Polyoxometalates Impact as Anticancer Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Types of POMs
2.2. Types of Cancers
2.3. POMs Effects
2.3.1. Cell Viability
2.3.2. Effect of POMs on the Cell Cycle
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-Fu | Fluorouracil |
| A2780 | human ovarian cancer cell line |
| A2780cis | cisplatin-resistant human ovarian cancer cell line |
| A2780cisR | cisplatin-resistant human ovarian cancer cell line |
| A549 | human lung cancer cell line |
| Ac | actinium |
| AGS | human stomach cancer cell line |
| As | arsenic |
| BEAS-2B | non-tumor human epithelial cell line of the bronchial epithelium |
| BGC-823 | endocervical adenocarcinoma cell line related to human papillomavirus |
| Co | cobalt |
| CoV10 | Na4Co6V10O28.18H2O |
| CT26 | murine colorectal carcinoma cell line that is from a BALB/c mouse. |
| CDDP | cisplatin or cis-diamminedichloroplatinum(II) |
| CDK | cyclin dependent kinase |
| CDKN2A | cyclin-dependent kinase inhibitor 2A |
| DHE | dihydroethidium |
| DHR-123 | dihydrorhodamine-123 oxidation test |
| DNA | deoxyribonucleic acid |
| DU145 | human prostate cancer cell line |
| ΔΨm | mitochondrial membrane potential |
| E. coli | Escherichia coli |
| H1299 | non-small cell lung carcinoma human cell line |
| H22 | hepatoma-22, Mouse liver tumor cell line |
| HDACI | histone Deacetylase Inhibitor |
| Hep-A-22 | mouse liver cancer lineage |
| HEK-293T | transformed human embryonic kidney cell line |
| HepG2 | human hepatocarcinoma cell line |
| HL-60 | human leukemia cell line |
| HPOMs | heteropolyoxometalates |
| HT29 | human colon adenocarcinoma cell line |
| HUVEC | human umbilical vein endothelial cells |
| IC50 | 50% inhibitory concentration |
| LNCaP | androgen-sensitive prostate adenocarcinoma human cell line |
| MC3T3-E1 | osteoblast precursor cell line sub-line derived from Mus musculus (Mouse) calvaria |
| MCF-7 | human breast cancer cell line |
| MDA-MB-231 | human breast cancer epithelial cell lineage |
| MG-63 | cell that has fibroblast morphology isolated from the bone of a patient with osteosarcoma |
| MMP | mitochondrial membrane potential |
| Mn | manganese |
| Mo | molybdenum |
| MoO42− | molybdate |
| Na | sodium |
| NaB | sodium butyrate |
| Nb | niobium |
| O2− | anion superoxide |
| OVCAR-3 | human ovarian cancer cell line |
| P | phosphor |
| PAC-320 | {(n-Bu)Sn(OH)}3GeW9O34]4−·26H2O |
| PbPd12 | Na12[PbIVO8Pd12(PO4)8]·38H2O |
| Pd13 | Na8[Pd13As8O34(OH)6].42H2O |
| Pd13L | Na6[Pd13(PhAsO3)8]·23H2O |
| POM | polyoxometalate |
| POM@Sio2 | K2Na[AsIIIMo6O21(O2CCH2NH3)3]·6H2O |
| POMs | polyoxometalates |
| POMos | polyoxomolybdates |
| PONMs | noble polyoxo metalates |
| POPs | polyoxopaladates |
| POVs | polyoxovanadates |
| POTs | polyoxotungstates |
| PS | phosphatidylserine |
| PW9Cu | K7Na3[Cu4(H2O)2(PW9034)2]20H2O |
| ROS | oxigen-reactive species |
| SAHA | suberoylanilide acid—Vorinostat |
| SARS-CoV | severe acute respiratory syndrome coronavirus |
| SH-SY5Y | subline cloned three times from neuroblastoma cell line SK-N-SH (ATCC HTB-11) |
| SK-OV-3 | ovarian cancer human epithelial cell line |
| SMMC-7721 | endocervical adenocarcinoma cell line related to human papillomavirus |
| SnPd12 | Na12[SnIVO8Pd12(PO4)8]·43H2O |
| SrPd12 | Na4[SrPd12O6(OH)3(PhAsO3)6(OAc)3] 2NaOAc·32H2O |
| Ta | tantalum |
| Tc | technetium |
| U937 | histiocytic lymphoma human cell line |
| UMR106 | epithelial cell isolated from the bone of a mouse with osteosarcoma |
| V | vanadium |
| V18 | K12[V18O42(H2O)] 6H2O |
| VMOP-31 | 6{V5O9Cl(COO)4} |
| W | tungsten |
References
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in Cancer Nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, E.L.M. Lithium as a Neuroprotective Agent for Bipolar Disorder: An Overview. Cell. Mol. Neurobiol. 2022, 42, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Vosahlikova, M.; Roubalova, L.; Cechova, K.; Kaufman, J.; Musil, S.; Miksik, I.; Alda, M.; Svoboda, P. Na+/K+-ATPase and Lipid Peroxidation in Forebrain Cortex and Hippocampus of Sleep-Deprived Rats Treated with Therapeutic Lithium Concentration for Different Periods of Time. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109953. [Google Scholar] [CrossRef] [PubMed]
- Bertinat, R.; Westermeier, F.; Gatica, R.; Nualart, F. Sodium Tungstate: Is It a Safe Option for a Chronic Disease Setting, Such as Diabetes? J. Cell. Physiol. 2019, 234, 51–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.J.S.A.; Gois, P.M.P.; Gasser, G. Unveiling the Potential of Transition Metal Complexes for Medicine: Translational in Situ Activation of Metal-Based Drugs from Bench to in Vivo Applications. ChemBioChem 2021, 22, 1740–1742. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and Possible Benefits in the Light of a Comprehensive Overview of Its Pharmacotoxicological Mechanisms and Multi-Applications with a Summary of Further Research Trends. J. Trace. Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Yeo, C.; Ooi, K.; Tiekink, E. Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy? Molecules 2018, 23, 1410. [Google Scholar] [CrossRef] [Green Version]
- Soria-Carrera, H.; Atrián-Blasco, E.; de la Fuente, J.M.; Mitchell, S.G.; Martín-Rapún, R. Polyoxometalate–Polypeptide Nanoassemblies as Peroxidase Surrogates with Antibiofilm Properties. Nanoscale 2022, 14, 5999–6006. [Google Scholar] [CrossRef]
- Soria-Carrera, H.; Franco-Castillo, I.; Romero, P.; Martín, S.; Fuente, J.M.; Mitchell, S.G.; Martín-Rapún, R. On-POM Ring-Opening Polymerisation of N-Carboxyanhydrides. Angew. Chem. Int. Ed. Engl. 2021, 60, 3449–3453. [Google Scholar] [CrossRef]
- Aureliano, M.; Marques-da-Silva, D.; Serrano, A.; Martins, J.; Faleiro, L.; Fonseca, C.; Fraqueza, G.; Lagoa, R. Chapter 7, Polyoxometalates with anticancer, antibacterial and antiviral activities. In Polyoxometalates: Advances, Properties, and Applications, 1st ed.; Rubio, L.R., Artetxe, B., Gutiérrez-Zorrilla, J.M., Vilas, J.L., Eds.; Jenny Stanford Publishing: Singapore, 2022; ISBN 9781003277446/9789814968140. [Google Scholar]
- Pimpão, C.; da Silva, I.V.; Mósca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef] [Green Version]
- Gumerova, N.; Krivosudský, L.; Fraqueza, G.; Breibeck, J.; Al-Sayed, E.; Tanuhadi, E.; Bijelic, A.; Fuentes, J.; Aureliano, M.; Rompel, A. The P-Type ATPase Inhibiting Potential of Polyoxotungstates. Metallomics 2018, 10, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+- and Ca2+-ATPase Activities by Phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Fraqueza, G.; Berrocal, M.; Cordoba-Granados, J.J.; Gumerova, N.I.; Rompel, A.; Gutierrez-Merino, C.; Mata, A.M. Inhibition of SERCA and PMCA Ca2+-ATPase Activities by Polyoxotungstates. J. Inorg. Biochem. 2022, 236, 111952. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as Potential Next-Generation Metallodrugs in the Combat Against Cancer. Angew. Chem. Int. Ed. Engl. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [Green Version]
- Bijelic, A.; Aureliano, M.; Rompel, A. The Antibacterial Activity of Polyoxometalates: Structures, Antibiotic Effects and Future Perspectives. Chem. Comm. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [Green Version]
- Čolović, M.B.; Lacković, M.; Lalatović, J.; Mougharbel, A.S.; Kortz, U.; Krstić, D.Z. Polyoxometalates in Biomedicine: Update and Overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with Emerging Biomedical Activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ Interactions with Proteins: An Overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Guedes, G.; Wang, S.; Santos, H.A.; Sousa, F.L. Polyoxometalate Composites in Cancer Therapy and Diagnostics. Eur. J. Inorg. Chem. 2020, 2020, 2121–2132. [Google Scholar] [CrossRef]
- Aureliano, M. The Future Is Bright for Polyoxometalates. BioChem 2022, 2, 8–26. [Google Scholar] [CrossRef]
- Aureliano, M. Decavanadate: A Journey in a Search of a Role. Dalton Trans. 2009, 9093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aureliano, M.; Fraqueza, G.; Ohlin, C.A. Ion Pumps as Biological Targets for Decavanadate. Dalton Trans. 2013, 42, 11770. [Google Scholar] [CrossRef]
- Crans, D.C.; Smee, J.J.; Gaidamauskas, E.; Yang, L. The Chemistry and Biochemistry of Vanadium and the Biological Activities Exerted by Vanadium Compounds. Chem. Rev. 2004, 104, 849–902. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Crans, D.C. Decavanadate (V10O286-) and Oxovanadates: Oxometalates with Many Biological Activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, G.; Aureliano, M.; Garribba, E. Rationalizing the Decavanadate(V) and Oxidovanadium(IV) Binding to G-Actin and the Competition with Decaniobate(V) and ATP. Inorg. Chem. 2021, 60, 334–344. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [Green Version]
- Treviño, S.; González-Vergara, E. Metformin-Decavanadate Treatment Ameliorates Hyperglycemia and Redox Balance of the Liver and Muscle in a Rat Model of Alloxan-Induced Diabetes. New J. Chem. 2019, 43, 17850–17862. [Google Scholar] [CrossRef]
- Treviño, S.; Velázquez-Vázquez, D.; Sánchez-Lara, E.; Diaz-Fonseca, A.; Flores-Hernandez, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Metforminium Decavanadate as a Potential Metallopharmaceutical Drug for the Treatment of Diabetes Mellitus. Oxid. Med. Cell. Longev. 2016, 2016, 6058705. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Lombardo, I.; Sánchez-Lara, E.; Pérez-Benítez, A.; Mendoza, Á.; Bernès, S.; González-Vergara, E. Synthesis of Metforminium(2+) Decavanadates—Crystal Structures and Solid-State Characterization. Eur. J. Inorg. Chem. 2014, 2014, 4581–4588. [Google Scholar] [CrossRef]
- Chatkon, A.; Chatterjee, P.B.; Sedgwick, M.A.; Haller, K.J.; Crans, D.C. Counterion Affects Interaction with Interfaces: The Antidiabetic Drugs Metformin and Decavanadate. Eur. J. Inorg. Chem. 2013, 2013, 1859–1868. [Google Scholar] [CrossRef]
- Sánchez-Lara, E.; Treviño, S.; Sánchez-Gaytán, B.L.; Sánchez-Mora, E.; Eugenia Castro, M.; Meléndez-Bustamante, F.J.; Méndez-Rojas, M.A.; González-Vergara, E. Decavanadate Salts of Cytosine and Metformin: A Combined Experimental-Theoretical Study of Potential Metallodrugs against Diabetes and Cancer. Front. Chem. 2018, 6, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, I.E.; Porro, V.; Astrada, S.; Egusquiza, M.G.; Cabello, C.I.; Bollati-Fogolin, M.; Etcheverry, S.B. Polyoxometalates as Antitumor Agents: Bioactivity of a New Polyoxometalate with Copper on a Human Osteosarcoma Model. Chem. Biol. Interact. 2014, 222, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yamase, T. Polyoxometalates Active against Tumors, Viruses, and Bacteria. Prog. Mol. Subcell. Biol. 2013, 54, 65–116. [Google Scholar] [CrossRef] [PubMed]
- Van Rompuy, L.S.; Parac-Vogt, T.N. Interactions between Polyoxometalates and Biological Systems: From Drug Design to Artificial Enzymes. Curr. Opin. Biotechnol. 2019, 58, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, W.; Hu, Q.; Yan, H.; Zeng, Y. Synthesis and Evaluation of Pyridinium Polyoxometalates as Anti-HIV-1 Agents. Bioorg. Med. Chem. Lett. 2017, 27, 2357–2359. [Google Scholar] [CrossRef]
- Francese, R.; Civra, A.; Rittà, M.; Donalisio, M.; Argenziano, M.; Cavalli, R.; Mougharbel, A.S.; Kortz, U.; Lembo, D. Anti-Zika Virus Activity of Polyoxometalates. Antiviral. Res. 2019, 163, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Enderle, A.G.; Bosso, M.; Groß, R.; Heiland, M.; Bollini, M.; Culzoni, M.J.; Kirchhoff, F.; Münch, J.; Streb, C. Increased in Vitro Anti-HIV Activity of Caffeinium-Functionalized Polyoxometalates. ChemMedChem 2021, 16, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Mahdavi, M.; Zendehcheshm, S. Can Polyoxometalates (POMs) Prevent of Coronavirus 2019-NCoV Cell Entry? Interaction of POMs with TMPRSS2 and Spike Receptor Domain Complexed with ACE2 (ACE2-RBD): Virtual Screening Approaches. Inform. Med. Unlocked 2022, 29, 100902. [Google Scholar] [CrossRef] [PubMed]
- Raza, R.; Matin, A.; Sarwar, S.; Barsukova-Stuckart, M.; Ibrahim, M.; Kortz, U.; Iqbal, J. Polyoxometalates as Potent and Selective Inhibitors of Alkaline Phosphatases with Profound Anticancer and Amoebicidal Activities. Dalton Trans. 2012, 41, 14329. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Wang, J.F.; Han, Q.; Shangguan, P.; Liu, L.L.; Chen, L.J.; Zhao, J.W.; Streb, C.; Song, Y.F. Multicomponent Self-Assembly of a Giant Heterometallic Polyoxotungstate Supercluster with Antitumor Activity. Angew. Chem. Int. Ed. Engl. 2021, 60, 11153–11157. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Gao, H.; Yan, M.; Li, S.; Li, X.; Dai, Z.; Liu, S. Polyoxometalate-Based Organic–Inorganic Hybrids as Antitumor Drugs. Small 2015, 11, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yang, L.; Liu, Y.; Qin, X.; Zhou, Y.; Zhou, Y.; Liu, J. Mo Polyoxometalate Nanoparticles Inhibit Tumor Growth and Vascular Endothelial Growth Factor Induced Angiogenesis. Sci. Technol. Adv. Mater. 2014, 15, 035010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulmier, A.; Feng, X.; Oms, O.; Mialane, P.; Rivière, E.; Shin, C.J.; Yao, J.; Kubo, T.; Furuta, T.; Oldfield, E.; et al. Anticancer Activity of Polyoxometalate-Bisphosphonate Complexes: Synthesis, Characterization, in Vitro and in Vivo Results. Inorg. Chem. 2017, 56, 7558–7565. [Google Scholar] [CrossRef]
- Li, D.; Gao, X.; Gu, J.; Tian, Y.; Liu, Y.; Jin, Z.; Yan, D.; Chen, Y.G.; Zhu, X. A Novel Application of Ti-Substituted Polyoxometalates: Anti-Inflammatory Activity in OVA-Induced Asthma Murine Model. Bioinorg. Chem. Appl. 2016, 2016, 3239494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Li, M.; Zhang, Y.; Wang, R.; Song, Y.; Zhao, W.; Lin, S. A Supramolecular Complex Based on a Gd-Containing Polyoxometalate and Food-Borne Peptide for MRI/CT Imaging and NIR-Triggered Photothermal Therapy. Dalton Trans. 2021, 50, 8076–8083. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Artetxe, B.; Vivanco, M.d.M.; Gutiérrez-Zorrilla, J.M.; Vilas-Vilela, J.L. Chitosan Nanogels as Nanocarriers of Polyoxometalates for Breast Cancer Therapies. Carbohydr. Polym. 2019, 213, 159–167. [Google Scholar] [CrossRef]
- Ma, M.; Gao, N.; Sun, Y.; Du, X.; Ren, J.; Qu, X. Redox-Activated Near-Infrared-Responsive Polyoxometalates Used for Photothermal Treatment of Alzheimer’s Disease. Adv. Healthc. Mater. 2018, 7, 1800320. [Google Scholar] [CrossRef]
- Li, M.; Guan, Y.; Zhao, A.; Ren, J.; Qu, X. Using Multifunctional Peptide Conjugated Au Nanorods for Monitoring β-Amyloid Aggregation and Chemo-Photothermal Treatment of Alzheimer’s Disease. Theranostics 2017, 7, 2996–3006. [Google Scholar] [CrossRef]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Duan, T.; Xu, C.; Qu, X. Transition-Metal-Substituted Polyoxometalate Derivatives as Functional Anti-Amyloid Agents for Alzheimer’s Disease. Nat. Commun. 2014, 5, 3422. [Google Scholar] [CrossRef] [Green Version]
- Bâlici, Ş.; Şuşman, S.; Rusu, D.; Nicula, G.Z.; Soriţău, O.; Rusu, M.; Biris, A.S.; Matei, H. Differentiation of Stem Cells into Insulin-Producing Cells under the Influence of Nanostructural Polyoxometalates. J. Appl. Toxicol. 2016, 36, 373–384. [Google Scholar] [CrossRef]
- Amante, C.; De Sousa-Coelho, A.L.; Aureliano, M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828. [Google Scholar] [CrossRef]
- Fonseca, C.; Aureliano, M. Biological Activity of Gold Compounds against Viruses and Parasitosis: A Systematic Review. BioChem 2022, 2, 145–159. [Google Scholar] [CrossRef]
- Aureliano, M.; Mitchell, S.G.; Yin, P. Editorial: Emerging polyoxometalates with biological, biomedical, and health applications. Front. Chem. 2022, 10, 977317. [Google Scholar] [CrossRef] [PubMed]
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrao, G.; Goncalves, J.; Sanchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915. [Google Scholar] [CrossRef]
- Faleiro, L.; Marques, A.; Martins, J.; Jordão, L.; Nogueira, I.; Gumerova, N.I.; Rompel, A.; Aureliano, M. The Preyssler-Type Polyoxotungstate Exhibits Anti-Quorum Sensing, Antibiofilm, and Antiviral Activities. Biology 2022, 11, 994. [Google Scholar] [CrossRef]
- Zhai, F.; Wang, X.; Li, D.; Zhang, H.; Li, R.; Song, L. Synthesis and Biological Evaluation of Decavanadate Na4Co(H2O)6V10O28·18H2O. Biomed. Pharmacother. 2009, 63, 51–55. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, B.; Qi, Y.; Guo, S.; Tian, R.; Sun, J.; Zhao, M. The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)]·6H2O on Breast Cancer Cell Lines. Molecules 2017, 22, 1535. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Gan, H.; Zhao, Y.; Li, W.; Wu, Y.; Yan, X.; Wang, Y.; Li, J.; Li, J.; Wang, X. Self-Assembly and Antitumor Activity of a Polyoxovanadate-Based Coordination Nanocage. Chem-Eur. J. 2019, 25, 15326–15332. [Google Scholar] [CrossRef]
- Li, C.; Cao, H.; Sun, J.; Tian, R.; Li, D.; Qi, Y.; Yang, W.; Li, J. Antileukemic Activity of an Arsenomolybdate in the Human HL-60 and U937 Leukemia Cells. J. Inorg. Biochem. 2017, 168, 67–75. [Google Scholar] [CrossRef]
- Cao, H.; Li, C.; Qi, W.; Meng, X.; Tian, R.; Qi, Y.; Yang, W.; Li, J. Synthesis, Cytotoxicity and Antitumour Mechanism Investigations of Polyoxometalate Doped Silica Nanospheres on Breast Cancer MCF-7 Cells. PLoS ONE 2017, 12, e0181018. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Qi, W.; Cao, H.; Qi, Y.; Zhang, S.; Xu, S.; Sun, J.; Guo, S. BSA-Binding Properties and Anti-Proliferative Effects of Amino Acids Functionalized Polyoxomolybdates. Biomed. Pharmacother. 2016, 79, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Gupta, R.; Singh, B.; Sharma, D.; Singh, M. Effective Inhibitory Activity against MCF-7, A549 and HepG2 Cancer Cells by a Phosphomolybdate Based Hybrid Solid. Dalton Trans. 2020, 49, 7069–7077. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Gupta, R.; Vaghasiya, K.; Verma, R.K.; Sharma, D.; Singh, M. In Vitro Anti-Tumoral and Anti-Bacterial Activity of an Octamolybdate Cluster-Based Hybrid Solid Incorporated with a Copper Picolinate Complex. ACS Appl. Bio. Mater. 2020, 3, 4025–4035. [Google Scholar] [CrossRef] [PubMed]
- Isakovic, A.M.; Čolović, M.B.; Ma, T.; Ma, X.; Jeremic, M.; Gerić, M.; Gajski, G.; Misirlic-Dencic, S.; Kortz, U.; Krstić, D. Selected Polyoxopalladates as Promising and Selective Antitumor Drug Candidates. J. Biol. Inorg. Chem. 2021, 26, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.M.; Duan, X.; Yao, S.; Wang, Z.; Lin, Z.; Li, Y.G.; Long, L.S.; Wang, E.B.; Lin, W. Cation-Mediated Optical Resolution and Anticancer Activity of Chiral Polyoxometalates Built from Entirely Achiral Building Blocks. Chem. Sci. 2016, 7, 4220–4229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Tao, L.; Zhang, F.; Zhang, Y.; Liu, Y.; Xu, H.; Diao, G.; Ni, L. Transition Metal Substituted Sandwich-Type Polyoxometalates with a Strong Metal-C (Imidazole) Bond as Anticancer Agents. Chem. Commun. 2019, 55, 1096–1099, Correction in Chem. Commun. 2020, 56, 2364. [Google Scholar]
- Dong, Z.; Yang, Y.; Liu, S.; Lu, J.; Huang, B.; Zhang, Y. HDAC Inhibitor PAC-320 Induces G2/M Cell Cycle Arrest and Apoptosis in Human Prostate Cancer. Oncotarget 2018, 9, 512–523. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Tan, R.; Cao, J.; Yang, Y.; Kong, C.; Du, J.; Zhu, S.; Zhang, Y.; Lu, J.; Huang, B.; et al. Discovery of Polyoxometalate-Based HDAC Inhibitors with Profound Anticancer Activity in Vitro and in Vivo. Eur. J. Med. Chem. 2011, 46, 2477–2484. [Google Scholar] [CrossRef]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA Approval Summary: Vorinostat for Treatment of Advanced Primary Cutaneous T-Cell Lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef]
- Wang, X.; Wei, S.; Zhao, C.; Li, X.; Jin, J.; Shi, X.; Su, Z.; Li, J.; Wang, J. Promising application of polyoxometalates in the treatment of cancer, infectious diseases and Alzheimer’s disease. J. Biol. Inorg. Chem. 2022, 27, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Xu, K.; Zhao, C.; Song, X.; Li, J.; Li, L.; Nie, W.; Bao, H.; Wang, J.; Niu, F.; et al. Genotoxicity and acute and subchronic toxicity studies of a bioactive polyoxometalate in Wistar rats. BMC Pharmacol. Toxicol. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinčić, M.; Čolović, M.B.; Sarić Matutinović, M.; Ćetković, M.; Kravić Stevović, T.; Mougharbel, A.S.; Todorović, J.; Ignjatović, S.; Radosavljević, B.; Milisavljević, M.; et al. In vivo toxicity evaluation of two polyoxotungstates with potential antidiabetic activity using Wistar rats as a model system. RSC Adv. 2020, 10, 2846–2855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steens, N.; Ramadan, A.M.; Absillis, G.; Parac-Vogt, T.N. Hydrolytic cleavage of DNA-model substrates promoted by polyoxidovanadates. Dalton Trans. 2010, 39, 585–592. [Google Scholar] [CrossRef]
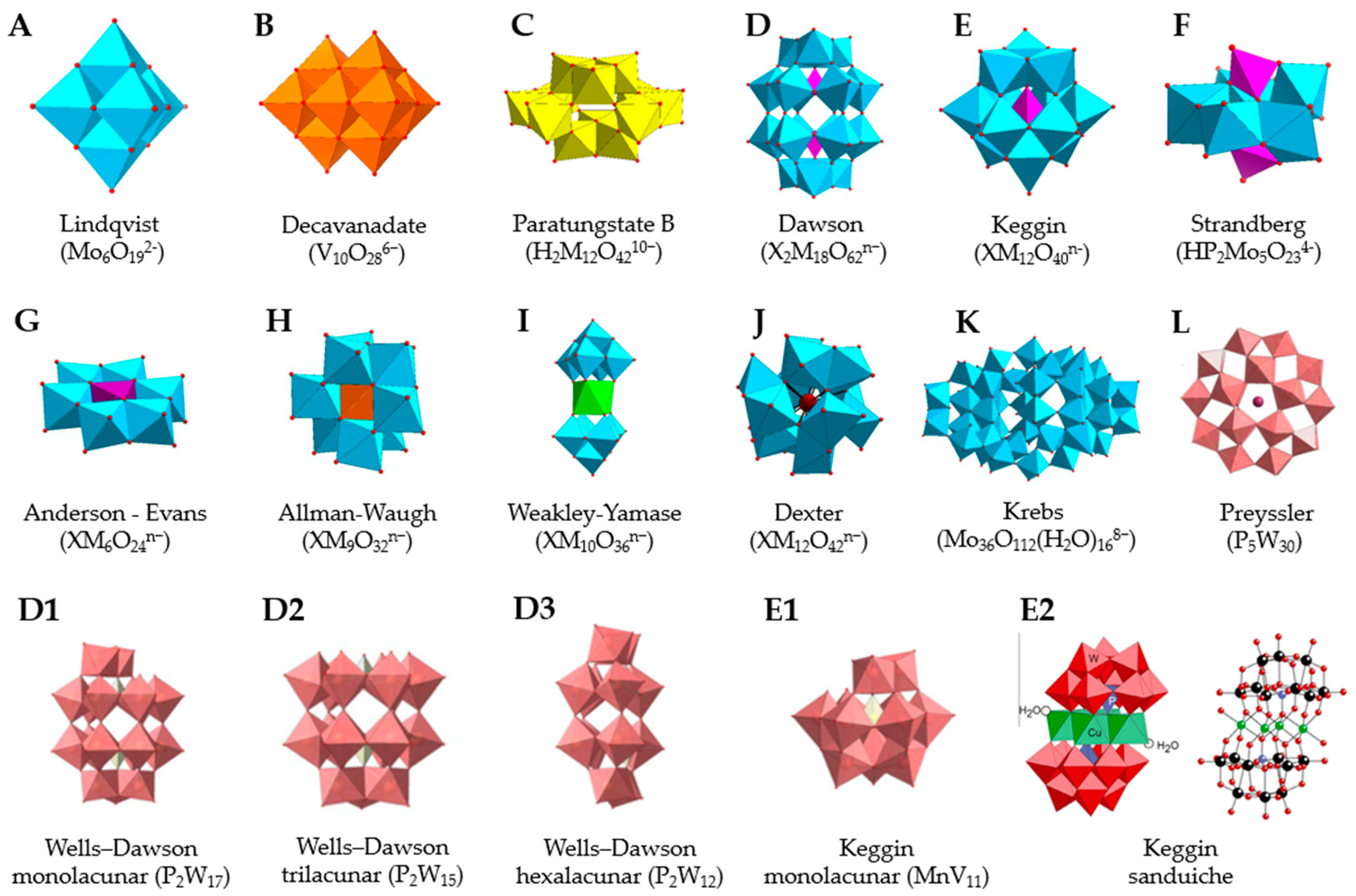




| POM Formula (Abbreviation) | Structure | Structure Type | Ref. |
|---|---|---|---|
| POVs | |||
| Na4Co6V10O28.18H2O (CoV10) Na3(12H2O)H3V10O28.2H2O (NaV10) |  | Decavanadate | [23,57] |
| K12[V18O42(H2O)] 6H2O (V18) |  | Keggin | [58] |
| 6{V5O9Cl(COO)4} (VMOP-31) |  | Lindqvist | [59] |
| POMos | |||
| K2Na[AsIIIMo6O21(O2CCH2NH3)3] 6H2O—(compound 1) |  | Anderson–Evans | [62] |
| K2Na2[γ-Mo8O26(O2CCH2NH3)2] 6H2O—(compound 2) |  | ||
| [{4,4′-H2bpy}{4,4′-Hbpy}2{H2P2Mo5O23}].5H2O |  | Strandberg | [63] |
| [(Cu(pic)2)2(Mo8O26)]·8H2O |  | Anderson–Evans | [64] |
| K2Na[AsIIIMo6O21(O2CCH2NH3)3]·6H2O (POM@SiO2) |  | Anderson–Evans | [61,62] |
| K2Na[AsIIIMo6O21(O2CCH2NH3)3]·6H2O |  | Anderson–Evans | [60,62] |
| POPds | |||
| Na8[Pd13As8O34(OH)6].42H2O (Pd13) |  | Unidentified | [65] |
| Na4[SrPd12O6(OH)3(PhAsO3)6(OAc)3] 2NaOAc·32H2O (SrPd12) |  | ||
| Na6[Pd13(PhAsO3)8]·23H2O (Pd13L) |  | ||
| Na12[SnIVO8Pd12(PO4)8]·43H2O (SnPd12) |  | ||
| Na12[PbIVO8Pd12(PO4)8]·38H2O (PbPd12) |  | ||
| POTs | |||
| K7Na3[Cu4(H2O)2(PW9034)2]20H2O (PW9Cu) |  | Keggin sandwich | [33] |
| {CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}15 |  | Homochiral | [66] |
| {(n-Bu)Sn(OH)}3GeW9O34]4−·26H2O (PAC-320) |  | Keggin | [68,69] |
| H2[(CH3)4N]4− {[Na(H2O)4][Na0.7Ni5.3(imi)2(Himi)(H2O)2(SbW9O33)2]} 10H2O (C25N10Na1.7Ni5.3O82Sb2W18) H3[(CH3)4N]4[Na0.7Co5.3(imi)2(Himi)(H2O)2(SbW9O33)2] 12H2O (C25N10Na0.7Co5.3O76Sb2W18) |  | Keggin sandwich | [67] |
| Formula or Abbreviation | Cell Line | IC50 (µM) | Ref. | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| POVs | |||||
| VMOP-31 | A549 | 2.64 | 0.74 | 0.55 | [59] |
| BEAS-2B | 15.46 | 3.13 | 1.84 | ||
| HL-60 | 14.66 | 2.04 | 1.61 | ||
| V18 | MCF-7 | 45.95 | 11.95 | 12.49 | [58] |
| VMOP-31 | MCF-7 | 1.52 | 0.63 | 0.53 | [59] |
| V18 | MDA-MB-231 | >500 | 360.32 | 135.66 | [58] |
| CoV10 | SK-OV-3 | <0.24 * | [57] | ||
| NaV10 | SK-OV-3 | 18.90 * | |||
| Co(Ac)2 | SK-OV-3 | 50.90 * | |||
| CoV10 | SMMC-7721 | <0.26 * | |||
| NaV10 | SMMC-7721 | 9.56 * | |||
| Co(Ac)2 | SMMC-7721 | 44.90 * | |||
| VMOP-31 | SMMC-7721 | 2.66 | 1.17 | 0.75 | [59] |
| POMos | |||||
| K2Na[AsIIIMo6O21(O2CCH2NH3)3] 6H2O | A549 | 332.87 | 180.40 | [62] | |
| K2Na2[γ-Mo8O26(O2CCH2NH3)2] | A549 | 1072.02 | 330.18 | ||
| [{4,4′-H2bpy}{4,4′-Hbpy}2{H2P2Mo5O23}].5H2O | 4549 | 25.17 | [63] | ||
| [(Cu(pic)2)2(Mo8O26)] 8H2O | A549 | 25.00 | [64] | ||
| [{4,4′-H2bpy}{4,4′-Hbpy}2{H2P2Mo5O23}].5H2O | HepG2 | 33.79 | [63] | ||
| [(Cu(pic)2)2(Mo8O26)]·8H2O | HepG2 | 21.56 | [64] | ||
| K2Na[AsIIIMo6O21(O2CCH2NH3)3] 6H2O | HL-60 | 8.61 | 0.13 | 19.49 | [60] |
| HUVEC | 889.18 | ||||
| K2Na[AsIIIMo6O21(O2CCH2NH3)3]·6H2O (POM@SiO2) | MCF-7 | 40.00 * | 10.80 * | 1.70 * | [61] |
| [{4,4′-H2bpy}{4,4′-Hbpy}2{H2P2Mo5O23}].5H2O | MCF-7 | 32.11 | [63] | ||
| [(Cu(pic)2)2(Mo8O26)] ·8H2O | MCF-7 | 24.24 | [64] | ||
| K2Na[AsIIIMo6O21(O2CCH2NH3)3] 6H2O | U937 | 14.50 | 5.65 | 2.73 | [60] |
| POPds | |||||
| PbPd12 | SH-SY5Y | >>100 | >>100 | [65] | |
| Pd13 | SH-SY5Y | 7.20 | 4.40 | ||
| Pd13L | SH-SY5Y | 63.80 | 21.40 | ||
| SnPd12 | SH-SY5Y | >>100 | >>100 | ||
| SrPd12 | SH-SY5Y | 75.80 | 76.70 | ||
| POTs | |||||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | A2780 | 0.77 | [66] | ||
| {Sb9W21} | A2780 | 4.44 | |||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | A2780cis | 4.35 | |||
| {Sb9W21} | A2780cis | 29.02 | |||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | A549 | 12.65 | |||
| C25N10Na1.7Ni5.3O82Sb2W18 | A549 | 39.75 | [67] | ||
| C25N10Na1.7Ni5.3O82Sb2W18 | AGS | 1.75 | |||
| C25N10Na0.7Ni5.3O76Sb2W18 | AGS | 1.42 | |||
| {Sb8W36} | AGS | 2.86 | |||
| {SbW9} | AGS | 26.22 | |||
| C25N10Na1.7Ni5.3O82Sb2W18 | BGC-823 | 22.27 | |||
| C25N10Na0.7Ni5.3O76Sb2W18 | BGC-823 | 20.51 | |||
| {Sb8W36} | BGC-823 | 8.68 | |||
| {SbW9} | BGC-823 | 188.28 | |||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | CT26 | 14.72 | [66] | ||
| PAC-320 | DU145 | 4.55 | [68] | ||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | HT29 | 15.60 | [66] | ||
| C25N10Na1.7Ni5.3O82Sb2W18 | H1299 | 63.23 | [67] | ||
| C25N10Na1.7Ni5.3O82Sb2W18 | HEK293T | 114.76 | |||
| C25N10Na0.7Ni5.3O76Sb2W18 | HEK293T | 103.09 | |||
| C25N10Na1.7Ni5.3O82Sb2W18 | HepG2 | 42.98 | |||
| PAC-320 | LNCaP | 5.64 | [68] | ||
| PW9Cu | MC3T3-E1 | 92.00 | [33] | ||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | MCF-7 | 12.24 | [66] | ||
| PW9Cu | MG-63 | 22.00 | [33] | ||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | OVCAR-3 | 1.78 | [66] | ||
| {Sb9W21} | OVCAR-3 | 8.80 | [67] | ||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− | SK-OV-3 | 15.02 | [66] | ||
| C25N10Na1.7Ni5.3O82Sb2W18 | SMMC-7721 | 48.29 | [67] | ||
| PW9Cu | UMR106 | 81.00 | [33] | ||
| Compound | Cell Line | IC50 µM | Ref. | ||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | |||
| ATRA | HL-60 | 20.76 | [60] | ||
| U937 | 14.85 | ||||
| Cisplatin | SH-SY5Y | 28.40 | 11.60 | [65] | |
| MG-63 | 43.00 | [33] | |||
| AGS | 17.44 | [67] | |||
| BGC-823 | 5.78 | ||||
| MTX | HepG2 | 42.03 | [63] | ||
| A549 | 26.93 | ||||
| MCF-7 | 49.79 | ||||
| TSA | LNCaP | 98.14 | [68] | ||
| NaB | 3.46 | ||||
| TSA | DU145 | 59.45 | |||
| NaB | 1.20 | ||||
| Compound | Cell Line | Cell Cycle Arrest | Ref | ||
|---|---|---|---|---|---|
| G1 | S | G2/M | |||
| POVs | |||||
| VMOP-31 | SMMC-77221 |  |  | [59] | |
| CoV10 | SMMC-77221 |  | [57] | ||
| V18 | MCF-7 |  | [58] | ||
| POMos | |||||
| K2Na[AsIIIMo6O21(O2CCH2NH3)3] 6H2O—1 | A549 |  | [62] | ||
| K2Na2[γ-Mo8O26(O2CCH2NH3)2]—2 | A549 |  | |||
| [{4,4′-H2bpy}{4,4′-Hbpy}2{H2P2Mo5O23}].5H2O | A549 |  | [63] | ||
| HepG2 |  | ||||
| MCF-7 |  | ||||
| [(Cu(pic)2)2(Mo8O26)] 8H2O | A549 |  | [64] | ||
| HepG2 |  | ||||
| MCF-7 |  | ||||
| K2Na[AsIIIMo6O21(O2CCH2NH3)3] 6H2O | HL-60 |  | [60] | ||
| U937 |  | ||||
| POM@SiO2 | MCF-7 |  | [61] | ||
| POPds | |||||
| Pd13 | SH-SY5Y |  | [65] | ||
| Pd13L |  | ||||
| SrPd12 |  | ||||
| POTs | |||||
| [Co(H2O)6{CoSb6O4(H2O)3[Co(hmta)SbW8O31]3}]13− (1) | A2780 |  | [66] | ||
| A2780cis |  | ||||
| C25N10Na1.7Ni5.3O82Sb2W18—1 | AGS |  | [67] | ||
| BGC-823 |  | ||||
| PAC-320 | DU145 |  | [68] | ||
| LNCaP |  | ||||
| PW9Cu | MG-63 |  | [33] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, F.; Aureliano, M. Polyoxometalates Impact as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 5043. https://doi.org/10.3390/ijms24055043
Carvalho F, Aureliano M. Polyoxometalates Impact as Anticancer Agents. International Journal of Molecular Sciences. 2023; 24(5):5043. https://doi.org/10.3390/ijms24055043
Chicago/Turabian StyleCarvalho, Fátima, and Manuel Aureliano. 2023. "Polyoxometalates Impact as Anticancer Agents" International Journal of Molecular Sciences 24, no. 5: 5043. https://doi.org/10.3390/ijms24055043
APA StyleCarvalho, F., & Aureliano, M. (2023). Polyoxometalates Impact as Anticancer Agents. International Journal of Molecular Sciences, 24(5), 5043. https://doi.org/10.3390/ijms24055043








