Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant
Abstract
:1. Introduction
2. Reproductive Aging
2.1. Aging of the Ovaries
2.2. Aging of the Uterus
3. Oxidative Stress in Female Reproduction
3.1. Oxidative Stress in the Ovaries and Uterus
3.2. Oxidative Stress in the In Vitro Production of Embryos
4. Mesenchymal Stem Cells
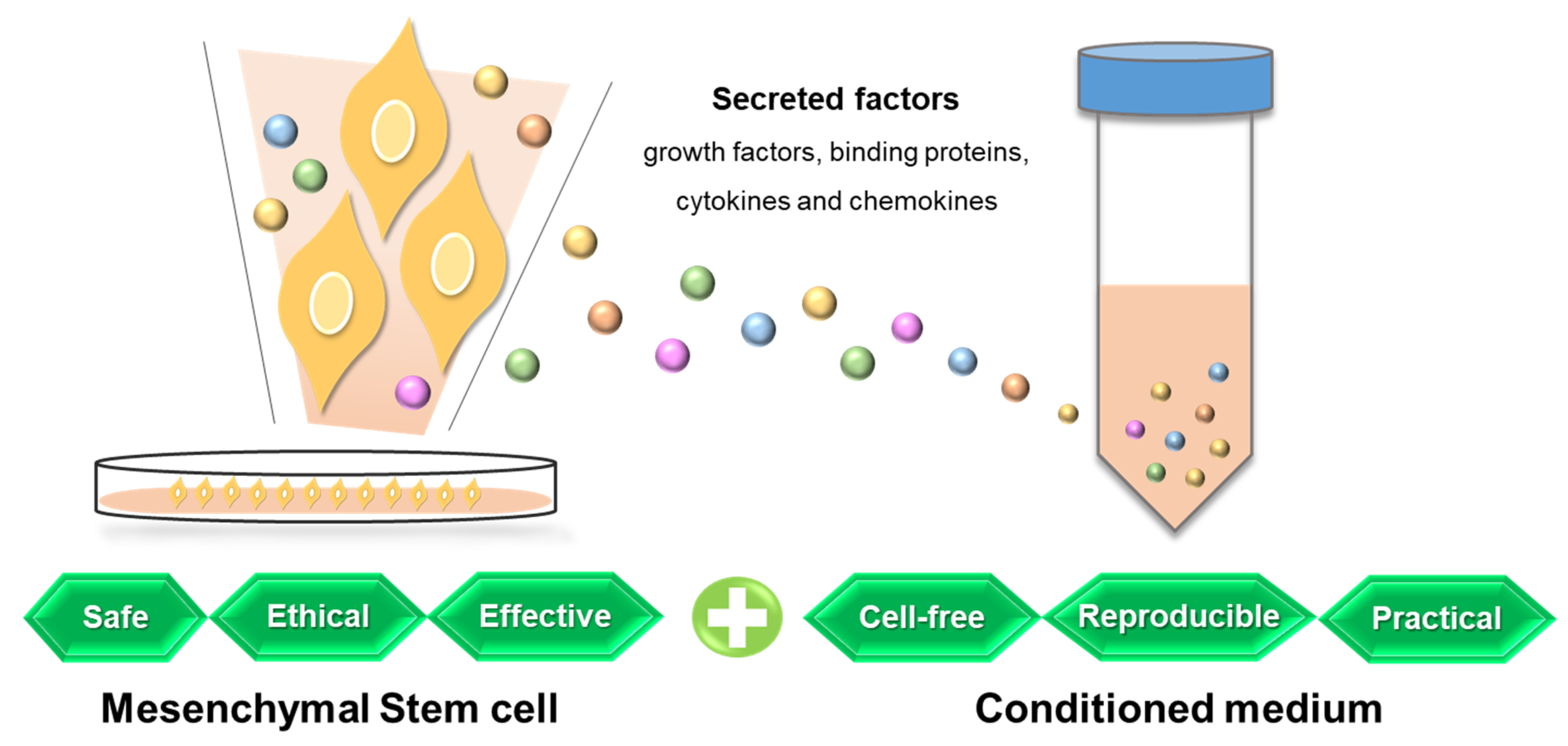
5. Mesenchymal Stem Cell Conditioned Medium
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cimadomo, D.; Fabozzi, G.; Vaiarelli, A.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. Impact of Maternal Age on Oocyte and Embryo Competence. Front. Endocrinol. 2018, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Guzeloglu-Kayisli, O.; Basar, M.; Arici, A. Basic aspects of implantation. Reprod. Biomed. Online 2007, 15, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Djahanbakhch, O.; Ezzati, M.; Zosmer, A. Reproductive ageing in women. J. Pathol. 2007, 211, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.P.; Ying, Y.; Wu, H.T.; Zhou, C.Q.; Xu, Y.W.; Wang, Q.; Li, J.; Shen, X.T.; Li, J. Comparison of Endocrine Profile and In Vitro Fertilization Outcome in Patients with PCOS, Ovulatory PCO, or Normal Ovaries. Int. J. Endocrinol. 2012, 2012, 492803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnhart, K.; Dunsmoor-Su, R.; Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 2002, 77, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Consonni, D.; Dridi, D.; Bracco, B.; Frattaruolo, M.P.; Somigliana, E. Uterine adenomyosis and in vitro fertilization outcome: A systematic review and meta-analysis. Hum. Reprod. 2014, 29, 964–977. [Google Scholar] [CrossRef] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders-A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Sohal, R.S. Role of oxidative stress and protein oxidation in the aging process. Free Radic. Biol. Med. 2002, 33, 37–44. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Buffenstein, R.; Edrey, Y.H.; Yang, T.; Mele, J. The oxidative stress theory of aging: Embattled or invincible? Insights from non-traditional model organisms. Age 2008, 30, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [Green Version]
- Tokmak, A.; Yildirim, G.; Sarikaya, E.; Cinar, M.; Bogdaycioglu, N.; Yilmaz, F.M.; Yilmaz, N. Increased oxidative stress markers may be a promising indicator of risk for primary ovarian insufficiency: A cross-sectional case control study. Rev. Bras. Ginecol. Obstet. 2015, 37, 411–416. [Google Scholar] [CrossRef]
- Behrman, H.R.; Kodaman, P.H.; Preston, S.L.; Gao, S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001, 8, S40–S42. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Dechend, R.; Poston, L. Oxidative stress and preeclampsia: Rationale for antioxidant clinical trials. Hypertension 2004, 44, 374–380. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]
- Duhig, K.; Chappell, L.C.; Shennan, A.H. Oxidative stress in pregnancy and reproduction. Obstet. Med. 2016, 9, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of Oxidative Stress on Age-Associated Decline in Oocyte Developmental Competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, R217–R227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamil, M.; Debbarh, H.; Aboulmaouahib, S.; Aniq Filali, O.; Mounaji, K.; Zarqaoui, M.; Saadani, B.; Louanjli, N.; Cadi, R. Reactive oxygen species in reproduction: Harmful, essential or both? Zygote 2020, 28, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Paramio, M.T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Casper, R.F.; Diez-Juan, A.; Simon, C.; Domar, A.D.; Frydman, R. Aging and the environment affect gamete and embryo potential: Can we intervene? Fertil. Steril. 2016, 105, 548–559. [Google Scholar] [CrossRef] [Green Version]
- du Plessis, S.S.; Makker, K.; Desai, N.R.; Agarwal, A. Impact of oxidative stress on IVF. Expert Rev. Obstet. Gynecol. 2008, 3, 539–554. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- Cagnone, G.; Sirard, M.A. The embryonic stress response to in vitro culture: Insight from genomic analysis. Reproduction 2016, 152, R247–R261. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Park, J.S. Usage of Human Mesenchymal Stem Cells in Cell-based Therapy: Advantages and Disadvantages. Dev. Reprod. 2017, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Parekkadan, B.; Milwid, J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunawardena, T.N.A.; Rahman, M.T.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media derived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019, 13, 569–586. [Google Scholar] [CrossRef]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, S. Aging: The Biology of Senescence. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Khalil, A.; Syngelaki, A.; Maiz, N.; Zinevich, Y.; Nicolaides, K.H. Maternal age and adverse pregnancy outcome: A cohort study. Ultrasound Obstet. Gynecol. 2013, 42, 634–643. [Google Scholar] [CrossRef]
- Kenny, L.C.; Lavender, T.; McNamee, R.; O’Neill, S.M.; Mills, T.; Khashan, A.S. Advanced maternal age and adverse pregnancy outcome: Evidence from a large contemporary cohort. PLoS ONE 2013, 8, e56583. [Google Scholar] [CrossRef] [Green Version]
- Lean, S.C.; Derricott, H.; Jones, R.L.; Heazell, A.E.P. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186287. [Google Scholar] [CrossRef] [Green Version]
- American Society for Reproductive Medicine. Assisted Reproductive Technologies (booklet). In Patient Fact Sheets and Booklets on Reproductivefacts.org; American Society for Reproductive Medicine: Birmingham, AL, USA, 2018. [Google Scholar]
- Li, Q.; Geng, X.; Zheng, W.; Tang, J.; Xu, B.; Shi, Q. Current understanding of ovarian aging. Sci. China Life Sci. 2012, 55, 659–669. [Google Scholar] [CrossRef]
- Amanvermez, R.; Tosun, M. An Update on Ovarian Aging and Ovarian Reserve Tests. Int. J. Fertil. Steril. 2016, 9, 411–415. [Google Scholar] [CrossRef]
- Nelson, S.M.; Telfer, E.E.; Anderson, R.A. The ageing ovary and uterus: New biological insights. Hum. Reprod. Update 2013, 19, 67–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Case, A.; Reproductive, E.; Infertility, C. Advanced reproductive age and fertility. J. Obstet. Gynaecol. Can. 2011, 33, 1165–1175. [Google Scholar] [CrossRef]
- Kawamara, K.; Kelsey, T.; Hiraike, O. Editorial: Ovarian Ageing: Pathophysiology and Recent Development of Maintaining Ovarian Reserve. Front. Endocrinol. 2020, 11, 591764. [Google Scholar] [CrossRef]
- Hale, G.E.; Burger, H.G. Hormonal changes and biomarkers in late reproductive age, menopausal transition and menopause. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 7–23. [Google Scholar] [CrossRef]
- Seifer, D.B.; DeJesus, V.; Hubbard, K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil. Steril. 2002, 78, 1046–1048. [Google Scholar] [CrossRef]
- Tatone, C.; Carbone, M.C.; Falone, S.; Aimola, P.; Giardinelli, A.; Caserta, D.; Marci, R.; Pandolfi, A.; Ragnelli, A.M.; Amicarelli, F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 2006, 12, 655–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, G.I.; Jurisicova, A.; Matikainen, T.; Moriyama, T.; Kim, M.R.; Takai, Y.; Pru, J.K.; Kolesnick, R.N.; Tilly, J.L. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J. 2005, 19, 860–862. [Google Scholar] [CrossRef] [Green Version]
- Gordo, A.C.; Rodrigues, P.; Kurokawa, M.; Jellerette, T.; Exley, G.E.; Warner, C.; Fissore, R. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol. Reprod. 2002, 66, 1828–1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenlaub-Ritter, U.; Vogt, E.; Yin, H.; Gosden, R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod. Biomed. Online 2004, 8, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.E.; Goodwin, P.; Klein, N.A.; Soules, M.R. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum. Reprod. 1996, 11, 2217–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Practice Committee of the American Society for Reproductive. Aging and infertility in women. Fertil. Steril. 2006, 86, S248–S252. [Google Scholar] [CrossRef] [PubMed]
- Kinugawa, C.; Murakami, T.; Okamura, K.; Yajima, A. Telomerase activity in normal ovaries and premature ovarian failure. Tohoku J. Exp. Med. 2000, 190, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Barritt, J.A.; Cohen, J.; Brenner, C.A. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reprod. Biomed. Online 2000, 1, 96–100. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.M.; Desquiret-Dumas, V.; Ferre-L’Hotellier, V.; Moriniere, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; Luderer, U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 2011, 84, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Defining ovarian reserve to better understand ovarian aging. Reprod. Biol. Endocrinol. 2011, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Balasch, J. Ageing and infertility: An overview. Gynecol. Endocrinol. 2010, 26, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Flamigni, C.; Borini, A.; Violini, F.; Bianchi, L.; Serrao, L. Oocyte donation: Comparison between recipients from different age groups. Hum. Reprod. 1993, 8, 2088–2092. [Google Scholar] [CrossRef]
- Cano, F.; Simon, C.; Remohi, J.; Pellicer, A. Effect of aging on the female reproductive system: Evidence for a role of uterine senescence in the decline in female fecundity. Fertil. Steril. 1995, 64, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.H.; Ho, P.C. Ageing and ART: A waste of time and money? Best Pract. Res. Clin. Obstet. Gynaecol. 2007, 21, 5–20. [Google Scholar] [CrossRef]
- Simmen, R.C.; Heard, M.E.; Simmen, A.M.; Montales, M.T.; Marji, M.; Scanlon, S.; Pabona, J.M. The Kruppel-like factors in female reproductive system pathologies. J. Mol. Endocrinol. 2015, 54, R89–R101. [Google Scholar] [CrossRef] [Green Version]
- Elmes, M.; Szyszka, A.; Pauliat, C.; Clifford, B.; Daniel, Z.; Cheng, Z.; Wathes, C.; McMullen, S. Maternal age effects on myometrial expression of contractile proteins, uterine gene expression, and contractile activity during labor in the rat. Physiol. Rep. 2015, 3, e12305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanikawa, N.; Ohtsu, A.; Kawahara-Miki, R.; Kimura, K.; Matsuyama, S.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Age-associated mRNA expression changes in bovine endometrial cells in vitro. Reprod. Biol. Endocrinol. 2017, 15, 63. [Google Scholar] [CrossRef] [Green Version]
- Woods, L.; Perez-Garcia, V.; Kieckbusch, J.; Wang, X.; DeMayo, F.; Colucci, F.; Hemberger, M. Decidualisation and placentation defects are a major cause of age-related reproductive decline. Nat. Commun. 2017, 8, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, L.; Hernandez-Garcia, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregon, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mironczuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A. Reduced glutathione: A radioprotector or a modulator of DNA-repair activity? Nutrients 2013, 5, 525–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Du, B.; Takahashi, K.; Ishida, G.M.; Nakahara, K.; Saito, H.; Kurachi, H. Usefulness of intraovarian artery pulsatility and resistance indices measurement on the day of follicle aspiration for the assessment of oocyte quality. Fertil. Steril. 2006, 85, 366–370. [Google Scholar] [CrossRef]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Pathak, D.; Venkatesh, S.; Kriplani, A.; Ammini, A.C.; Dada, R. Chromosomal abnormalities & oxidative stress in women with premature ovarian failure (POF). Indian J. Med. Res. 2012, 135, 92–97. [Google Scholar] [CrossRef] [PubMed]
- American College of, O.; Gynecologists’ Committee on Practice, B.-G. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet. Gynecol. 2018, 131, e157–e171. [Google Scholar] [CrossRef]
- Hilali, N.; Vural, M.; Camuzcuoglu, H.; Camuzcuoglu, A.; Aksoy, N. Increased prolidase activity and oxidative stress in PCOS. Clin. Endocrinol. 2013, 79, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid. Med. Cell. Longev. 2017, 2017, 4371714. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.D.; Clarke, C.L. Physiological action of progesterone in target tissues. Endocr. Rev. 1997, 18, 502–519. [Google Scholar] [CrossRef] [Green Version]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. Biomed. Online 2012, 25, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Ghafourifar, P.; Richter, C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997, 418, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Rizk, B.; Badr, M.; Talerico, C. Oxidative Stress and The Endometrium. In Studies on Women’s Health; Agarwal, A., Aziz, N., Rizk, B., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 61–74. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Tanaka, T. Xanthine oxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil. Steril. 2001, 75, 785–790. [Google Scholar] [CrossRef]
- Al-Jameil, N.; Aziz Khan, F.; Fareed Khan, M.; Tabassum, H. A brief overview of preeclampsia. J. Clin. Med. Res. 2014, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Yung, H.W.; Cindrova-Davies, T.; Charnock-Jones, D.S. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009, 30 (Suppl. A), S43–S48. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Allamaneni, S.S. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online 2004, 9, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, Y.; Noda, Y.; Mori, T.; Nakano, M. Increased generation of reactive oxygen species in embryos cultured in vitro. Free Radic. Biol. Med. 1993, 15, 69–75. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, B.; Sajko, M.C.; Vlaisavljevic, V. A prospective, randomized trial on the effect of atmospheric versus reduced oxygen concentration on the outcome of intracytoplasmic sperm injection cycles. Fertil. Steril. 2010, 94, 511–519. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Jung, S.K.; Kim, H.J.; Kim, C.L.; Lee, J.H.; You, J.Y.; Lee, E.S.; Lim, J.M.; Yun, S.J.; Song, J.Y.; Cha, S.H. Enhancing effects of serum-rich and cytokine-supplemented culture conditions on developing blastocysts and deriving porcine parthenogenetic embryonic stem cells. J. Vet. Sci. 2014, 15, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Kelley, R.L.; Gardner, D.K. Addition of interleukin-6 to mouse embryo culture increases blastocyst cell number and influences the inner cell mass to trophectoderm ratio. Clin. Exp. Reprod. Med. 2017, 44, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Poverini, R.; Lisi, R.; Lisi, F.; Berlinghieri, V.; Bielli, W.; Carfagna, P.; Costantino, A.; Iacomino, D.; Nicodemo, G. Common medium versus advanced IVF medium for cryopreserved oocytes in heterologous cycles. Cell Death Discov. 2018, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Raheem, K.A. Cytokines, growth factors and macromolecules as mediators of implantation in mammalian species. Int. J. Vet. Sci. Med. 2018, 6, S6–S14. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, R.; Shankar, M.B.; Munuswamy, D. Effect of alpha-tocopherol supplementation on in vitro maturation of sheep oocytes and in vitro development of preimplantation sheep embryos to the blastocyst stage. J. Assist. Reprod. Genet. 2010, 27, 483–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelrazik, H.; Sharma, R.; Mahfouz, R.; Agarwal, A. L-carnitine decreases DNA damage and improves the in vitro blastocyst development rate in mouse embryos. Fertil. Steril. 2009, 91, 589–596. [Google Scholar] [CrossRef]
- Zullo, G.; Albero, G.; Neglia, G.; De Canditiis, C.; Bifulco, G.; Campanile, G.; Gasparrini, B. L-ergothioneine supplementation during culture improves quality of bovine in vitro-produced embryos. Theriogenology 2016, 85, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Cambra, J.M.; Martinez, C.A.; Rodriguez-Martinez, H.; Martinez, E.A.; Cuello, C.; Gil, M.A. N-(2-mercaptopropionyl)-glycine enhances in vitro pig embryo production and reduces oxidative stress. Sci. Rep. 2020, 10, 18632. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, B.M.; Devaraj, M.; Gupta, P.S.; Ravindra, J.P.; Nandi, S. Effect of taurine and melatonin in the culture medium on buffalo in vitro embryo development. Reprod. Domest. Anim. 2009, 44, 12–16. [Google Scholar] [CrossRef]
- Ochota, M.; Pasieka, A.; Nizanski, W. Superoxide dismutase and taurine supplementation improves in vitro blastocyst yield from poor-quality feline oocytes. Theriogenology 2016, 85, 922–927. [Google Scholar] [CrossRef]
- Wang, X.; Falcone, T.; Attaran, M.; Goldberg, J.M.; Agarwal, A.; Sharma, R.K. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil. Steril. 2002, 78, 1272–1277. [Google Scholar] [CrossRef]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A.; International Society for Cellular, T. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Jt. Surg. Am. 1998, 80, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Southerland, S.S.; Souza, J.; Calcutt, A.F.; Cartledge, R.G. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am. Surg. 1999, 65, 22–26. [Google Scholar] [CrossRef]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Zvaifler, N.J.; Marinova-Mutafchieva, L.; Adams, G.; Edwards, C.J.; Moss, J.; Burger, J.A.; Maini, R.N. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000, 2, 477–488. [Google Scholar] [CrossRef] [Green Version]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [Green Version]
- In ‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [CrossRef]
- Alsalameh, S.; Amin, R.; Gemba, T.; Lotz, M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004, 50, 1522–1532. [Google Scholar] [CrossRef]
- In ‘t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.; Claas, F.H.; Fibbe, W.E.; Kanhai, H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef]
- Fan, C.G.; Tang, F.W.; Zhang, Q.J.; Lu, S.H.; Liu, H.Y.; Zhao, Z.M.; Liu, B.; Han, Z.B.; Han, Z.C. Characterization and neural differentiation of fetal lung mesenchymal stem cells. Cell Transplant. 2005, 14, 311–321. [Google Scholar] [CrossRef] [Green Version]
- McGuckin, C.P.; Forraz, N.; Baradez, M.O.; Navran, S.; Zhao, J.; Urban, R.; Tilton, R.; Denner, L. Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 2005, 38, 245–255. [Google Scholar] [CrossRef]
- Alviano, F.; Fossati, V.; Marchionni, C.; Arpinati, M.; Bonsi, L.; Franchina, M.; Lanzoni, G.; Cantoni, S.; Cavallini, C.; Bianchi, F.; et al. Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Biol. 2007, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.A.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.T.; Nguyen Thi Phuong, T.; Tien, N.L.B.; Tran, D.K.; Minh, L.B.; Thanh, V.V.; Gia Anh, P.; Pham, V.H.; Thi Nga, V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J. Clin. Med. 2019, 8, 917. [Google Scholar] [CrossRef] [Green Version]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin. Biol. Ther. 2009, 9, 879–887. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, B.S.; Kim, H.K.; Park, J.S.; Kim, K.J.; Choi, J.S.; Chung, S.J.; Kim, D.D.; Sung, J.H. Evidence supporting antioxidant action of adipose-derived stem cells: Protection of human dermal fibroblasts from oxidative stress. J. Dermatol. Sci. 2008, 49, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.C.; Lee, P.Y.; Cheng, H.; Tsai, C.H.; Ma, H.; Tarng, D.C. Adipose-derived stem cells exhibit antioxidative and antiapoptotic properties to rescue ischemic acute kidney injury in rats. Plast. Reconstr. Surg. 2013, 132, 940e–951e. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Jo, S.H.; Kim, W.H.; Kweon, O.K. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toda, A.; Okabe, M.; Yoshida, T.; Nikaido, T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J. Pharmacol. Sci. 2007, 105, 215–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.Y.; Lee, K.B.; Kim, M.K. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014, 47, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Tamagawa, T.; Oi, S.; Ishiwata, I.; Ishikawa, H.; Nakamura, Y. Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum. Cell 2007, 20, 77–84. [Google Scholar] [CrossRef]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef]
- de Godoy, M.A.; Saraiva, L.M.; de Carvalho, L.R.P.; Vasconcelos-Dos-Santos, A.; Beiral, H.J.V.; Ramos, A.B.; Silva, L.R.P.; Leal, R.B.; Monteiro, V.H.S.; Braga, C.V.; et al. Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J. Biol. Chem. 2018, 293, 1957–1975. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; McTaggart, S.J.; Johnson, D.W.; Gobe, G.C. Original article anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy 2012, 14, 162–172. [Google Scholar] [CrossRef]
- Guillen, M.I.; Platas, J.; Perez Del Caz, M.D.; Mirabet, V.; Alcaraz, M.J. Paracrine Anti-inflammatory Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in Human Monocytes. Front. Physiol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Laporte, C.; Tubbs, E.; Cristante, J.; Gauchez, A.S.; Pesenti, S.; Lamarche, F.; Cottet-Rousselle, C.; Garrel, C.; Moisan, A.; Moulis, J.M.; et al. Human mesenchymal stem cells improve rat islet functionality under cytokine stress with combined upregulation of heme oxygenase-1 and ferritin. Stem Cell Res. Ther. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Song, S.; Song, L.; Bi, Y.; Zhu, K.; Qiao, X.; Wang, H.; Gao, C.; Cai, H.; Ji, G. Mesenchymal stem cells alleviate aging in vitro and in vivo. Ann. Transl. Med. 2022, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Jin, J.; Lv, X.; Tao, J.; Wang, R.; Miao, D. Anti-aging Effect of Transplanted Amniotic Membrane Mesenchymal Stem Cells in a Premature Aging Model of Bmi-1 Deficiency. Sci. Rep. 2015, 5, 13975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Dong, Z.; Peng, Z.; Lu, F. Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by D-galactose. PLoS ONE 2014, 9, e97573. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Qian, C.; Ding, C.; Meng, Q.; Zou, Q.; Li, H. Fetal liver mesenchymal stem cells restore ovarian function in premature ovarian insufficiency by targeting MT1. Stem Cell Res. Ther. 2019, 10, 362. [Google Scholar] [CrossRef]
- Lu, X.; Bao, H.; Cui, L.; Zhu, W.; Zhang, L.; Xu, Z.; Man, X.; Chu, Y.; Fu, Q.; Zhang, H. hUMSC transplantation restores ovarian function in POI rats by inhibiting autophagy of theca-interstitial cells via the AMPK/mTOR signaling pathway. Stem Cell Res. Ther. 2020, 11, 268. [Google Scholar] [CrossRef]
- Seok, J.; Park, H.; Choi, J.H.; Lim, J.Y.; Kim, K.G.; Kim, G.J. Placenta-Derived Mesenchymal Stem Cells Restore the Ovary Function in an Ovariectomized Rat Model via an Antioxidant Effect. Antioxidants 2020, 9, 591. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.O.; Choi, S.-M.; Kim, H.-S. Mesenchymal stem cell-derived secretome and microvesicles as a cell-free therapeutics for neurodegenerative disorders. Tissue Eng. Regen. Med. 2013, 10, 93–101. [Google Scholar] [CrossRef]
- Yang, D.; Wang, W.; Li, L.; Peng, Y.; Chen, P.; Huang, H.; Guo, Y.; Xia, X.; Wang, Y.; Wang, H.; et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS ONE 2013, 8, e59020. [Google Scholar] [CrossRef]
- Bakondi, B.; Shimada, I.S.; Perry, A.; Munoz, J.R.; Ylostalo, J.; Howard, A.B.; Gregory, C.A.; Spees, J.L. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol. Ther. 2009, 17, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Cantinieaux, D.; Quertainmont, R.; Blacher, S.; Rossi, L.; Wanet, T.; Noel, A.; Brook, G.; Schoenen, J.; Franzen, R. Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: An original strategy to avoid cell transplantation. PLoS ONE 2013, 8, e69515. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.P.; Chio, C.C.; Cheong, C.U.; Chao, C.M.; Cheng, B.C.; Lin, M.T. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. 2013, 124, 165–176. [Google Scholar] [CrossRef]
- Mishra, P.J.; Mishra, P.J.; Banerjee, D. Cell-free derivatives from mesenchymal stem cells are effective in wound therapy. World J. Stem Cells 2012, 4, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wei, C.; Cheng, K.; Han, B.; Yan, J.; Zhang, M.; Peng, C.; Liu, Y. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J. Surg. Res. 2013, 183, 907–915. [Google Scholar] [CrossRef]
- van Koppen, A.; Joles, J.A.; van Balkom, B.W.; Lim, S.K.; de Kleijn, D.; Giles, R.H.; Verhaar, M.C. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS ONE 2012, 7, e38746. [Google Scholar] [CrossRef]
- Inukai, T.; Katagiri, W.; Yoshimi, R.; Osugi, M.; Kawai, T.; Hibi, H.; Ueda, M. Novel application of stem cell-derived factors for periodontal regeneration. Biochem. Biophys. Res. Commun. 2013, 430, 763–768. [Google Scholar] [CrossRef]
- Kawai, T.; Katagiri, W.; Osugi, M.; Sugimura, Y.; Hibi, H.; Ueda, M. Secretomes from bone marrow-derived mesenchymal stromal cells enhance periodontal tissue regeneration. Cytotherapy 2015, 17, 369–381. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, F.; Borsari, V.; Sartori, M.; Orciani, M.; Mattioli-Belmonte, M.; Fini, M. The use of cell conditioned medium for musculoskeletal tissue regeneration. J. Cell. Physiol. 2018, 233, 4423–4442. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vilchez, T.; Sierra-Sanchez, A.; Sanchez-Diaz, M.; Quinones-Vico, M.I.; Sanabria-de-la-Torre, R.; Martinez-Lopez, A.; Arias-Santiago, S. Mesenchymal Stromal Cell-Conditioned Medium for Skin Diseases: A Systematic Review. Front. Cell Dev. Biol. 2021, 9, 654210. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.D.; Basalova, N.A.; Kirpatovsky, V.I.; Ohobotov, D.A.; Grigorieva, O.A.; Balabanyan, V.Y.; Kamalov, A.A.; Efimenko, A.Y. Application of rat cryptorchidism model for the evaluation of mesenchymal stromal cell secretome regenerative potential. Biomed. Pharmacother. 2019, 109, 1428–1436. [Google Scholar] [CrossRef]
- Jin, S.E.; Sung, J.H. Hair regeneration using adipose-derived stem cells. Histol. Histopathol. 2016, 31, 249–256. [Google Scholar] [CrossRef]
- Kay, A.G.; Long, G.; Tyler, G.; Stefan, A.; Broadfoot, S.J.; Piccinini, A.M.; Middleton, J.; Kehoe, O. Mesenchymal Stem Cell-Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis. Sci. Rep. 2017, 7, 18019. [Google Scholar] [CrossRef] [Green Version]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al-Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M.; et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ra, K.; Oh, H.J.; Kim, G.A.; Kang, S.K.; Ra, J.C.; Lee, B.C. High Frequency of Intravenous Injection of Human Adipose Stem Cell Conditioned Medium Improved Embryo Development of Mice in Advanced Maternal Age through Antioxidant Effects. Animals 2020, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Ra, K.; Oh, H.J.; Kim, E.Y.; Kang, S.K.; Ra, J.C.; Kim, E.H.; Lee, B.C. Anti-Oxidative Effects of Human Adipose Stem Cell Conditioned Medium with Different Basal Medium during Mouse Embryo In Vitro Culture. Animals 2020, 10, 1414. [Google Scholar] [CrossRef]
- Ra, K.; Oh, H.J.; Kim, E.Y.; Kang, S.K.; Ra, J.C.; Kim, E.H.; Park, S.C.; Lee, B.C. Comparison of Anti-Oxidative Effect of Human Adipose- and Amniotic Membrane-Derived Mesenchymal Stem Cell Conditioned Medium on Mouse Preimplantation Embryo Development. Antioxidants 2021, 10, 268. [Google Scholar] [CrossRef]
- Akbari, H.; Eftekhar Vaghefi, S.H.; Shahedi, A.; Habibzadeh, V.; Mirshekari, T.R.; Ganjizadegan, A.; Mollaei, H.; Ahmadi, M.; Nematollahi-Mahani, S.N. Mesenchymal Stem Cell-Conditioned Medium Modulates Apoptotic and Stress-Related Gene Expression, Ameliorates Maturation and Allows for the Development of Immature Human Oocytes after Artificial Activation. Genes 2017, 8, 371. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Yan, L.; Xin, Z.; Hao, J.; Liu, W.; Wang, S.; Liao, S.; Wang, H.; Yang, X. Protective effects of human umbilical cord mesenchymal stem cell-derived conditioned medium on ovarian damage. J. Mol. Cell. Biol. 2020, 12, 372–385. [Google Scholar] [CrossRef]
- Kalehoei, E.; Moradi, M.; Azadbakht, M.; Zhaleh, H.; Parvini, M.; Cheraghbaeigi, S.; Saghari, S. In vitro maturation medium supplementation: Utilization of repaglinide, L-carnitine, and mesenchymal stem cell-conditioned medium to improve developmental competence of oocytes derived from endometriosis mouse models. Braz. J. Med. Biol. Res. 2022, 55, e11948. [Google Scholar] [CrossRef]
- Park, A.; Oh, H.J.; Ji, K.; Choi, E.M.; Kim, D.; Kim, E.; Kim, M.K. Effect of Passage Number of Conditioned Medium Collected from Equine Amniotic Fluid Mesenchymal Stem Cells: Porcine Oocyte Maturation and Embryo Development. Int. J. Mol. Sci. 2022, 23, 6569. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.; Kwon, Y.; Park, K.S.; Jeong, J.H.; Choi, S.J.; Bang, S.I.; Chang, J.W.; Lee, C. Comparative Proteomic Analysis of the Mesenchymal Stem Cells Secretome from Adipose, Bone Marrow, Placenta and Wharton’s Jelly. Int. J. Mol. Sci. 2021, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, F.; Yan, B.; Xiao, D.J.; Wang, Y.S.; Liu, H. Comparison of the Cytokine Profile in Mesenchymal Stem Cells from Human Adipose, Umbilical Cord, and Placental Tissues. Cell. Reprogram. 2021, 23, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Elman, J.S.; Li, M.; Wang, F.; Gimble, J.M.; Parekkadan, B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J. Inflamm. 2014, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Lei, D.; Ouyang, W.; Ren, J.; Li, H.; Hu, J.; Huang, S. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed. Res. Int. 2014, 2014, 109389. [Google Scholar] [CrossRef] [Green Version]
- Konala, V.B.R.; Bhonde, R.; Pal, R. Secretome studies of mesenchymal stromal cells (MSCs) isolated from three tissue sources reveal subtle differences in potency. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 689–700. [Google Scholar] [CrossRef]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 4290. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Guo, S.; Wei, C.; Li, H.; Chen, L.; Yin, C.; Zhang, C. The Comparison of Adipose Stem Cell and Placental Stem Cell in Secretion Characteristics and in Facial Antiaging. Stem Cells Int. 2016, 2016, 7315830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shologu, N.; Scully, M.; Laffey, J.G.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.J.; Song, H.S.; Bhang, S.; Lee, S.; Kang, B.G.; Lee, J.C.; An, J.; Cha, C.I.; Nam, D.H.; Kim, B.S.; et al. Therapeutic effects of human adipose stem cell-conditioned medium on stroke. J. Neurosci. Res. 2012, 90, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Lee, S.; Shin, J.Y.; Lee, T.J.; Jang, H.K.; Kim, B.S. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol. Ther. 2014, 22, 862–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, S.M.; Hassan, Z.M.; Hossein-Khannazer, N.; Pourfathollah, A.A.; Soudi, S. Investigating the route of administration and efficacy of adipose tissue-derived mesenchymal stem cells and conditioned medium in type 1 diabetic mice. Inflammopharmacology 2020, 28, 585–601. [Google Scholar] [CrossRef]

| Antioxidants | Target | Effects of Treatment | Reference |
|---|---|---|---|
| α-tocopherol | Ovine embryos | ↑ Cleavage rate ↑ Embryo developmental rate ↑ Blastocyst formation ↑ Total cell number of blastocyst | [104] |
| L-Carnitine | Mouse embryo | ↑ Blastocyst development rate ↓ DNA damage | [105] |
| L-ergothioneine | Bovine embryos | ↑ Blastocyst hatching rate ↑ ICM: total cells ratio ↓ Apoptotic cells | [106] |
| N-(2-mercaptopropionyl)-glycine | Porcine embryo | ↑ Blastocyst formation ↓ Intracellular reactive oxygen ↓ Oxidative stress-related gene expression | [107] |
| Melatonin & Taurine | Buffalo embryo | ↑ meiotic maturation rate ↑ Transferable embryo yield | [108] |
| SOD & Taurine | Feline embryo | ↑ Oocyte maturation ↓ Oocyte degeneration ↑ Blastocyst number and quality | [109] |
| Vitamin C &E | Mouse embryo | ↓ Embryo toxicity ↑ Blastocyst development rate | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ra, K.; Park, S.C.; Lee, B.C. Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant. Int. J. Mol. Sci. 2023, 24, 5053. https://doi.org/10.3390/ijms24055053
Ra K, Park SC, Lee BC. Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant. International Journal of Molecular Sciences. 2023; 24(5):5053. https://doi.org/10.3390/ijms24055053
Chicago/Turabian StyleRa, Kihae, Se Chang Park, and Byeong Chun Lee. 2023. "Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant" International Journal of Molecular Sciences 24, no. 5: 5053. https://doi.org/10.3390/ijms24055053





