The Chemokine Receptor CCR1 Mediates Microglia Stimulated Glioma Invasion
Abstract
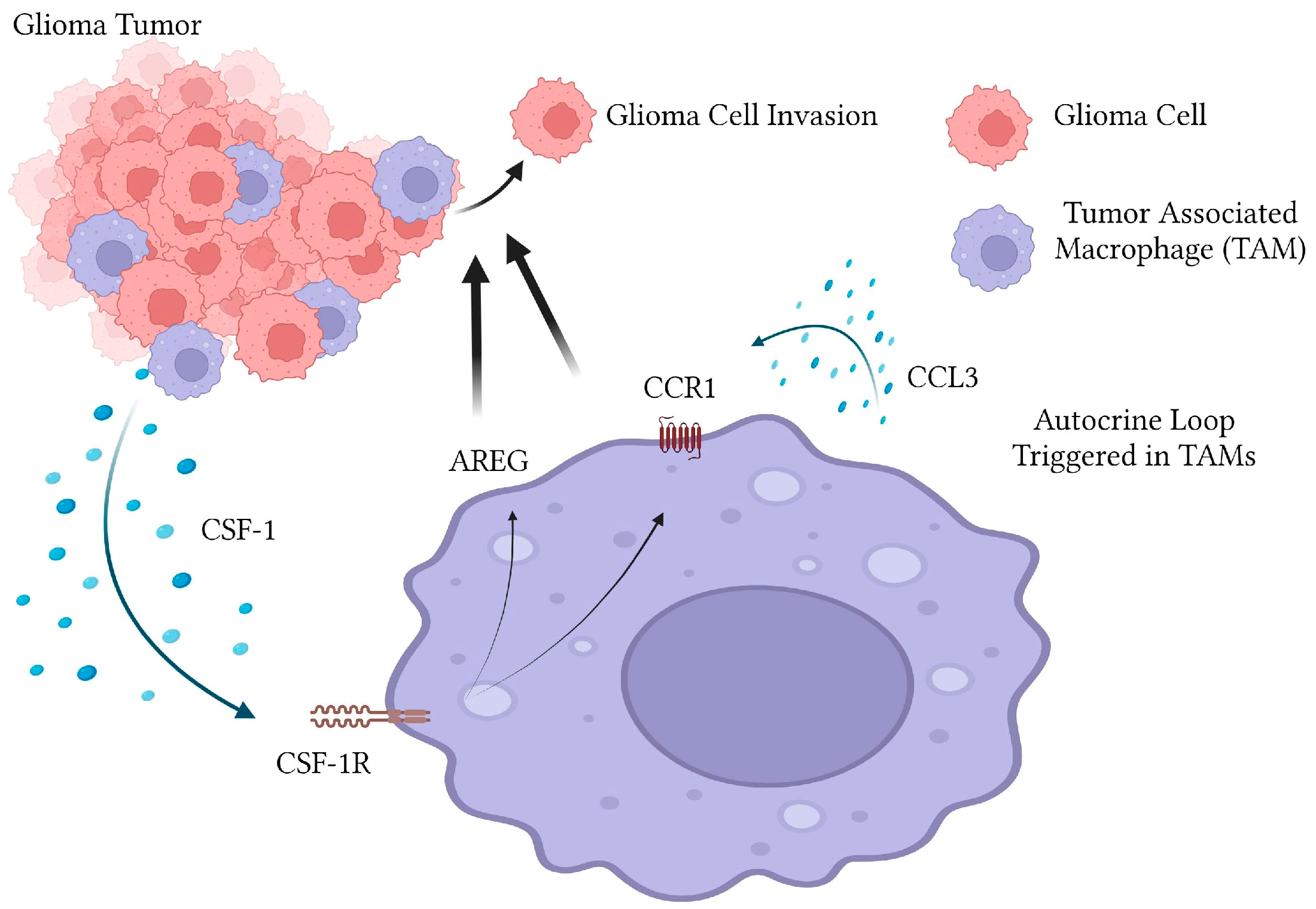
:1. Introduction
2. Results
2.1. Pharmacological Inhibition of CCR1 Prevents Microglial-Activation of Glioma Invasion
2.2. Glioma Conditioned Media Induces CCR1 Expression in Microglia
2.3. Glioma Conditioned Media Induces CCR1 Ligand Expression in Microglia
2.4. CSF-1R Signaling Partially Controls CCR1 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Glioma Invasion Assays
4.3. Quantitative RT-PCR
4.4. Immunofluorescence
4.5. ELISA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Van den Bent, M.J.; Weller, M.; Wen, P.Y.; Kros, J.M.; Aldape, K.; Chang, S. A Clinical Perspective on the 2016 WHO Brain Tumor Classification and Routine Molecular Diagnostics. Neuro-Oncology 2017, 19, 614–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Xiao, M.; Li, X.; Xin, L.; Song, J.; Zhan, Q.; Wang, C.; Zhang, Q.; Yuan, X.; Tan, Y.; et al. Origin, Activation, and Targeted Therapy of Glioma-Associated Macrophages. Front. Immunol. 2022, 13, 974996. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.S.; Vinnakota, K.; van Rooijen, N.; Kiwit, J.; Synowitz, M.; Glass, R.; Kettenmann, H. Minocycline Reduces Glioma Expansion and Invasion by Attenuating Microglial MT1-MMP Expression. Brain. Behav. Immun. 2011, 25, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, M.; Markovic, D.; Gabrusiewicz, K.; Synowitz, M.; Glass, R.; Zawadzka, M.; Wesolowska, A.; Kettenmann, H.; Kaminska, B. The Invasion Promoting Effect of Microglia on Glioblastoma Cells Is Inhibited by Cyclosporin A. Brain 2007, 130, 476–489. [Google Scholar] [CrossRef]
- Markovic, D.S.; Glass, R.; Synowitz, M.; Van Rooijen, N.; Kettenmann, H. Microglia Stimulate the Invasiveness of Glioma Cells by Increasing the Activity of Metalloprotease-2. J. Neuropathol. Exp. Neurol. 2005, 64, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Markovic, D.S.; Vinnakota, K.; Chirasani, S.; Synowitz, M.; Raguet, H.; Stock, K.; Sliwa, M.; Lehmann, S.; Kälin, R.; van Rooijen, N.; et al. Gliomas Induce and Exploit Microglial MT1-MMP Expression for Tumor Expansion. Proc. Natl. Acad. Sci. USA 2009, 106, 12530–12535. [Google Scholar] [CrossRef] [Green Version]
- Coniglio, S.J.; Segall, J.E. Review: Molecular Mechanism of Microglia Stimulated Glioblastoma Invasion. Matrix Biol. 2013, 32, 372–380. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Valilou, S.F.; Soltani, A.; Ahmadi, S.; Abarghan, Y.J. Tumor-Associated Macrophages: Role in Cancer Development and Therapeutic Implications. Cell Oncol. 2019, 42, 591–608. [Google Scholar] [CrossRef]
- Carron, E.C.; Homra, S.; Rosenberg, J.; Coffelt, S.B.; Kittrell, F.; Zhang, Y.; Creighton, C.J.; Fuqua, S.A.; Medina, D.; Machado, H.L. Macrophages Promote the Progression of Premalignant Mammary Lesions to Invasive Cancer. Oncotarget 2017, 8, 50731–50746. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Tirado, C.; Entenberg, D.; Li, J.; Qian, B.Z.; Condeelis, J.S.; Pollard, J.W. Interleukin 4 Controls the Pro-Tumoral Role of Macrophages in Mammary Cancer Pulmonary Metastasis in Mice. Cancers 2022, 14, 4336. [Google Scholar] [CrossRef]
- Hesketh, A.J.; Maloney, C.; Behr, C.A.; Edelman, M.C.; Glick, R.D.; Al-Abed, Y.; Symons, M.; Soffer, S.Z.; Steinberg, B.M. The Macrophage Inhibitor CNI-1493 Blocks Metastasis in a Mouse Model of Ewing Sarcoma through Inhibition of Extravasation. PLoS ONE 2015, 10, e0145197. [Google Scholar] [CrossRef]
- Cannarile, M.A.; Weisser, M.; Jacob, W.; Jegg, A.-M.; Ries, C.H.; Rüttinger, D. Colony-Stimulating Factor 1 Receptor (CSF1R) Inhibitors in Cancer Therapy. J. Immunother. Cancer 2017, 5, 53. [Google Scholar] [CrossRef]
- De Palma, M.; Lewis, C.E. Cancer: Macrophages Limit Chemotherapy. Nature 2011, 472, 303–304. [Google Scholar] [CrossRef]
- Strachan, D.C.; Ruffell, B.; Oei, Y.; Bissell, M.J.; Coussens, L.M.; Pryer, N.; Daniel, D. CSF1R Inhibition Delays Cervical and Mammary Tumor Growth in Murine Models by Attenuating the Turnover of Tumor-Associated Macrophages and Enhancing Infiltration by CD8(+) T Cells. Oncoimmunology 2013, 2, e26968. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, T.; Doughty-Shenton, D.; Cassetta, L.; Fragkogianni, S.; Brownlie, D.; Kato, Y.; Carragher, N.; Pollard, J.W. Monocytes Differentiate to Immune Suppressive Precursors of Metastasis-Associated Macrophages in Mouse Models of Metastatic Breast Cancer. Front. Immunol. 2018, 8, 2004. [Google Scholar] [CrossRef] [Green Version]
- Boimel, P.J.; Smirnova, T.; Zhou, Z.N.; Wyckoff, J.; Park, H.; Coniglio, S.J.; Qian, B.-Z.; Stanley, E.R.; Cox, D.; Pollard, J.W.; et al. Contribution of CXCL12 Secretion to Invasion of Breast Cancer Cells. Breast Cancer Res. 2012, 14, R23. [Google Scholar] [CrossRef] [Green Version]
- Ide, H.; Seligson, D.B.; Memarzadeh, S.; Xin, L.; Horvath, S.; Dubey, P.; Flick, M.B.; Kacinski, B.M.; Palotie, A.; Witte, O.N. Expression of Colony-Stimulating Factor 1 Receptor during Prostate Development and Prostate Cancer Progression. Proc. Natl. Acad. Sci. USA 2002, 99, 14404–14409. [Google Scholar] [CrossRef] [Green Version]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Goswami, S.; Sahai, E.; Wyckoff, J.B.; Cammer, M.; Cox, D.; Pixley, F.J.; Stanley, E.R.; Segall, J.E.; Condeelis, J.S. Macrophages Promote the Invasion of Breast Carcinoma Cells via a Colony-Stimulating Factor-1/Epidermal Growth Factor Paracrine Loop. Cancer Res. 2005, 65, 5278–5283. [Google Scholar] [CrossRef] [Green Version]
- Coniglio, S.J.; Eugenin, E.; Dobrenis, K.; Stanley, E.R.; West, B.L.; Symons, M.H.; Segall, J.E. Microglial Stimulation of Glioblastoma Invasion Involves Epidermal Growth Factor Receptor (EGFR) and Colony Stimulating Factor 1 Receptor (CSF-1R) Signaling. Mol. Med. 2012, 18, 519–527. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R Inhibition Alters Macrophage Polarization and Blocks Glioma Progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butowski, N.; Colman, H.; De Groot, J.F.; Omuro, A.M.; Nayak, L.; Wen, P.Y.; Cloughesy, T.F.; Marimuthu, A.; Haidar, S.; Perry, A.; et al. Orally Administered Colony Stimulating Factor 1 Receptor Inhibitor PLX3397 in Recurrent Glioblastoma: An Ivy Foundation Early Phase Clinical Trials Consortium Phase II Study. Neuro. Oncol. 2015, 18, nov245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monestime, S.; Lazaridis, D. Pexidartinib (TURALIOTM): The First FDA-Indicated Systemic Treatment for Tenosynovial Giant Cell Tumor. Drugs R D 2020, 20, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.R.P.; Lee, R.J.; West, B.L.; Green, K.N. Characterizing Newly Repopulated Microglia in the Adult Mouse: Impacts on Animal Behavior, Cell Morphology, and Neuroinflammation. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Luster, A.D. Chemokines and Their Receptors: Drug Targets in Immunity and Inflammation. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 171–197. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of Chemokine Receptors in Breast Cancer Metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O.; Nomiyama, H. The Chemokine and Chemokine Receptor Superfamilies and Their Molecular Evolution. Genome Biol. 2006, 7, 243. [Google Scholar] [CrossRef]
- Kielian, T.; van Rooijen, N.; Hickey, W.F. MCP-1 Expression in CNS-1 Astrocytoma Cells: Implications for Macrophage Infiltration into Tumors in Vivo. J. Neurooncol. 2002, 56, 1–12. [Google Scholar] [CrossRef]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 Inhibition Reduces Tumor Myeloid Cells and Unmasks a Checkpoint Inhibitor Effect to Slow Progression of Resistant Murine Gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138. [Google Scholar] [CrossRef]
- Merritt, J.R.; Liu, J.; Quadros, E.; Morris, M.L.; Liu, R.; Zhang, R.; Jacob, B.; Postelnek, J.; Hicks, C.M.; Chen, W.; et al. Novel Pyrrolidine Ureas as C-C Chemokine Receptor 1 (CCR1) Antagonists. J. Med. Chem. 2009, 52, 1295–1301. [Google Scholar] [CrossRef]
- Coniglio, S.; Miller, I.; Symons, M.; Segall, J.E. Coculture Assays to Study Macrophage and Microglia Stimulation of Glioblastoma Invasion. J. Vis. Exp. 2016, 116, 53990. [Google Scholar] [CrossRef]
- Coniglio, S.J.; Segall, J.E. Microglial-Stimulation of Glioma Invasion Involves the EGFR Ligand Amphiregulin. PLoS ONE 2021, 16, e0260252. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Dang, W.Q.; Cao, M.F.; Xiao, J.F.; Lv, S.Q.; Jiang, W.J.; Yao, X.H.; Lu, H.M.; Miao, J.Y.; et al. CCL8 Secreted by Tumor-Associated Macrophages Promotes Invasion and Stemness of Glioblastoma Cells via ERK1/2 Signaling. Lab. Investig. 2019, 8, 619–629. [Google Scholar] [CrossRef]
- Pham, K.; Luo, D.; Liu, C.; Harrison, J.K. CCL5, CCR1 and CCR5 in Murine Glioblastoma: Immune Cell Infiltration and Survival Rates Are Not Dependent on Individual Expression of Either CCR1 or CCR5. J. Neuroimmunol. 2012, 246, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-Induced Chemokine Cascade Promotes Breast Cancer Metastasis by Enhancing Retention of Metastasis-Associated Macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Swamydas, M.; Ricci, K.; Rego, S.L.; Dréau, D. Mesenchymal Stem Cell-Derived CCL-9 and CCL-5 Promote Mammary Tumor Cell Invasion and the Activation of Matrix Metalloproteinases. Cell Adh. Migr. 2013, 7, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Barbai, T.; Fejős, Z.; Puskas, L.G.; Tímár, J.; Rásó, E. The Importance of Microenvironment: The Role of CCL8 in Metastasis Formation of Melanoma. Oncotarget 2015, 6, 29111–29128. [Google Scholar] [CrossRef] [Green Version]
- Itatani, Y.; Kawada, K.; Fujishita, T.; Kakizaki, F.; Hirai, H.; Matsumoto, T.; Iwamoto, M.; Inamoto, S.; Hatano, E.; Hasegawa, S.; et al. Loss of SMAD4 from Colorectal Cancer Cells Promotes CCL15 Expression to Recruit CCR1+ Myeloid Cells and Facilitate Liver Metastasis. Gastroenterology 2013, 145, 1064–1075.e11. [Google Scholar] [CrossRef] [Green Version]
- Inamoto, S.; Itatani, Y.; Yamamoto, T.; Minamiguchi, S.; Hirai, H.; Iwamoto, M.; Hasegawa, S.; Taketo, M.M.; Sakai, Y.; Kawada, K. Loss of SMAD4 Promotes Colorectal Cancer Progression by Accumulation of Myeloid-Derived Suppressor Cells through the CCL15-CCR1 Chemokine Axis. Clin. Cancer Res. 2016, 22, 492–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, H.; Fujishita, T.; Kurimoto, K.; Miyachi, H.; Kitano, S.; Inamoto, S.; Itatani, Y.; Saitou, M.; Maekawa, T.; Taketo, M.M. CCR1-Mediated Accumulation of Myeloid Cells in the Liver Microenvironment Promoting Mouse Colon Cancer Metastasis. Clin. Exp. Metastasis 2014, 31, 977–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, Z.; Zheng, B.; Shi, Y.; Duan, M.; Ma, L.; Wang, Z.-C.; Dong, L.-Q.; Dong, P.-P.; Shi, J.-Y.; et al. CCL15 Recruits Suppressive Monocytes to Facilitate Immune Escape and Disease Progression in Hepatocellular Carcinoma. Hepatology 2019, 69, 143–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messex, J.K.; Adams, K.L.A.; Hawkins, W.G.; Denardo, D.; Bardeesy, N.; Billadeau, D.D.; Liou, G.Y. Oncogenic Kras-Mediated Cytokine CCL15 Regulates Pancreatic Cancer Cell Migration and Invasion through ROS. Cancers 2022, 14, 2153. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Itatani, Y.; Inamoto, S.; Okamura, R.; Iwamoto, M.; Miyamoto, E.; Chen-Yoshikawa, T.F.; Hirai, H.; Hasegawa, S.; et al. Loss of SMAD4 Promotes Lung Metastasis of Colorectal Cancer by Accumulation of CCR1 + Tumor-Associated Neutrophils through CCL15-CCR1 Axis. Clin. Cancer Res. 2017, 23, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Akram, I.G.; Georges, R.; Hielscher, T.; Adwan, H.; Berger, M.R. The Chemokines CCR1 and CCRL2 Have a Role in Colorectal Cancer Liver Metastasis. Tumor Biol. 2016, 37, 2461–2471. [Google Scholar] [CrossRef]
- Kitamura, T.; Kometani, K.; Hashida, H.; Matsunaga, A.; Miyoshi, H.; Hosogi, H.; Aoki, M.; Oshima, M.; Hattori, M.; Takabayashi, A.; et al. SMAD4-Deficient Intestinal Tumors Recruit CCR1+ Myeloid Cells That Promote Invasion. Nat. Genet. 2007, 39, 467–475. [Google Scholar] [CrossRef]
- Kiyasu, Y.; Kawada, K.; Hirai, H.; Ogawa, R.; Hanada, K.; Masui, H.; Nishikawa, G.; Yamamoto, T.; Mizuno, R.; Itatani, Y.; et al. Disruption of CCR1-Mediated Myeloid Cell Accumulation Suppresses Colorectal Cancer Progression in Mice. Cancer Lett. 2020, 487, 53–62. [Google Scholar] [CrossRef]
- Kitamura, T.; Fujishita, T.; Loetscher, P.; Revesz, L.; Hashida, H.; Kizaka-Kondoh, S.; Aoki, M.; Taketo, M.M. Inactivation of Chemokine (C-C Motif) Receptor 1 (CCR1) Suppresses Colon Cancer Liver Metastasis by Blocking Accumulation of Immature Myeloid Cells in a Mouse Model. Proc. Natl. Acad. Sci. USA 2010, 107, 13063–13068. [Google Scholar] [CrossRef] [Green Version]
- Dobrenis, K. Microglia in Cell Culture and in Transplantation Therapy for Central Nervous System Disease. Methods 1998, 16, 320–344. [Google Scholar] [CrossRef]
- Illig, C.R.; Chen, J.; Wall, M.J.; Wilson, K.J.; Ballentine, S.K.; Rudolph, M.J.; DesJarlais, R.L.; Chen, Y.; Schubert, C.; Petrounia, I.; et al. Discovery of Novel FMS Kinase Inhibitors as Anti-Inflammatory Agents. Bioorg. Med. Chem. Lett. 2008, 18, 1642–1648. [Google Scholar] [CrossRef]
- Manthey, C.L.; Johnson, D.L.; Illig, C.R.; Tuman, R.W.; Zhou, Z.; Baker, J.F.; Chaikin, M.A.; Donatelli, R.R.; Franks, C.F.; Zeng, L.; et al. JNJ-28312141, a Novel Orally Active Colony-Stimulating Factor-1 Receptor/FMS-Related Receptor Tyrosine Kinase-3 Receptor Tyrosine Kinase Inhibitor with Potential Utility in Solid Tumors, Bone Metastases, and Acute Myeloid Leukemia. Mol. Cancer Ther. 2009, 8, 3151–3161. [Google Scholar] [CrossRef] [Green Version]




| Primer | Sequence |
|---|---|
| CCR1 Forward | 5′-AAGAGCCTGAAGCAGTGGAAG-3′ |
| CCR1 Reverse | 5′-GCAGCCATTTTGCCAGTG-3′ |
| CCL3 Forward | 5′ ACCACTGCCCTTGCTGTTC-3′ |
| CCL3 Reverse | 5′-TCTGCCGGTTTCTCTTAGTCAG-3′ |
| CCL5 Forward | 5′-GCTGCCCTCACCATCATCC-3′ |
| CCL5 Reverse | 5′-GTATTCTTGAACCCACTTCTTCTCTG-3′ |
| CCL6 Forward | 5′-GTGGCTGTCCTTGGGTCC-3′ |
| CCL6 Reverse | 5′-AGACCTGGGTTCCCCTCC-3′ |
| CCL9 Forward | 5′-CAACAGAGACAAAAGAAGTCCAGAG-3′ |
| CCL9 Reverse | 5′-CTTGCTGATAAAGATGATGCCC-3′ |
| GAPDH Forward | 5′-CTGGAGAAACCTGCCAAGTA-3′ |
| GAPDH Reverse | 5′-TGTTGCTGTAGCCGTATTCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeren, N.; Afzal, Z.; Morgan, S.; Marshall, G.; Uppiliappan, M.; Merritt, J.; Coniglio, S.J. The Chemokine Receptor CCR1 Mediates Microglia Stimulated Glioma Invasion. Int. J. Mol. Sci. 2023, 24, 5136. https://doi.org/10.3390/ijms24065136
Zeren N, Afzal Z, Morgan S, Marshall G, Uppiliappan M, Merritt J, Coniglio SJ. The Chemokine Receptor CCR1 Mediates Microglia Stimulated Glioma Invasion. International Journal of Molecular Sciences. 2023; 24(6):5136. https://doi.org/10.3390/ijms24065136
Chicago/Turabian StyleZeren, Nazende, Zobia Afzal, Sara Morgan, Gregory Marshall, Maithrayee Uppiliappan, James Merritt, and Salvatore J. Coniglio. 2023. "The Chemokine Receptor CCR1 Mediates Microglia Stimulated Glioma Invasion" International Journal of Molecular Sciences 24, no. 6: 5136. https://doi.org/10.3390/ijms24065136
APA StyleZeren, N., Afzal, Z., Morgan, S., Marshall, G., Uppiliappan, M., Merritt, J., & Coniglio, S. J. (2023). The Chemokine Receptor CCR1 Mediates Microglia Stimulated Glioma Invasion. International Journal of Molecular Sciences, 24(6), 5136. https://doi.org/10.3390/ijms24065136





