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef]
- Zhu, Z.; Chang, C.; Shu, Y.; Song, B. Transition to a Superpermeation Phase of Confined Water Induced by a Terahertz Electromagnetic Wave. J. Phys. Chem. Lett. 2020, 11, 256–262. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Turner, P. XMGRACE. 2005. Available online: https://plasma-gate.weizmann.ac.il/Grace/ (accessed on 4 December 2022).



| Secondary Structure (%) | Hydrogen Bonds (Number) | |||
|---|---|---|---|---|
| Structure | α-Helix | β-Sheet | ||
| Monomer | 59 | 50 | 0 | 99 |
| 57 | 32 | 0 | 100 | |
| 57 | 48 | 0 | 99 | |
| RSD | 0.94 | 8.06 | 0 | 0.47 |
| Monomer and Field | 31 | 13 | 2 | 71 |
| 18 | 2 | 2 | 64 | |
| 24 | 10 | 0 | 77 | |
| RSD | 5.31 | 4.64 | 0.94 | 5.31 |
| Oligomer | 50 | 0 | 46 | 208 |
| 47 | 0 | 44 | 201 | |
| 43 | 0 | 38 | 206 | |
| RSD | 2.87 | 0 | 3.40 | 2.94 |
| Oligomer and Field | 37 | 0 | 30 | 125 |
| 40 | 0 | 32 | 130 | |
| 36 | 0 | 29 | 140 | |
| RSD | 1.70 | 0 | 1.25 | 6.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Cheng, Y.; Wang, W.; Zhao, J.; Wang, Y.; Zhang, X.; Wang, M.; Shan, T.; He, M. Effects of Terahertz Radiation on the Aggregation of Alzheimer’s Aβ42 Peptide. Int. J. Mol. Sci. 2023, 24, 5039. https://doi.org/10.3390/ijms24055039
Wang L, Cheng Y, Wang W, Zhao J, Wang Y, Zhang X, Wang M, Shan T, He M. Effects of Terahertz Radiation on the Aggregation of Alzheimer’s Aβ42 Peptide. International Journal of Molecular Sciences. 2023; 24(5):5039. https://doi.org/10.3390/ijms24055039
Chicago/Turabian StyleWang, Lei, Yuanyuan Cheng, Wenxia Wang, Jinwu Zhao, Yinsong Wang, Xumei Zhang, Meng Wang, Tianhe Shan, and Mingxia He. 2023. "Effects of Terahertz Radiation on the Aggregation of Alzheimer’s Aβ42 Peptide" International Journal of Molecular Sciences 24, no. 5: 5039. https://doi.org/10.3390/ijms24055039
APA StyleWang, L., Cheng, Y., Wang, W., Zhao, J., Wang, Y., Zhang, X., Wang, M., Shan, T., & He, M. (2023). Effects of Terahertz Radiation on the Aggregation of Alzheimer’s Aβ42 Peptide. International Journal of Molecular Sciences, 24(5), 5039. https://doi.org/10.3390/ijms24055039







