Gene Expression Analysis of Immune Regulatory Genes in Circulating Tumour Cells and Peripheral Blood Mononuclear Cells in Patients with Colorectal Carcinoma
Abstract
:1. Introduction
2. Results
2.1. Enumeration of CTCs in Patients with CRC
2.2. Correction for Gene Expression by RT-PCR Due to Leukocyte Contamination in CTC-Enriched Fractions
2.3. Gene Expression Profile of CTC-Fractions and PBMCs
2.4. Clinical and Pathological Correlations
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Enrichment and Isolation of CTCs
4.3. Immunofluorescence Staining
4.4. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
4.5. RNA Extraction and cDNA Conversion
4.6. Quantitative Real-Time Polymerase Chain Reaction
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pantel, K.; Alix-Panabières, C.; Riethdorf, S. Cancer micrometastases. Nat. Rev. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef]
- Allen, J.E.; El-Deiry, W.S. Circulating tumor cells and colorectal cancer. Curr. Colorectal Cancer Rep. 2010, 6, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kallergi, G.; Vetsika, E.-K.; Aggouraki, D.; Lagoudaki, E.; Koutsopoulos, A.; Koinis, F.; Katsarlinos, P.; Trypaki, M.; Messaritakis, I.; Stournaras, C.; et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758834017750121. [Google Scholar] [CrossRef] [PubMed]
- Mohme, M.; Riethdorf, S.; Pantel, K. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 2016, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Agarwal, A.; Almquist, R.G.; Runyambo, D.; Park, S.; Bronson, E.; Boominathan, R.; Rao, C.; Anand, M.; Oyekunle, T.; et al. Expression of immune checkpoints on circulating tumor cells in men with metastatic prostate cancer. Biomark. Res. 2021, 9, 14. [Google Scholar] [CrossRef]
- Mostert, B.; Sieuwerts, A.M.; Bolt-de Vries, J.; Kraan, J.; Lalmahomed, Z.; van Galen, A.; van der Spoel, P.; de Weerd, V.; Ramírez-Moreno, R.; Smid, M.; et al. mRNA expression profiles in circulating tumor cells of metastatic colorectal cancer patients. Mol. Oncol. 2015, 9, 920–932. [Google Scholar] [CrossRef]
- Kong, S.L.; Liu, X.; Suhaimi, N.M.; Koh, K.J.H.; Hu, M.; Lee, D.Y.S.; Cima, I.; Phyo, W.M.; Lee, E.X.W.; Tai, J.A.; et al. Molecular characterization of circulating colorectal tumor cells defines genetic signatures for individualized cancer care. Oncotarget 2017, 8, 68026–68037. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Liu, H.; Wei, C.; Ru, H.; Qin, H.; Lai, H.; Meng, Y.; Wu, G.; Xie, W.; et al. Immune landscape and prognostic immune-related genes in KRAS-mutant colorectal cancer patients. J. Transl. Med. 2021, 19, 27. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, A.; Kim, S.K.; Chang, Y.S. MYC expression correlates with PD-L1 expression in non-small cell lung cancer. Lung Cancer 2017, 110, 63–67. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Thiem, A.; Hesbacher, S.; Kneitz, H.; di Primio, T.; Heppt, M.V.; Hermanns, H.M.; Goebeler, M.; Meierjohann, S.; Houben, R.; Schrama, D. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. J. Exp. Clin. Cancer Res. 2019, 38, 397. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Xia, X.; He, Y.Q.; Hu, Y.; Cremer, K.; Robert, A.; Liu, J.; Wang, F.; Ling, J.; Chiao, P.J.; et al. Regulation of PD-L1 expression in K-ras-driven cancers through ROS-mediated FGFR1 signaling. Redox. Biol. 2021, 38, 101780. [Google Scholar] [CrossRef]
- Fu, X.; Wang, X.; Duanmu, J.; Li, T.; Jiang, Q. KRAS mutations are negatively correlated with immunity in colon cancer. Aging 2020, 13, 750–768. [Google Scholar] [CrossRef]
- Ischenko, I.; D’Amico, S.; Rao, M.; Li, J.; Hayman, M.J.; Powers, S.; Petrenko, O.; Reich, N.C. KRAS drives immune evasion in a genetic model of pancreatic cancer. Nat. Commun. 2021, 12, 1482. [Google Scholar] [CrossRef]
- Zou, J.; Zhuang, M.; Yu, X.; Li, N.; Mao, R.; Wang, Z.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L.; et al. MYC inhibition increases PD-L1 expression induced by IFN-γ in hepatocellular carcinoma cells. Mol. Immunol. 2018, 101, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Fang, W.; Lin, Z.; Peng, P.; Wang, J.; Zhan, J.; Hong, S.; Huang, J.; Liu, L.; Sheng, J. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol. Immunother. 2017, 66, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Hamid, F.B.; Lu, C.T.; Matos, M.; Cheng, T.; Gopalan, V.; Lam, A.K. Enumeration, characterisation and clinicopathological significance of circulating tumour cells in patients with colorectal carcinoma. Cancer Genet. 2021, 254–255, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Maestro, M.L.; Puente, J.; Veganzones, S.; Alfonso, R.; Rafael, S.; García-Saenz, J.A.; Vidaurreta, M.; Martín, M.; Arroyo, M.; et al. Circulating tumor cells in colorectal cancer: Correlation with clinical and pathological variables. Ann. Oncol. 2008, 19, 935–938. [Google Scholar] [CrossRef]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.J.; Uhr, J.W.; Terstappen, L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef]
- Zhao, X.H.; Wang, Z.R.; Chen, C.L.; Di, L.; Bi, Z.F.; Li, Z.H.; Liu, Y.M. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J. Gastroenterol. 2019, 25, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.; Wu, M.-H.; Chang, P.-H.; Lin, H.-C.; Liao, C.-D.; Wu, S.-M.; Hung, T.-M.; Lin, C.-Y.; Chang, T.-C.; Tzu-Tsen, Y.; et al. The change in circulating tumor cells before and during concurrent chemoradiotherapy is associated with survival in patients with locally advanced head and neck cancer. Head Neck 2019, 41, 2676–2687. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.J.; Punt, C.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef]
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W.W.M. Circulating Tumor Cells at Each Follow-up Time Point during Therapy of Metastatic Breast Cancer Patients Predict Progression-Free and Overall Survival. Clin. Cancer Res. 2006, 12, 4218–4224. [Google Scholar] [CrossRef]
- Luo, C.; Cen, S.; Ding, G.; Wu, W. Mucinous colorectal adenocarcinoma: Clinical pathology and treatment options. Cancer Commun. 2019, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.; Ong, K.; Ho, Y.H. Colorectal mucinous adenocarcinoma: The clinicopathologic features and significance of p16 and p53 expression. Dis. Colon Rectum. 2006, 49, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Cree, I.A.; Charlton, P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Kadota, K.; Sima, C.S.; Arcila, M.E.; Hedvat, C.; Kris, M.G.; Jones, D.R.; Adusumilli, P.S.; Travis, W.D. KRAS Mutation Is a Significant Prognostic Factor in Early-stage Lung Adenocarcinoma. Am. J. Surg. Pathol. 2016, 40, 1579–1590. [Google Scholar] [CrossRef]
- Al-Sohaily, S.; Biankin, A.; Leong, R.; Kohonen-Corish, M.; Warusavitarne, J. Molecular pathways in colorectal cancer. J. Gastroenterol. Hepatol. 2012, 27, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.; Ong, K.; Ho, Y.H. hTERT expression in colorectal adenocarcinoma: Correlations with p21, p53 expressions and clinicopathological features. Int. J. Colorectal Dis. 2008, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Hensler, M.; Vančurová, I.; Becht, E.; Palata, O.; Strnad, P.; Tesařová, P.; Čabiňaková, M.; Švec, D.; Kubista, M.; Bartůňková, J.; et al. Gene expression profiling of circulating tumor cells and peripheral blood mononuclear cells from breast cancer patients. Oncoimmunology 2015, 5, e1102827. [Google Scholar] [CrossRef] [PubMed]
- Steinert, G.; Schölch, S.; Niemietz, T.; Iwata, N.; García, S.A.; Behrens, B.; Voigt, A.; Kloor, M.; Benner, A.; Bork, U.; et al. Immune Escape and Survival Mechanisms in Circulating Tumor Cells of Colorectal Cancer. Cancer Res. 2014, 74, 1694. [Google Scholar] [CrossRef] [PubMed]
- Hamid, F.B.; Gopalan, V.; Matos, M.; Lu, C.-T.; Lam, A.K.-y. Genetic Heterogeneity of Single Circulating Tumour Cells in Colorectal Carcinoma. Int. J. Mol. Sci. 2020, 21, 7766. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Smits, E.; Lardon, F.; Pauwels, P.; Deschoolmeester, V. Immune Checkpoint Modulation in Colorectal Cancer: What’s New and What to Expect. J. Immunol. Res. 2015, 2015, 158038. [Google Scholar] [CrossRef]
- Derakhshani, A.; Hashemzadeh, S.; Asadzadeh, Z.; Shadbad, M.A.; Rasibonab, F.; Safarpour, H.; Jafarlou, V.; Solimando, A.G.; Racanelli, V.; Singh, P.K.; et al. Cytotoxic T-Lymphocyte Antigen-4 in Colorectal Cancer: Another Therapeutic Side of Capecitabine. Cancers 2021, 13, 2414. [Google Scholar] [CrossRef]
- Fujiwara-Tani, R.; Sasaki, T.; Ohmori, H.; Luo, Y.; Goto, K.; Nishiguchi, Y.; Mori, S.; Nakashima, C.; Mori, T.; Miyagawa, Y.; et al. Concurrent Expression of CD47 and CD44 in Colorectal Cancer Promotes Malignancy. Pathobiology 2019, 86, 182–189. [Google Scholar] [CrossRef]
- Wellenstein, M.D.; de Visser, K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018, 48, 399–416. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Tada, H.; Takahashi, H.; Kuwabara-Yokobori, Y.; Ishii, H.; Ida, S.; Shino, M. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral Oncol. 2019, 89, 34–39. [Google Scholar] [CrossRef]
- Kondo, Y.; Hayashi, K.; Kawakami, K.; Miwa, Y.; Hayashi, H.; Yamamoto, M. KRAS mutation analysis of single circulating tumor cells from patients with metastatic colorectal cancer. BMC Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Zheng, Y.; Wittner, B.S.; Lee, R.J.; Zhu, H.; Broderick, K.T.; Desai, R.; Fox, D.B.; Brannigan, B.W.; Trautwein, J. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science 2015, 349, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, V.; Ebrahimi, F.; Islam, F.; Vider, J.; Qallandar, O.B.; Pillai, S.; Lu, C.-T.; Lam, A.K.-y. Tumour suppressor properties of miR-15a and its regulatory effects on BCL2 and SOX2 proteins in colorectal carcinomas. Exp. Cell Res. 2018, 370, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Gopalan, V.; Wahab, R.; Smith, R.A.; Qiao, B.; Lam, A.K.-Y. Stage dependent expression and tumor suppressive function of FAM134B (JK1) in colon cancer. Mol. Carcinog. 2017, 56, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
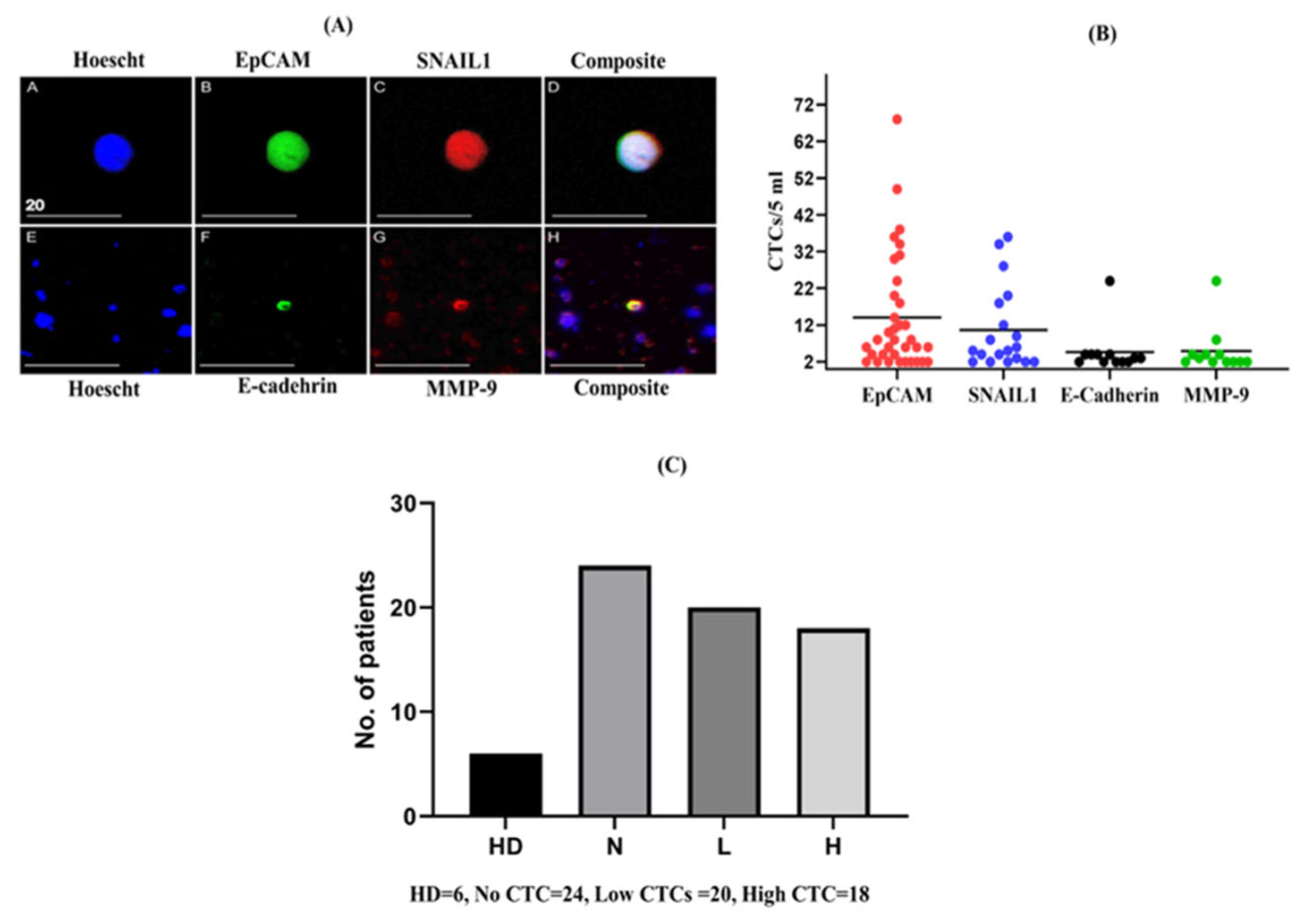
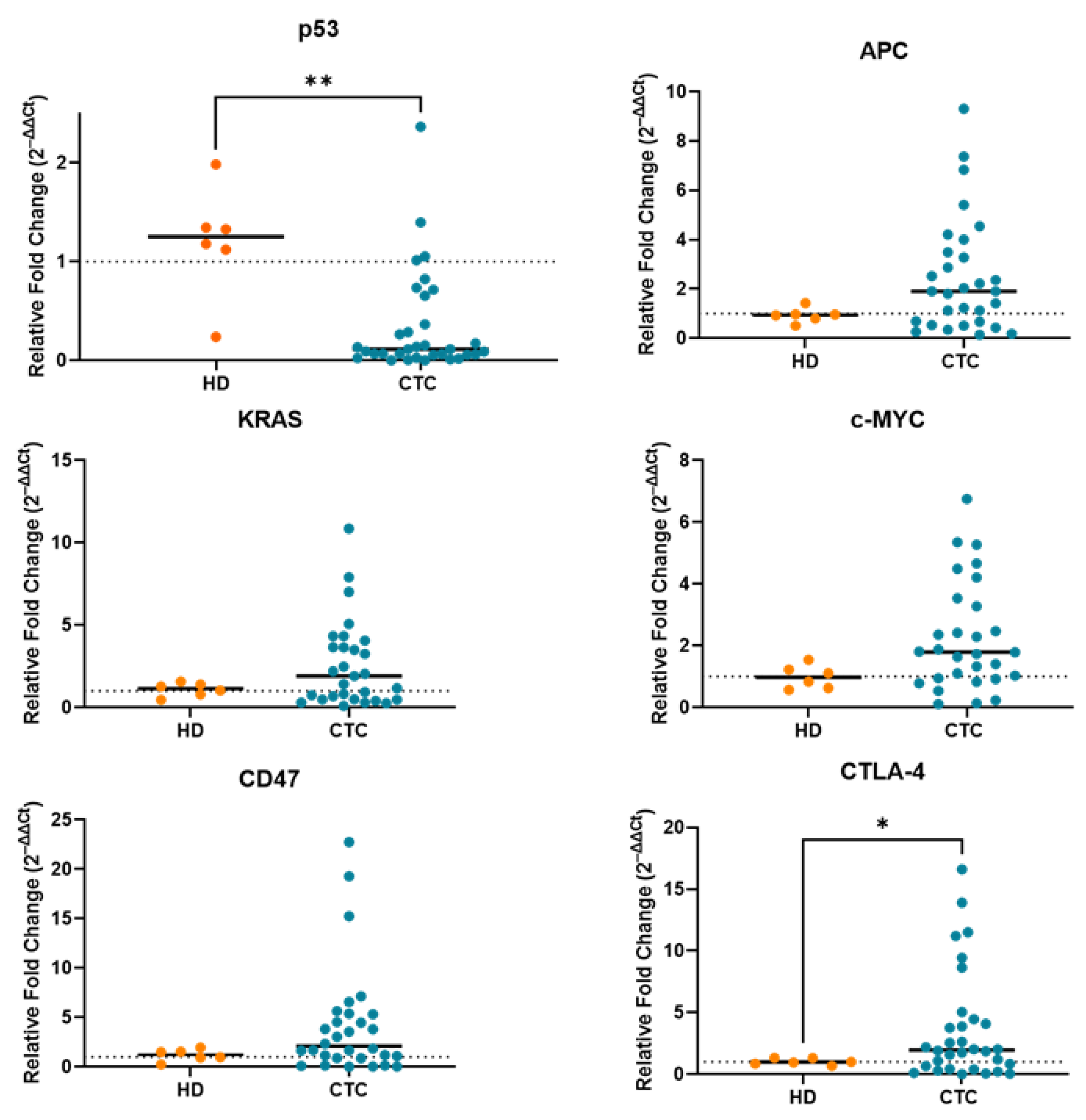
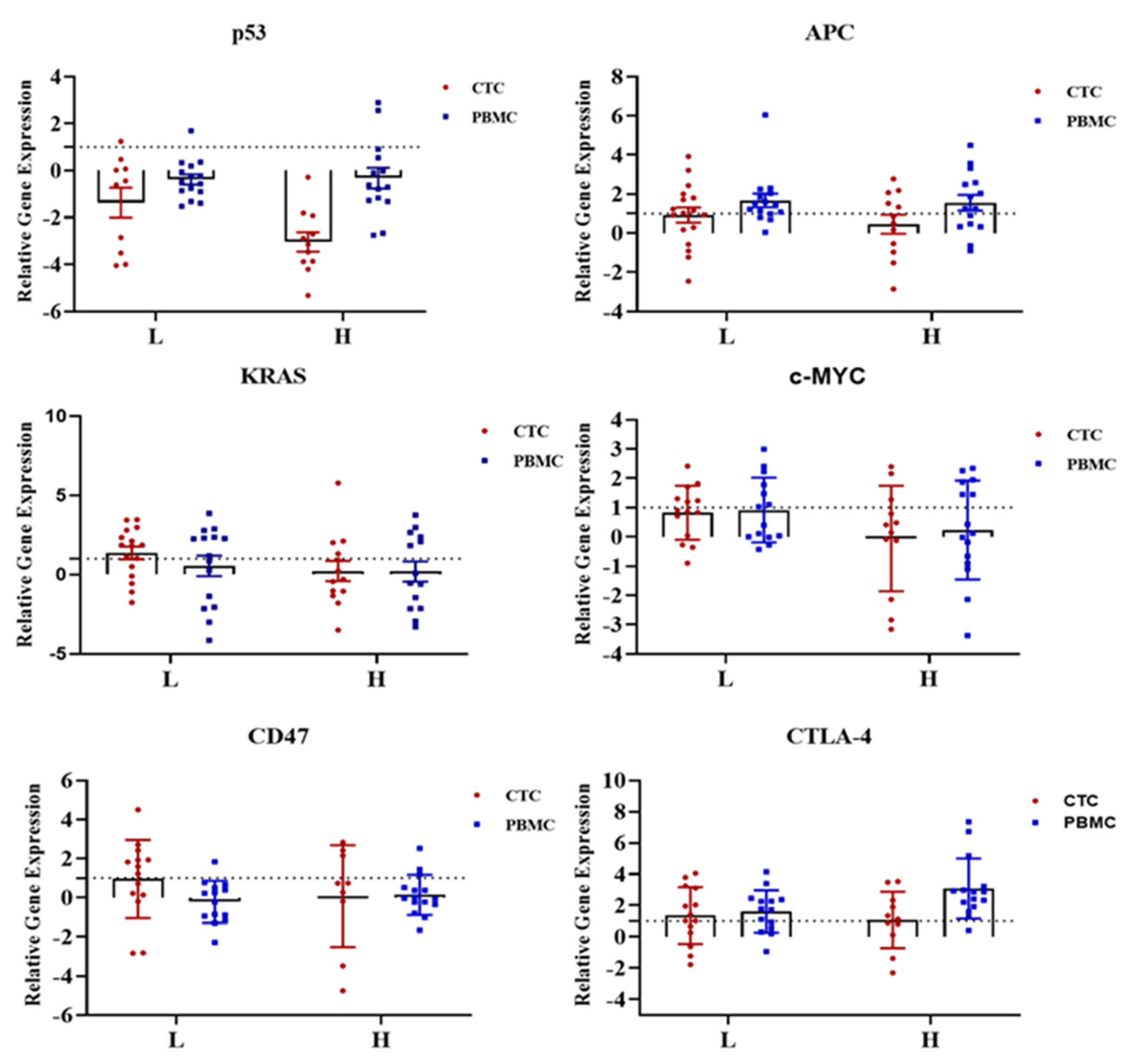



| Characteristics | Total (n = 62) | CTC = 0 (n = 24) | CTC < 10 (n = 20) | CTC ≥ 10 (n = 18) | p-Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 27 (43.55%) | 12 (44.44%) | 10 (37.04%) | 5 (18.52%) | 0.266 |
| Male | 35 (56.45%) | 12 (34.29%) | 10 (28.57%) | 13 (37.14%) | |
| Age | |||||
| ≤60 years | 13 (20.97%) | 4 (30.77%) | 6 (46.15%) | 3 (23.08%) | 0.483 |
| >60 years | 49 (79.03%) | 20 (40.82%) | 14 (28.57%) | 15 (30.61%) | |
| Size | |||||
| ≤50 mm | 44 (70.98%) | 19 (43.18%) | 16 (36.36%) | 9 (20.45%) | 0.051 |
| >50 mm | 18 (29.03%) | 5 (27.78%) | 4 (22.22%) | 9 (50%) | |
| Tumour perforation | |||||
| No | 58 (93.5%) | 23 (39.7%) | 20 (34.5) | 15 (25.9%) | 0.077 |
| With perforation | 4 (6.5%) | 1 (25%) | 0 (0.00%) | 3 (75%) | |
| Site | |||||
| Colon | 44 (70.97%) | 18 (40.91%) | 12 (27.27%) | 14 (31.82%) | 0.414 |
| Rectum | 18 (29.03%) | 6 (33.33%) | 8 (44.44%) | 4 (22.22%) | |
| Subtype | |||||
| Conventional | 51 (82.25%) | 21 (41.18%) | 19 (37.25%) | 11 (21.57%) | 0.019 |
| Mucinous | 11 (17.74%) | 3 (27.27%) | 1 (9.09%) | 7 (63.64%) | |
| Grade | |||||
| Well | 11 (17.74%) | 5 (45.45%) | 4 (36.36%) | 2 (18.18%) | 0.810 |
| Moderate | 46 (74.19%) | 18 (39.13%) | 14 (30.43%) | 14 (30.43%) | |
| Poor | 5 (8.06%) | 1 (20%) | 2 (40%) | 2 (40%) | |
| T stage | |||||
| I or II | 20 (32.26%) | 9 (45%) | 8 (40%) | 3 (15%) | 0.128 |
| III or IV | 42 (67.74%) | 15 (35.71%) | 12 (28.57%) | 15 (35.71%) | |
| Overall pathological stage | |||||
| I or II | 39 (62.9%) | 17 (43.59%) | 14 (35.90%) | 8 (20.51%) | 0.045 |
| III or IV | 23 (37.10%) | 7 (30.43%) | 6 (26.09%) | 10 (43.48%) | |
| Lymph node status | |||||
| Positive | 20 (32.26%) | 6 (30%) | 5 (25%) | 9 (45%) | 0.104 |
| Negative | 42 (67.74%) | 18 (42.86%) | 15 (35.71%) | 9 (21.43%) | |
| Distant metastasis | |||||
| Positive | 9 (14.52%) | 2 (22.22%) | 2 (22.22%) | 5 (55.56%) | 0.090 |
| Negative | 53 (85.48%) | 22 (30.2%) | 18 (69.8%) | 13 (24.53%) | |
| MSI status | |||||
| Stable | 52 (83.87%) | 20 (38.46%) | 16 (30.77%) | 16 (30.77%) | 0.643 |
| High | 10 (16.13%) | 4 (40%) | 4 (40%) | 2 (20%) |
| Characteristics | Total (29) | Low | High | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 11 (37.9%) | 4 (36.4%) | 7 (63.6%) | 0.313 |
| Male | 18 (62.1%) | 10 (55.6%) | 8 (44.4%) | |
| Age | ||||
| ≤60 years | 9 (31%) | 3 (33.3%) | 6 (66.7%) | 0.276 |
| >60 years | 20(69%) | 11 (55%) | 9 (45%) | |
| Size | ||||
| ≤50 mm | 21 (72.4%) | 8 (38.1%) | 13 (61.9%) | 0.071 |
| >50 mm | 8 (27.6%) | 6 (75.0%) | 2 (25.0%) | |
| Tumour perforation | ||||
| No | 26 (89.7%) | 11 (42.3%) | 15 (57.75%) | 0.029 |
| With perforation | 3 (10.3%) | 3 (100%) | 0 (0.0%) | |
| Site | ||||
| Colon | 16 (55.2%) | 9 (56.3%) | 7 (43.8%) | 0.339 |
| Rectum | 13 (44.8%) | 5 (38.5%) | 8 (61.5%) | |
| Grade | ||||
| Well | 6 (20.7%) | 2 (33.3%) | 4 (66.7%) | 0.082 |
| Moderate | 20 (69.0%) | 9(45.0%) | 11 (55.0%) | |
| Poor | 3 (10.3%) | 3 (100%) | 0 (0.0%) | |
| T stage | ||||
| I or II | 9 (31.0%) | 5 (55.6%) | 4 (44.4%) | 0.599 |
| III or IV | 20 (69.0%) | 9 (45.0%) | 11 (55.0%) | |
| Lymph node status | ||||
| Negative | 18 (62.1%) | 6 (33.3%) | 12 (66.7%) | 0.037 |
| Positive | 11 (37.9%) | 8 (72.7%) | 3 (27.3%) | |
| Distant metastasis | ||||
| Negative | 23 (79.3%) | 9 (39.1%) | 14(60.9%) | 0.046 |
| Positive | 6 (20.7%) | 5 (83.3%) | 1 (16.7%) | |
| Overall stage | ||||
| I or II | 16 (55.2%) | 4 (25.0%) | 12 (75.0%) | 0.004 |
| III or IV | 13 (44.8%) | 10 (76.9%) | 3 (23.1%) | |
| MSI status | ||||
| Stable | 26 (89.7%) | 12 (46.2%) | 14 (53.8%) | 0.498 |
| High | 3 (10.3%) | 2 (66.7%) | 1 (33.3%) | |
| CTC group | ||||
| Low | 16 (55.2%) | 5 (31.3%) | 11 (68.8%) | 0.039 |
| High | 13 (44.8%) | 9 (69.2%) | 4 (30.8%) |
| Characteristics | Total (n = 29) | Low | High | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 11 (37.9%) | 4 (36.4%) | 7 (63.6%) | 0.104 |
| Male | 18 (62.1%) | 2 (11.1%) | 16 (88.9%) | |
| Age | ||||
| ≤60 years | 8 (27.6%) | 3 (37.5%) | 5 (62.5%) | 0.185 |
| >60 years | 21(72.4%) | 3 (14.3%) | 18 (85.7%) | |
| Size | ||||
| ≤50 mm | 20 (69.0%) | 4 (20.0%) | 16 (80.0%) | 0.892 |
| >50 mm | 9 (31.0%) | 2 (22.2%) | 7 (77.8%) | |
| Tumour perforation | ||||
| No | 26 (89.7%) | 6 (23.1%) | 20 (76.9%) | 0.224 |
| With perforation | 3 (10.3%) | 0 (0.0%) | 3 (100.0%) | |
| Site | ||||
| Colon | 18 (62.1%) | 4 (22.2%) | 14 (77.8%) | 0.793 |
| Rectum | 11 (37.9%) | 2 (18.2%) | 9 (81.8%) | |
| Grade | ||||
| Well | 5 (17.2%) | 1 (20.0%) | 4 (80.0%) | 0.355 |
| Moderate | 20 (69.0%) | 5 (25.0%) | 15 (75.0%) | |
| Poor | 4 (13.8%) | 0 (0.0%) | 4 (100.0%) | |
| T stage | ||||
| I or II | 12 (41.40%) | 4 (33.3%) | 8 (66.7%) | 0.160 |
| III or IV | 17 (58.6%) | 2 (11.8%) | 15 (88.2%) | |
| Lymph node status | ||||
| Negative | 17 (58.6%) | 6 (35.3%) | 11 (64.7%) | 0.006 |
| Positive | 12 (41.4%) | 0 (0.0%) | 12 (100.0%) | |
| Distant metastasis | ||||
| Negative | 23 (79.3%) | 6 (26.1%) | 17 (73.9%) | 0.213 |
| Positive | 6 (20.7%) | 0 (0.0%) | 6 (100.0%) | |
| Overall stage | ||||
| I or II | 16 (55.2%) | 6 (37.5%) | 10 (62.5%) | 0.004 |
| III or IV | 13 (44.8%) | 0 (0.0%) | 13 (100.0%) | |
| MSI status | ||||
| Stable | 25 (86.2%) | 4 (16.0%) | 21 (84.0%) | 0.180 |
| High | 4 (13.8%) | 2 (50.0%) | 2 (50.0%) | |
| CTC group | ||||
| Low | 14 (48.3%) | 5 (35.7%) | 9 (64.3%) | 0.046 |
| High | 15 (51.7%) | 1 (6.7%) | 14 (93.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aktar, S.; Hamid, F.B.; Gamage, S.M.K.; Cheng, T.; Pakneshan, N.; Lu, C.T.; Islam, F.; Gopalan, V.; Lam, A.K.-y. Gene Expression Analysis of Immune Regulatory Genes in Circulating Tumour Cells and Peripheral Blood Mononuclear Cells in Patients with Colorectal Carcinoma. Int. J. Mol. Sci. 2023, 24, 5051. https://doi.org/10.3390/ijms24055051
Aktar S, Hamid FB, Gamage SMK, Cheng T, Pakneshan N, Lu CT, Islam F, Gopalan V, Lam AK-y. Gene Expression Analysis of Immune Regulatory Genes in Circulating Tumour Cells and Peripheral Blood Mononuclear Cells in Patients with Colorectal Carcinoma. International Journal of Molecular Sciences. 2023; 24(5):5051. https://doi.org/10.3390/ijms24055051
Chicago/Turabian StyleAktar, Sharmin, Faysal Bin Hamid, Sujani Madhurika Kodagoda Gamage, Tracie Cheng, Nahal Pakneshan, Cu Tai Lu, Farhadul Islam, Vinod Gopalan, and Alfred King-yin Lam. 2023. "Gene Expression Analysis of Immune Regulatory Genes in Circulating Tumour Cells and Peripheral Blood Mononuclear Cells in Patients with Colorectal Carcinoma" International Journal of Molecular Sciences 24, no. 5: 5051. https://doi.org/10.3390/ijms24055051
APA StyleAktar, S., Hamid, F. B., Gamage, S. M. K., Cheng, T., Pakneshan, N., Lu, C. T., Islam, F., Gopalan, V., & Lam, A. K.-y. (2023). Gene Expression Analysis of Immune Regulatory Genes in Circulating Tumour Cells and Peripheral Blood Mononuclear Cells in Patients with Colorectal Carcinoma. International Journal of Molecular Sciences, 24(5), 5051. https://doi.org/10.3390/ijms24055051






