Integrating Multi-Omics Analysis Reveals the Regulatory Mechanisms of White–Violet Mutant Flowers in Grape Hyacinth (Muscari latifolium)
Abstract
:1. Introduction
2. Results
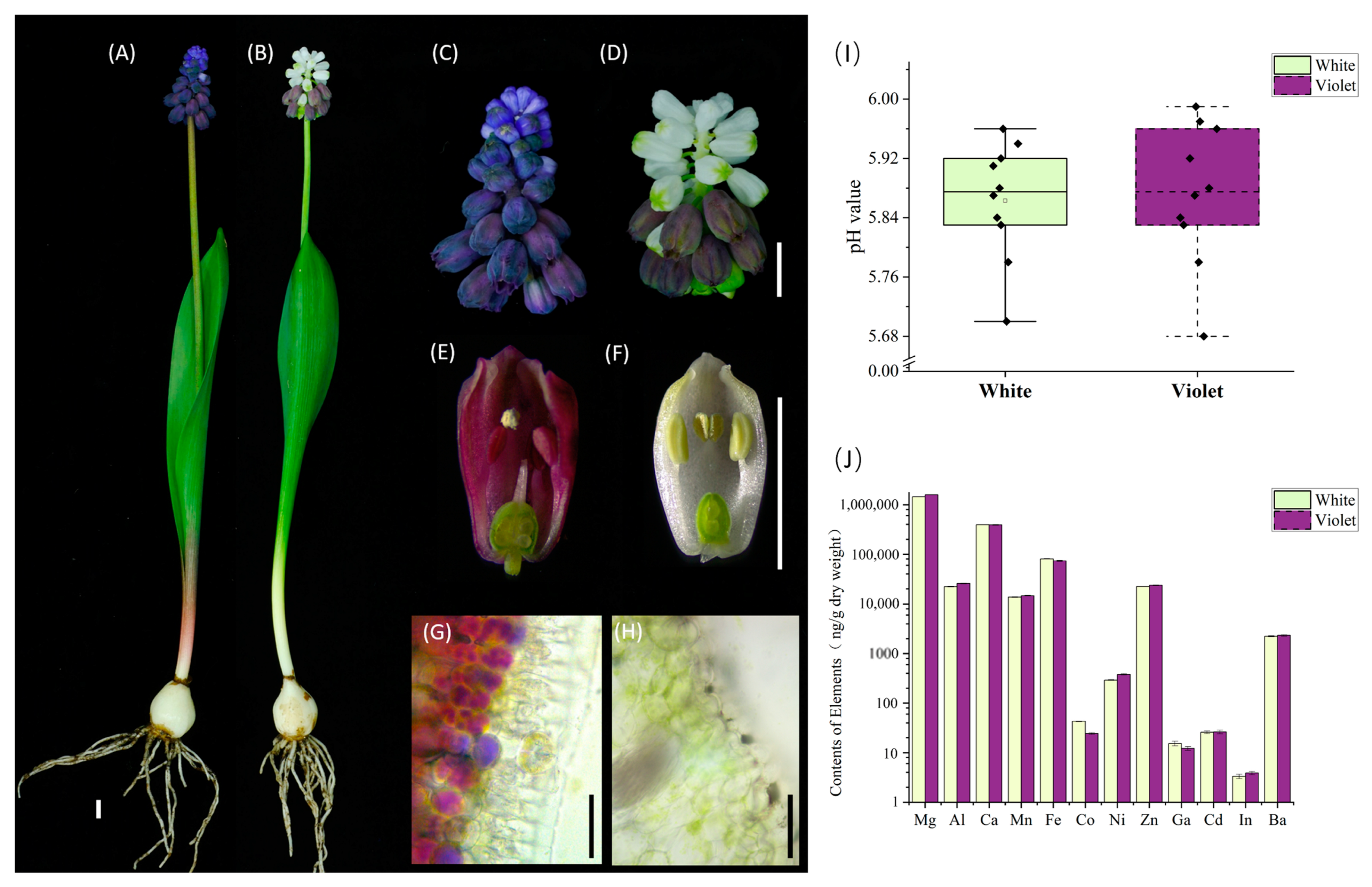
2.1. Phenotypic and Ionomics Analysis of M. latifolium White Flower and Violet Flower
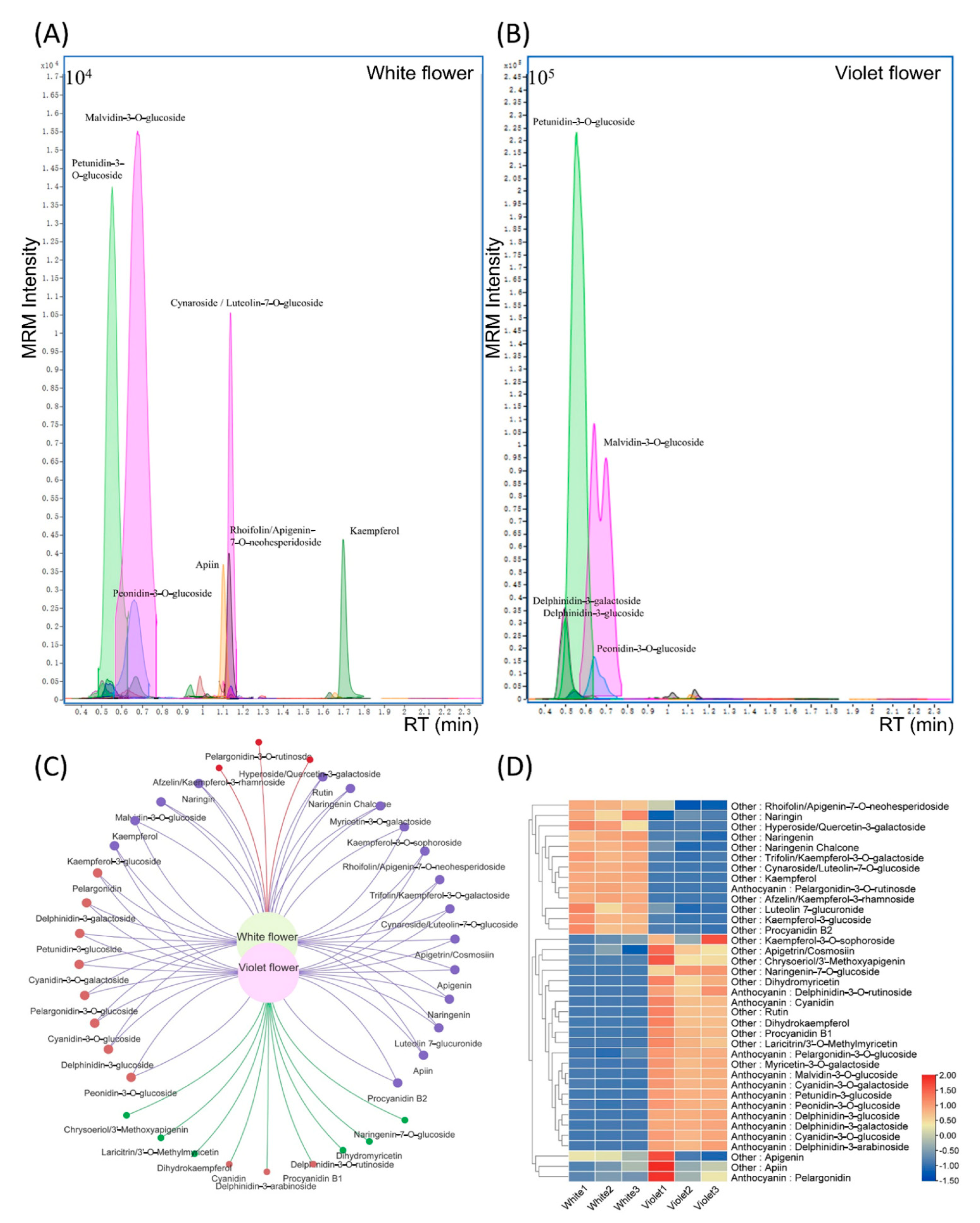
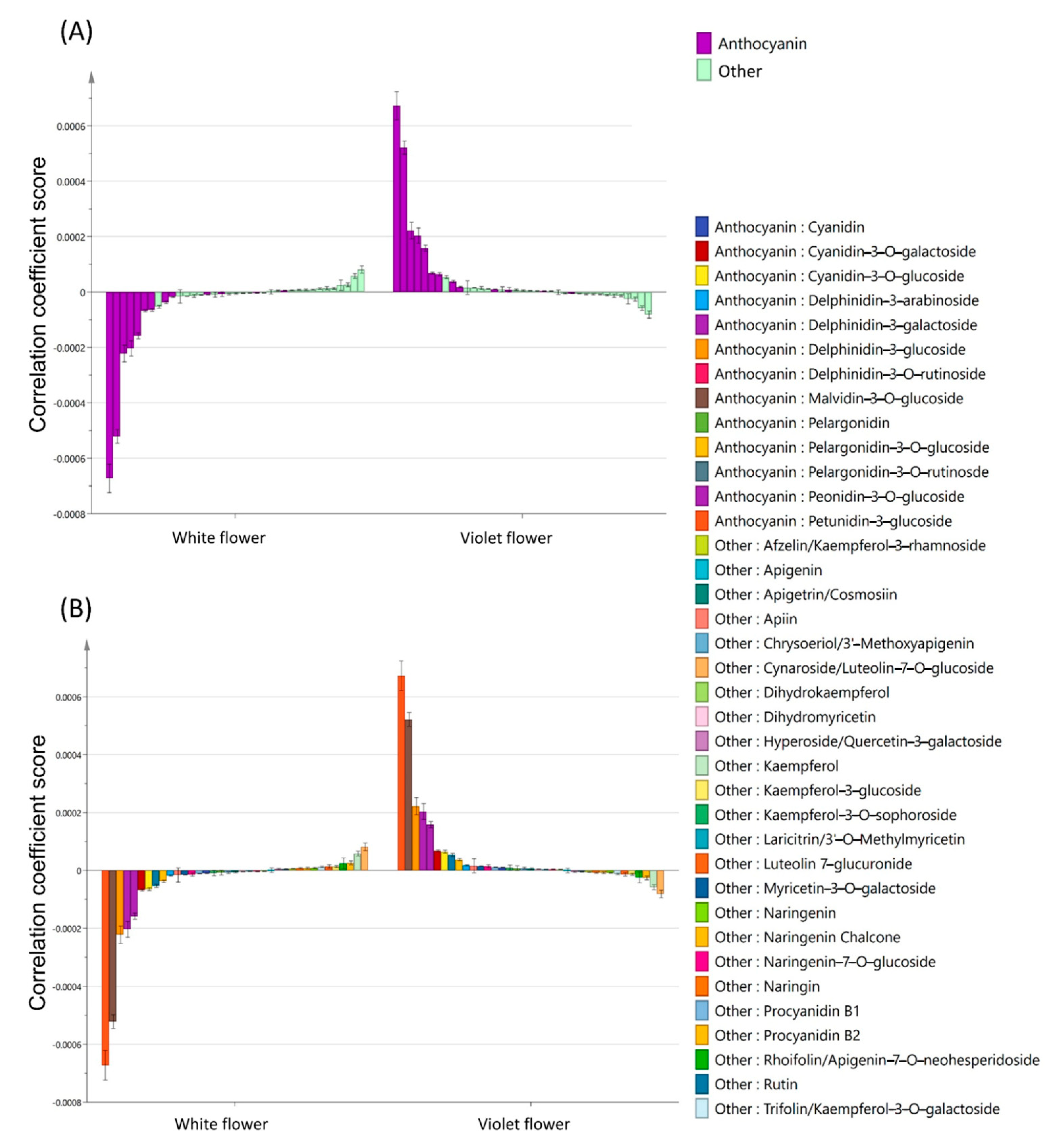
2.2. Targeted Metabolomics Analysis of White and Violet Flowers of M. latifolium
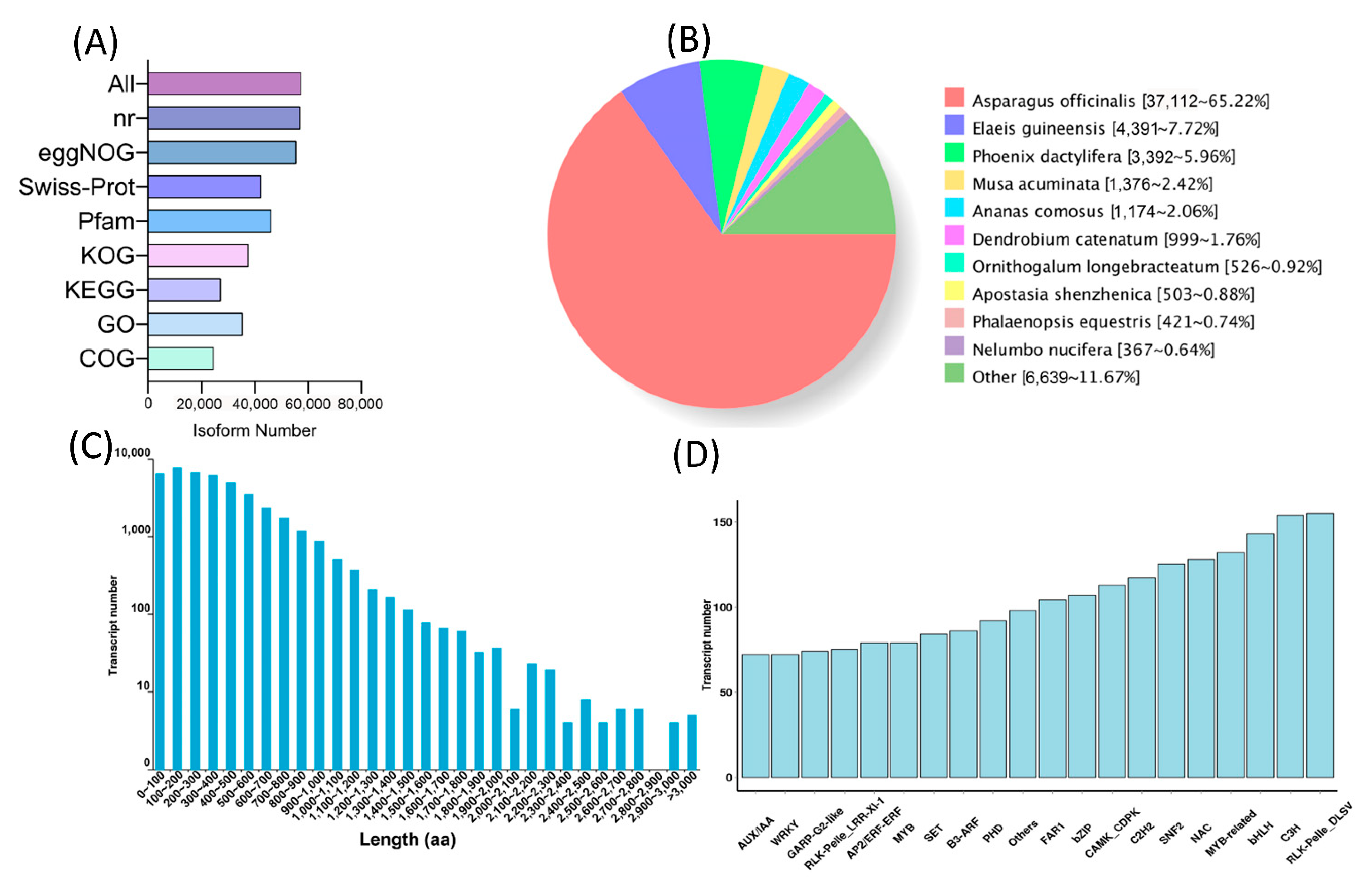
2.3. Full-Length Transcriptomics Analysis of White and Violet Flowers of M. latifolium
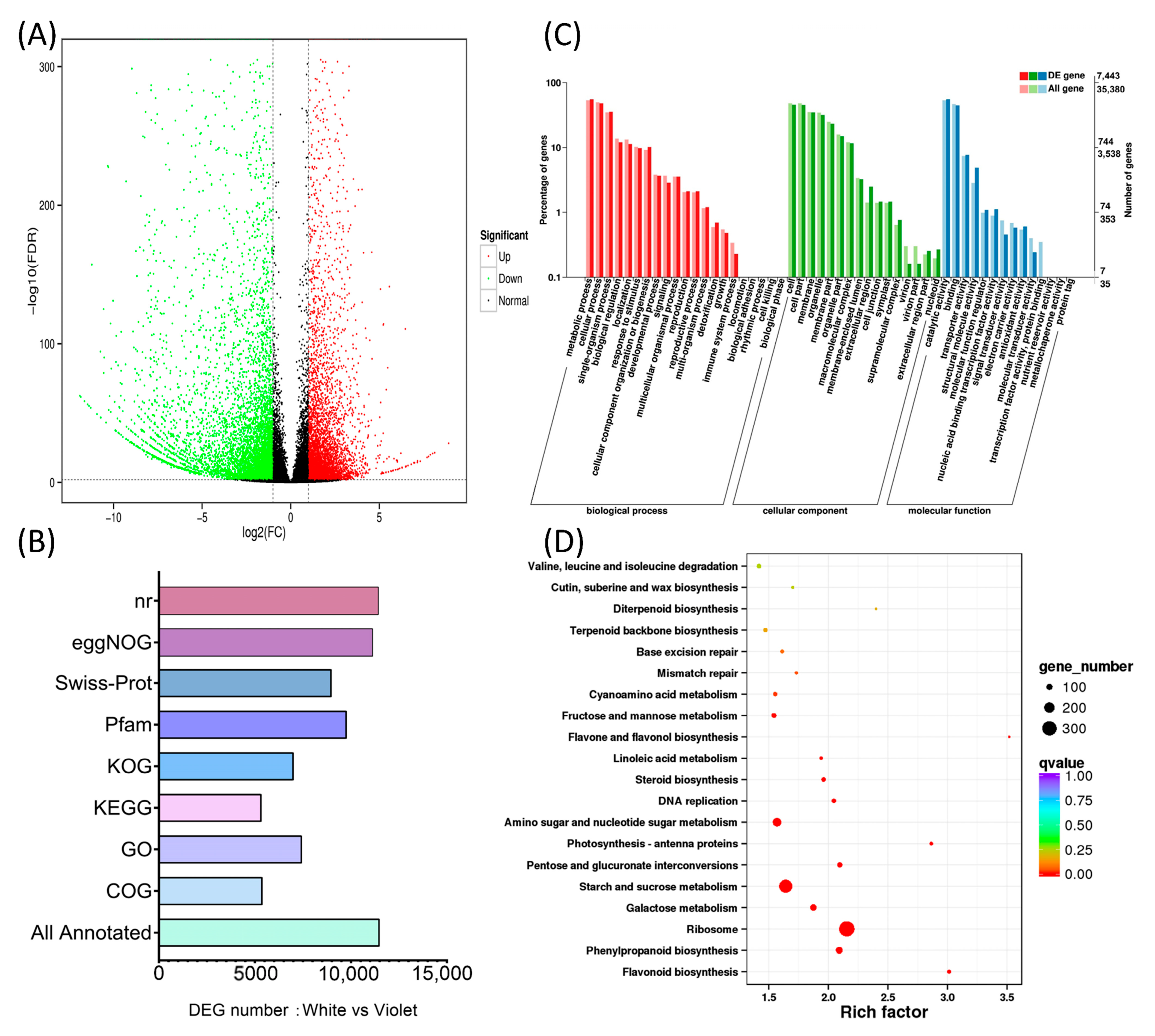
2.4. Differential Transcriptomics Analysis of White and Violet Flowers of M. latifolium
2.5. The Expression of the Flower Pigment Synthesis Pathway between White and Violet Flowers
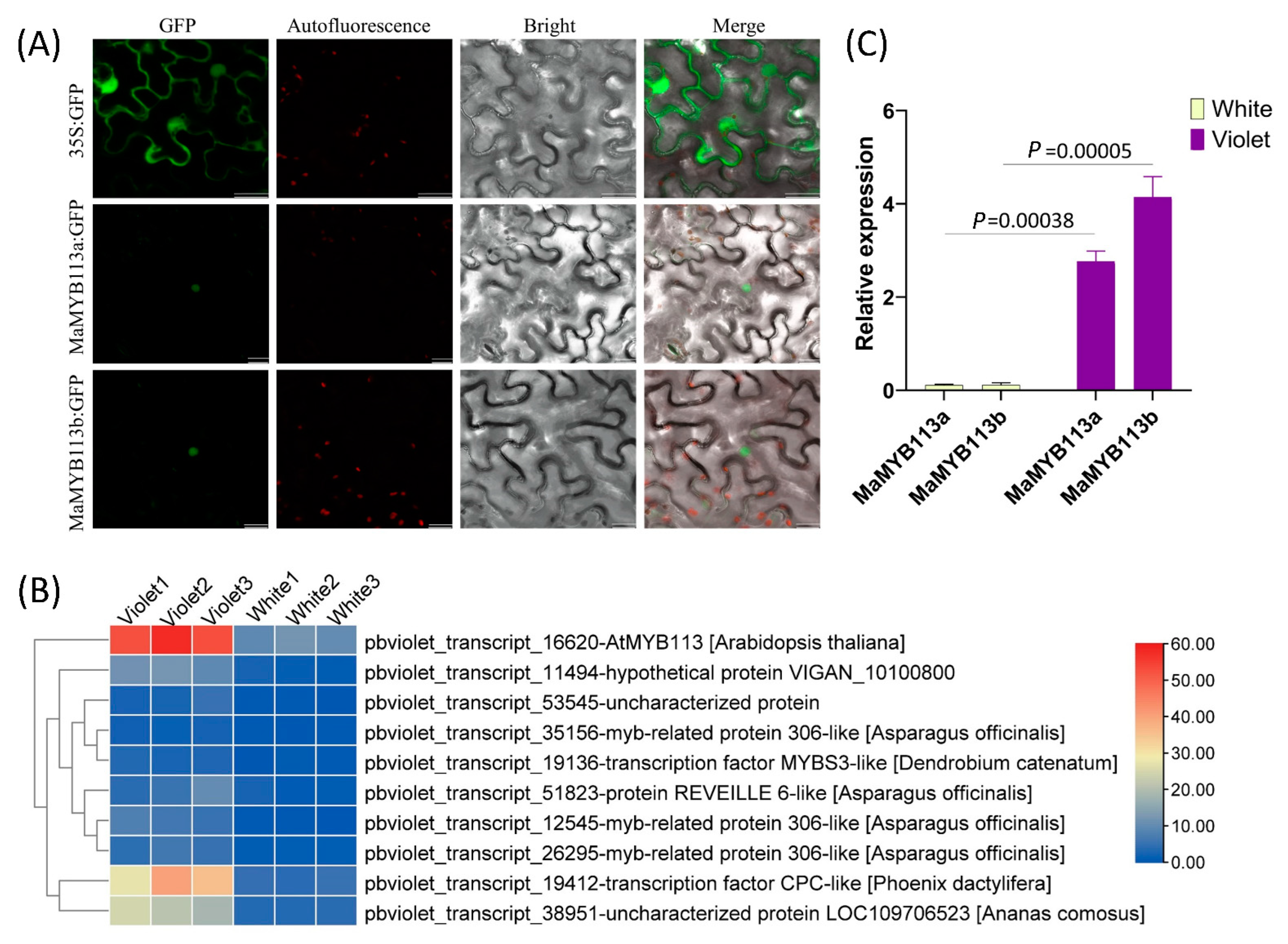
2.6. Differential Expression Analysis and Subcellular Localization of MaMYB113a/b
2.7. Phylogenetic Tree Analysis and Multiple Sequence Comparison of MaMYB113a/b
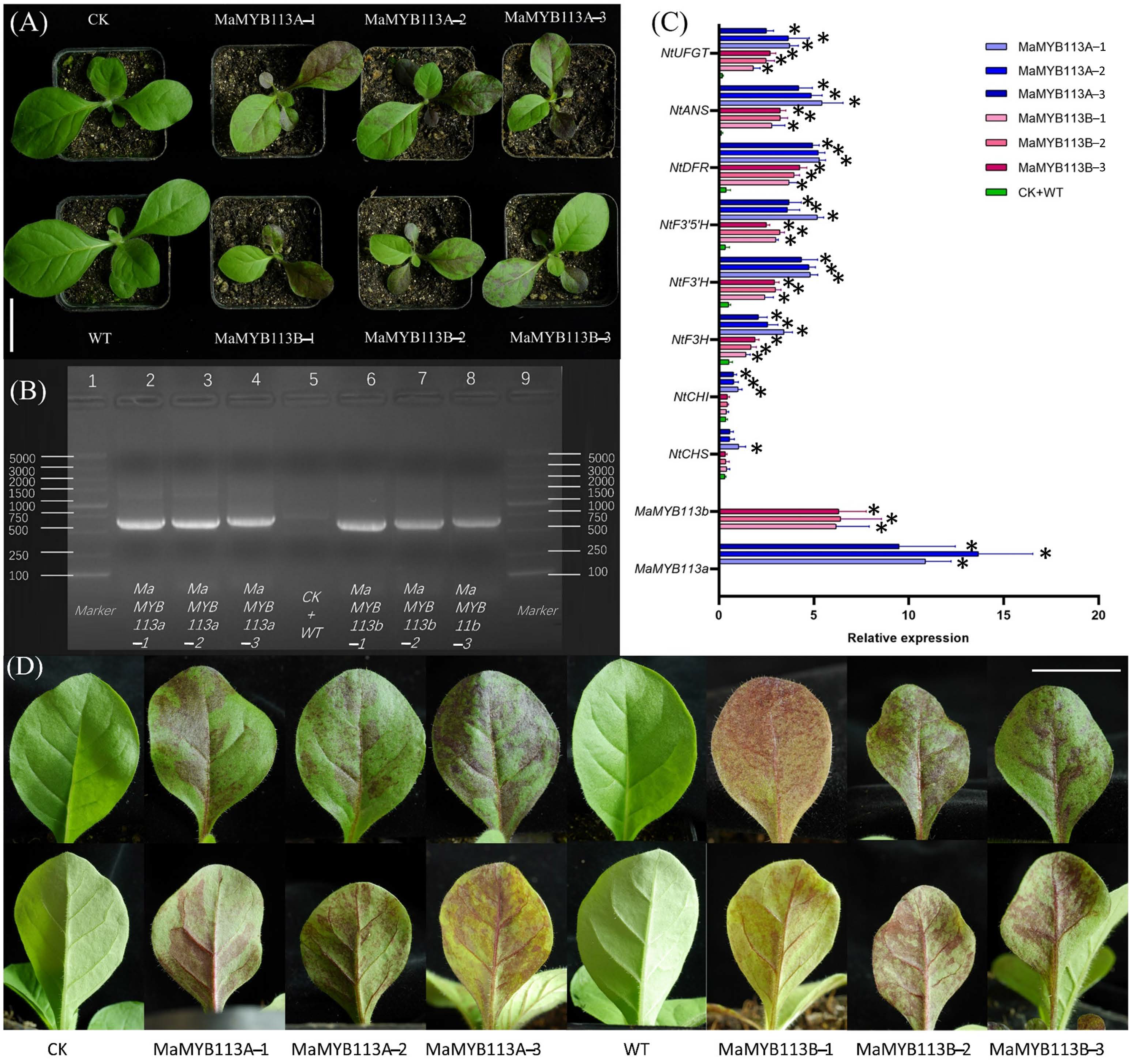
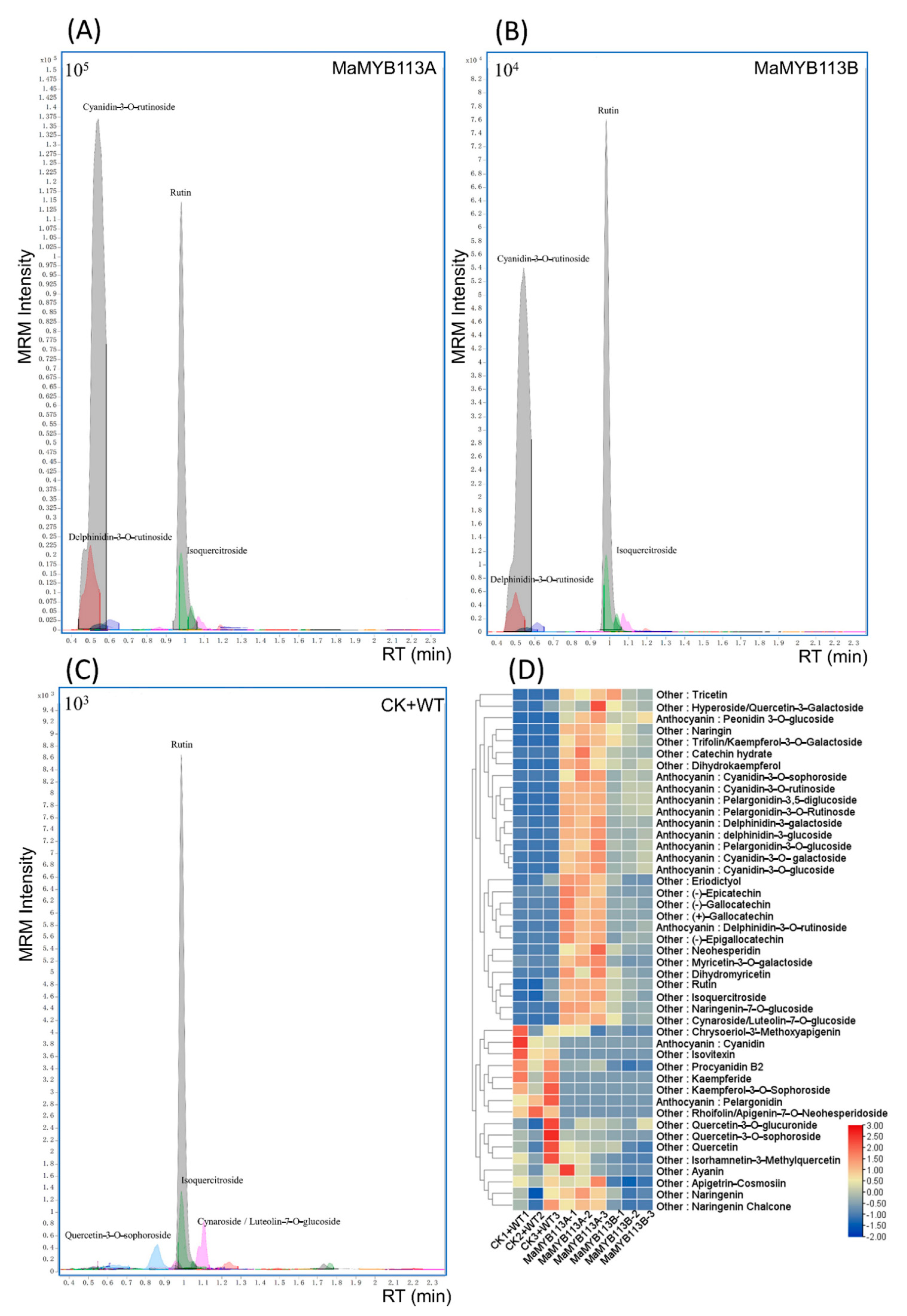
2.8. Transformation of MaMYB113a and MaMYB113b in Tobacco
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Microscopic Observation
4.3. Puncture pH of Tepals
4.4. Mass Spectrometry Analysis of Metal Elements in Tepals
4.5. Targeted Mass Spectrometry Analysis of Flower Color Related Compounds
4.6. RNA Extraction and cDNA Synthesis
4.7. Transcriptome Library Construction, Sequencing and Data Analysis
4.8. Phylogenetic Tree Analysis and Protein Sequence Alignment
4.9. MaMYB113a/b Subcellular Localisation
4.10. Tobacco Transformation
4.11. Real Time quantitative PCR (RT-qPCR)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.; Xu, Z.; Wang, W.; Mu, D.; Meng, X.; Lu, M.; Li, C. Advances on the coloring mechanism of double-color flowers in plants. HortScience 2022, 57, 1120–1127. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Albert, N.W.; Schwinn, K.E. From landing lights to mimicry: The molecular regulation of flower colouration and mechanisms for pigmentation patterning. Funct. Plant Biol. 2012, 39, 619–638. [Google Scholar] [CrossRef]
- Anwar, M.; Chen, L.; Xiao, Y.; Wu, J.; Zeng, L.; Li, H.; Wu, Q.; Hu, Z. Recent Advanced Metabolic and Genetic Engineering of Phenylpropanoid Biosynthetic Pathways. Int. J. Mol. Sci. 2021, 22, 9544. [Google Scholar] [CrossRef]
- Yan, H.; Pei, X.; Zhang, H.; Li, X.; Zhang, X.; Zhao, M.; Chiang, V.L.; Sederoff, R.R.; Zhao, X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021, 22, 3103. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, J.; Xia, Y.; Zhang, L.; Du, H. Evolution and functional diversification of R2R3-MYB transcription factors in plants. Hortic. Res. 2022, 9, uhac058. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kawachi, M.; Mori, M.; Maeshima, M.; Kondo, M.; Nishimura, M.; Kondo, T. The involvement of tonoplast proton pumps and Na+ (K+)/H+ exchangers in the change of petal color during flower opening of morning glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol. 2005, 46, 407–415. [Google Scholar] [CrossRef]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Shoji, K.; Momonoi, K.; Tsuji, T. Alternative expression of vacuolar iron transporter and ferritin genes leads to blue/purple coloration of flowers in Tulip cv. ‘Murasakizuisho’. Plant Cell Physiol. 2010, 51, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, H.D.; Jones, A.H.; Lariviere, C.M.; Mayhew, K.M.; Cain, J.B. Role of aluminum in red-to-blue color changes in Hydrangea macrophylla sepals. Biometals 2011, 24, 1005–1015. [Google Scholar] [CrossRef]
- Uysal, T.; Bozkurt, M.; Sezer, E.N.Ş.; Aksoy, A.; Ertuğrul, K. Karyomorphological Studies of Six Species of Turkish Muscari (Asparagaceae). Cytologia 2021, 86, 351–357. [Google Scholar] [CrossRef]
- Yilmaz, H.; Yilmaz, O.Y.; Akyüz, Y.F. Determining the factors affecting the distribution of Muscari latifolium, an endemic plant of Turkey, and a mapping species distribution model. Ecol. Evol. 2017, 7, 1112–1124. [Google Scholar] [CrossRef]
- Mori, S.; Asano, S.; Kobayashi, H.; Nakano, M. Analyses of anthocyanidins and anthocyanins in flowers of muscari spp. Bull. Fac. Agric. Niigata Univ. 2002, 55, 13–18. [Google Scholar]
- Qi, Y.; Lou, Q.; Li, H.; Yue, J.; Liu, Y.; Wang, Y. Anatomical and biochemical studies of bicolored flower development in Muscari latifolium. Protoplasma 2013, 250, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Lou, Q.; Liu, H. Method development for the identification of anthocyanins in Muscari latifolium flower. Acta Bot. Boreali-Occident. Sin. 2017, 37, 1031–1037. [Google Scholar]
- Lou, Q.; Wang, L.; Liu, H.; Liu, Y. Anthocyanin Profiles in Flowers of Grape Hyacinth. Molecules 2017, 22, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Liu, H.; Lou, Q.; Liu, Y. Ectopic expression of the grape hyacinth (Muscari armeniacum) R2R3-MYB transcription factor gene, MaAN2, induces anthocyanin accumulation in tobacco. Front. Plant. Sci. 2017, 8, 965. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Du, L.; Liu, H.; Liu, Y. A novel R2R3-MYB from grape hyacinth, MaMybA, which is different from MaAN2, confers intense and magenta anthocyanin pigmentation in tobacco. BMC Plant. Biol. 2019, 19, 390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, S.; Nakano, M.; Kondo, M.; Hoshi, Y.; Kobayashi, H.; Niigata, N. Isolation and characterization of a cytochrome P450 gene from Muscari armeniacum. Acta Hortic 2005, 673, 429–435. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D.; Cao, M.; Lin-Wang, K.; Cooney, J.M.; Jensen, D.J.; Austin, P.T.; Hunt, M.B.; Norling, C.; Hellens, R.P.; Schaffer, R.J.; et al. Environmental regulation of leaf colour in red 35S:PAP1 Arabidopsis thaliana. New Phytol. 2009, 182, 102–115. [Google Scholar] [CrossRef]
- Total elemental analysis of food samples for routine and research laboratories, using the Thermo Scientific iCAP RQ ICP-MS. Available online: https://tools.thermofisher.cn/content/sfs/brochures/AN-43326-ICP-MS-Elemental-Impurities-Food-AN43326-EN.pdf (accessed on 5 December 2022).
- Gordon, S.P.; Tseng, E.; Salamov, A.; Zhang, J.; Meng, X.; Zhao, Z.; Kang, D.; Underwood, J.; Grigoriev, I.V.; Figueroa, M.; et al. Widespread Polycistronic Transcripts in Fungi Revealed by Single-Molecule mRNA Sequencing. PLoS ONE 2015, 10, e0132628. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.Y.; Li, J.Q.; Wu, S.F.; Zhu, Y.P.; Chen, Y.W.; He, F.C. Integrated nr database in protein annotation system and its localization. Comput. Eng. 2006, 32, 71–74. [Google Scholar]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Koonin, E.V.; Fedorova, N.D.; Jackson, J.D.; Jacobs, A.R.; Krylov, D.M.; Makarova, K.S.; Mazumder, R.; Mekhedov, S.L.; Nikolskaya, A.N.; Rao, B.S.; et al. A comprehensive evolutionary classification of proteins encoded in complete eukaryotic genomes. Genome Biol. 2004, 5, R7. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal omega. Curr. Protoc. Bioinform. 2014, 48, 3.13.1–3.13.16. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Liu, H.; Lou, Q.; Ma, J.; Su, B.; Gao, Z.; Liu, Y. Cloning and Functional Characterization of Dihydroflavonol 4-Reductase Gene Involved in Anthocyanidin Biosynthesis of Grape Hyacinth. Int. J. Mol. Sci. 2019, 20, 4743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, F.Z.; You, L.J.; Yang, F.; Wang, L.N.; Guo, X.Q.; Gao, F.; Hua, C.; Tan, C.; Fang, L.; Shan, R.Q.; et al. CNGBdb: China National GeneBank DataBase. Yi Chuan 2020, 42, 799–809. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Li, Z.; Liu, Y. Integrating Multi-Omics Analysis Reveals the Regulatory Mechanisms of White–Violet Mutant Flowers in Grape Hyacinth (Muscari latifolium). Int. J. Mol. Sci. 2023, 24, 5044. https://doi.org/10.3390/ijms24055044
Ma J, Li Z, Liu Y. Integrating Multi-Omics Analysis Reveals the Regulatory Mechanisms of White–Violet Mutant Flowers in Grape Hyacinth (Muscari latifolium). International Journal of Molecular Sciences. 2023; 24(5):5044. https://doi.org/10.3390/ijms24055044
Chicago/Turabian StyleMa, Junren, Zhi Li, and Yali Liu. 2023. "Integrating Multi-Omics Analysis Reveals the Regulatory Mechanisms of White–Violet Mutant Flowers in Grape Hyacinth (Muscari latifolium)" International Journal of Molecular Sciences 24, no. 5: 5044. https://doi.org/10.3390/ijms24055044




