A Multi-Stage Bioprocess for the Expansion of Rodent Skin-Derived Schwann Cells in Computer-Controlled Bioreactors
Abstract
1. Introduction
2. Results
2.1. Pre-Expansion Cryopreservation
2.2. Inoculation
2.3. Expansion
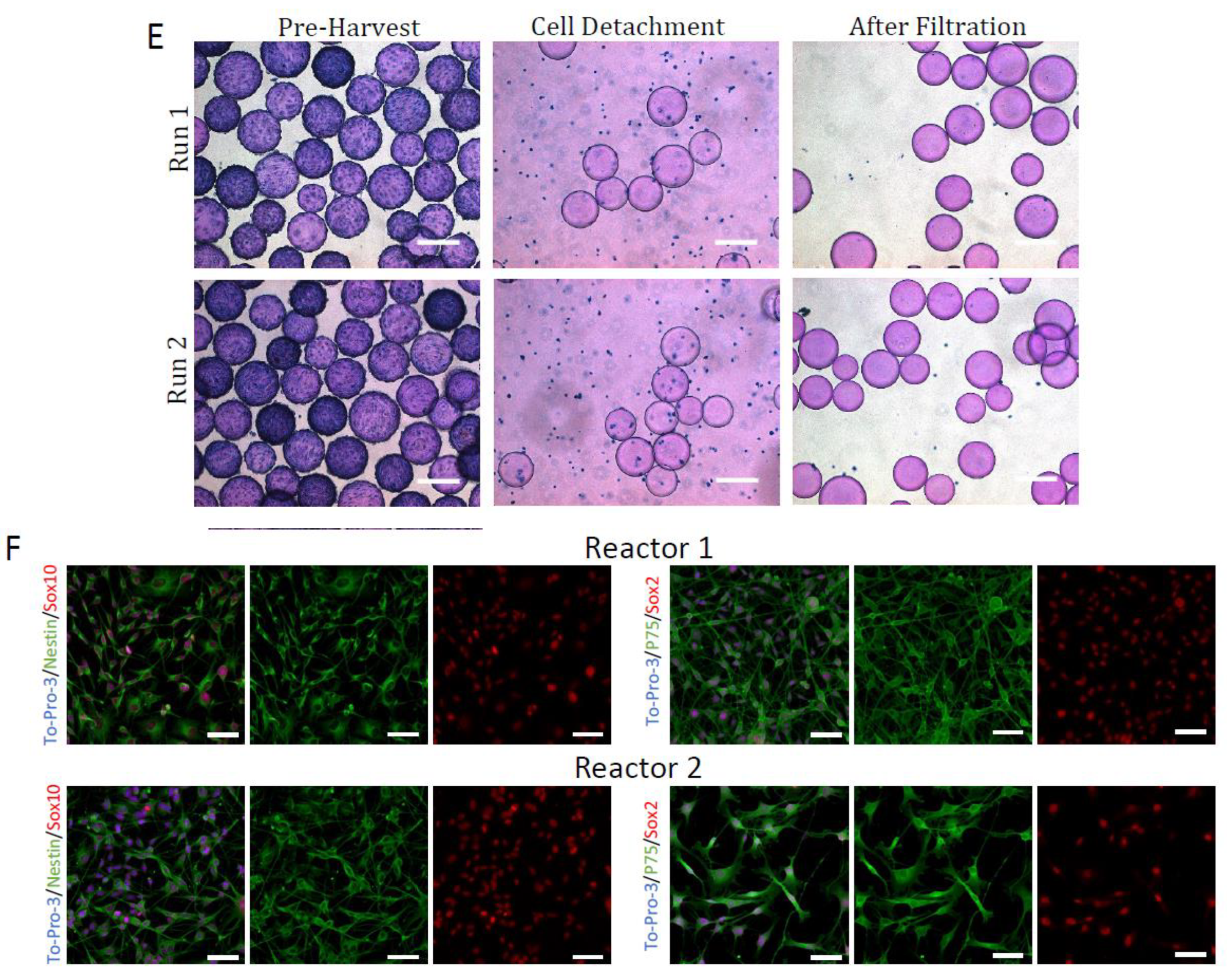
2.4. Harvest
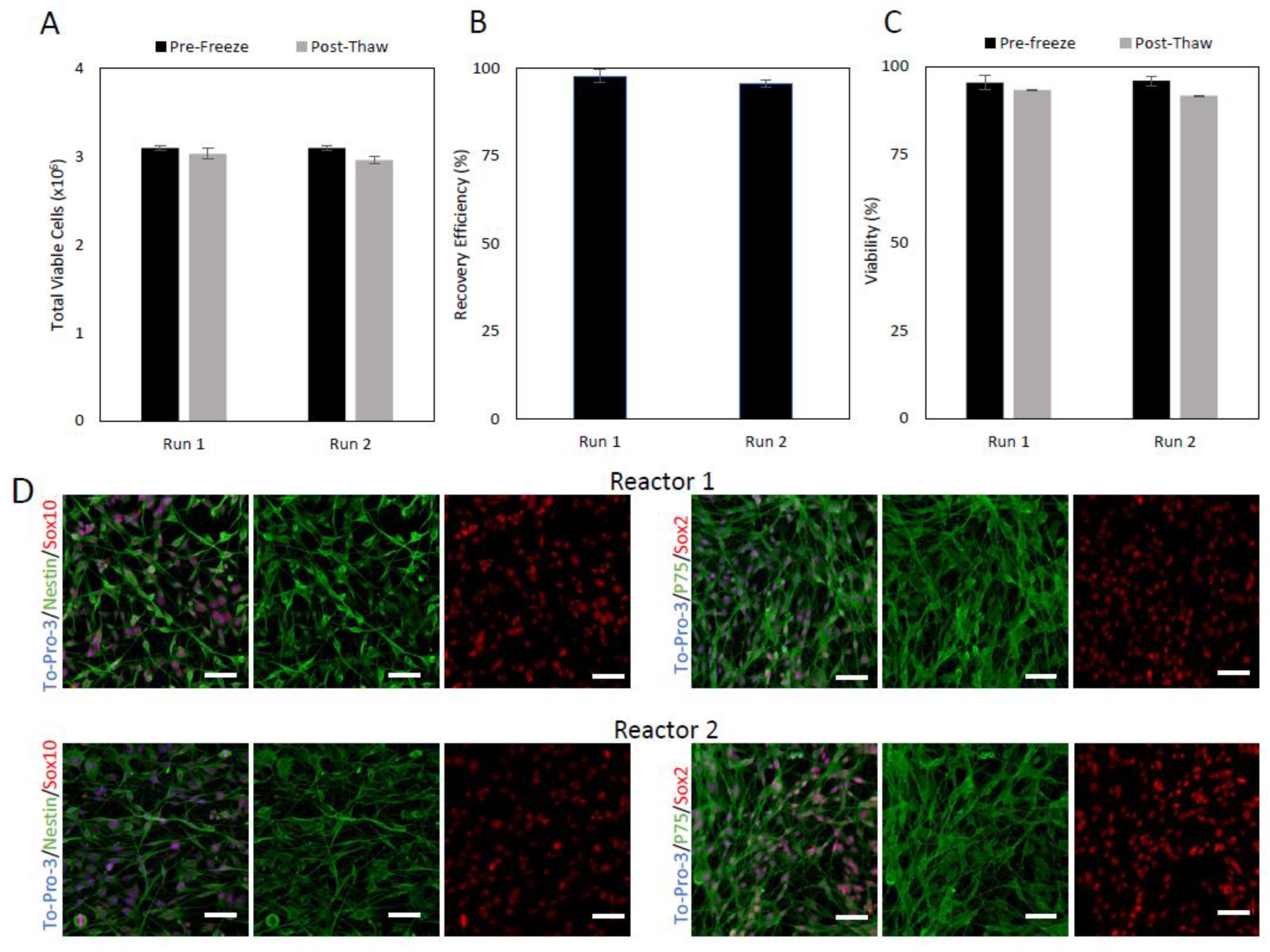
2.5. Post-Expansion Cryopreservation
2.6. Overall Process
3. Discussion
4. Materials and Methods
4.1. Adult Rat Skin-SC Isolation and Maintenance
4.2. Pre-Expansion Cryopreservation
4.3. Post-Expansion Cryopreservation
4.4. Culture Media
4.5. Static Culture
4.6. Microcarrier Preparation
4.7. Bioreactor Preparation
4.8. Bioreactor Culture
4.9. Metabolite Analysis
4.10. Sample Counting
4.11. Cell Harvest
4.12. Immunocytochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badner, A.; Siddiqui, A.M.; Fehlings, M.G. Spinal cord injuries: How could cell therapy help? Expert Opin. Biol. Ther. 2017, 17, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, F.A.; Diaz, A.; Errante, E.L.; Smartz, T.; Khan, A.; Silvera, R.; Brooks, A.E.; Lee, Y.-S.; Burks, S.S.; Levi, A.D. Systematic review of the therapeutic use of Schwann cells in the repair of peripheral nerve injuries: Advancements from animal studies to clinical trials. Front. Cell. Neurosci. 2022, 16, 929593. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Yousefifard, M.; Baikpour, M.; Rahimi-Movaghar, V.; Nasirinezhad, F.; Younesian, S.; Safari, S.; Ghelichkhani, P.; Jafari, A.M. The Efficacy of Schwann Cell Transplantation on Motor Function Recovery after Spinal Cord Injuries in Animal Models: A Systematic Review and Meta-Analysis. J. Chem. Neuroanat. 2016, 78, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The repair Schwann cell and its function in regenerating nerves. J. Physiol. 2016, 594, 3521–3531. [Google Scholar] [CrossRef]
- Berner, J.; Weiss, T.; Sorger, H.; Rifatbegovic, F.; Kauer, M.; Windhager, R.; Dohnal, A.; Ambros, P.F.; Ambros, I.M.; Boztug, K.; et al. Human repair-related Schwann cells adopt functions of antigen-presenting cells in vitro. Glia 2022, 70, 2361–2377. [Google Scholar] [CrossRef]
- Chu, T.; Baral, K.; Labit, E.; Rosin, N.; Sinha, S.; Umansky, D.; Alzahrani, S.; Arora, R.; Cao, L.; Rancourt, D.; et al. Comparison of human skin- and nerve-derived Schwann cells reveals many similarities and subtle genomic and functional differences. Glia 2022, 70, 2131–2156. [Google Scholar] [CrossRef]
- Deng, L.-X.; Walker, C.; Xu, X.-M. Schwann cell transplantation and descending propriospinal regeneration after spinal cord injury. Brain Res. 2014, 1619, 104–114. [Google Scholar] [CrossRef]
- Takami, T.; Oudega, M.; Bates, M.L.; Wood, P.M.; Kleitman, N.; Bunge, M.B. Schwann Cell But Not Olfactory Ensheathing Glia Transplants Improve Hindlimb Locomotor Performance in the Moderately Contused Adult Rat Thoracic Spinal Cord. J. Neurosci. 2002, 22, 6670–6681. [Google Scholar] [CrossRef]
- Kanno, H.; Pressman, Y.; Moody, A.; Berg, R.; Muir, E.M.; Rogers, J.H.; Ozawa, H.; Itoi, E.; Pearse, D.D.; Bunge, M.B. Combination of Engineered Schwann Cell Grafts to Secrete Neurotrophin and Chondroitinase Promotes Axonal Regeneration and Locomotion after Spinal Cord Injury. J. Neurosci. 2014, 34, 1838–1855. [Google Scholar] [CrossRef]
- Shakhbazau, A.; Mohanty, C.; Kumar, R.; Midha, R. Sensory recovery after cell therapy in peripheral nerve repair: Effects of naïve and skin precursor-derived Schwann cells. J. Neurosurg. 2014, 121, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, G.; Zhao, F.; Jin, X. Co-Transplantation of Schwann Cells and Bone Marrow Stromal Cells versus Single Cell Transplantation on Repairing Hemisected Spinal Cord Injury of Rats. Neural Regen. Res. 2010, 5, 805–813. [Google Scholar] [CrossRef]
- Pourheydar, B.; Joghataei, M.T.; Bakhtiari, M.; Mehdizadeh, M.; Yekta, Z.; Najafzadeh, N. Co-transplantation of Bone Marrow Stromal Cells with Schwann Cells Evokes Mechanical Allodynia in the Contusion Model of Spinal Cord Injury in Rats. Cell J. 2011, 13, 213–222. [Google Scholar] [PubMed]
- Walsh, S.K.; Kumar, R.; Grochmal, J.K.; Kemp, S.W.; Forden, J.; Midha, R. Fate of stem cell transplants in peripheral nerves. Stem Cell Res. 2012, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.K.; Gordon, T.; Addas, B.M.; Kemp, S.W.; Midha, R. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp. Neurol. 2010, 223, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.A.; Kim, K.-J.; Namgung, U. α6 and β1 Integrin Heterodimer Mediates Schwann Cell Interactions with Axons and Facilitates Axonal Regeneration after Peripheral Nerve Injury. Neuroscience 2018, 371, 49–59. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Wang, G.; Chen, L.; Chen, J.; Liu, Z.; Zhang, Z.; Shen, H.; Jin, Y.; Shen, Z. The effect of co-transplantation of nerve fibroblasts and Schwann cells on peripheral nerve repair. Int. J. Biol. Sci. 2017, 13, 1507–1519. [Google Scholar] [CrossRef]
- Gambhir, H.S.; Raharjo, E.; Forden, J.; Kumar, R.; Mishra, C.; Guo, G.F.; Grochmal, J.; Shapira, Y.; Midha, R. Improved method to track and precisely count Schwann cells post-transplantation in a peripheral nerve injury model. J. Neurosci. Methods 2016, 273, 86–95. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Biernaskie, J.; Toma, J.G.; Midha, R.; Miller, F.D. Skin-Derived Precursors Generate Myelinating Schwann Cells for the Injured and Dysmyelinated Nervous System. J. Neurosci. 2006, 26, 6651–6660. [Google Scholar] [CrossRef]
- Walsh, S.; Midha, R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg. Focus 2009, 26, E2. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Burks, S.S.; Anderson, K.D.; Dididze, M.; Khan, A.; Dietrich, W.D.; Levi, A.D. First human experience with autologous Schwann cells to supplement sciatic nerve repair: Report of 2 cases with long-term follow-up. Neurosurg. Focus 2017, 42, E2. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.V.; Deng, L.; Xu, X.-M. Human Schwann Cell Transplantation for Spinal Cord Injury: Prospects and Challenges in Translational Medicine. Front. Cell. Neurosci. 2021, 15, 690894. [Google Scholar] [CrossRef] [PubMed]
- Mirfeizi, L.; Stratton, J.A.; Kumar, R.; Shah, P.; Agabalyan, N.; Stykel, M.G.; Midha, R.; Biernaskie, J.; Kallos, M.S. Serum-free bioprocessing of adult human and rodent skin-derived Schwann cells: Implications for cell therapy in nervous system injury. J. Tissue Eng. Regen. Med. 2017, 11, 3385–3397. [Google Scholar] [CrossRef]
- Stratton, J.; Kumar, R.; Sinha, S.; Shah, P.; Stykel, M.; Shapira, Y.; Midha, R.; Biernaskie, J. Purification and Characterization of Schwann Cells from Adult Human Skin and Nerve. Eneuro 2017, 4, 4. [Google Scholar] [CrossRef]
- Walsh, S.; Biernaskie, J.; Kemp, S.; Midha, R. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience 2009, 164, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Biernaskie, J.; McKenzie, I.; Toma, J.G.; Miller, F.D. Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat. Protoc. 2006, 1, 2803–2812. [Google Scholar] [CrossRef]
- Guest, J.; Benavides, F.; Padgett, K.; Mendez, E.; Tovar, D. Technical aspects of spinal cord injections for cell transplantation. Clinical and translational considerations. Brain Res. Bull. 2011, 84, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.; Santamaria, A.J.; Benavides, F.D. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ Transplant. 2013, 18, 682–689. [Google Scholar] [CrossRef]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T.; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef]
- Riley, J.; Federici, T.; Park, J.; Suzuki, M.; Franz, K.; Tork, C.; Mchugh, J.; Teng, Q.; Boulis, N.M. Cervical Spinal Cord Therapeutics Delivery: Preclinical Safety Calidation of Stabilized Microinjection Platform. Neurosurgery 2010, 65, 754–762. [Google Scholar] [CrossRef]
- Peng, K.; Sant, D.; Andersen, N.; Silvera, R.; Camarena, V.; Piñero, G.; Graham, R.; Khan, A.; Xu, X.-M.; Wang, G.; et al. Magnetic separation of peripheral nerve-resident cells underscores key molecular features of human Schwann cells and fibroblasts: An immunochemical and transcriptomics approach. Sci. Rep. 2020, 10, 18433. [Google Scholar] [CrossRef] [PubMed]
- Kirouac, D.C.; Zandstra, P.W. The Systematic Production of Cells for Cell Therapies. Cell Stem Cell 2008, 3, 369–381. [Google Scholar] [CrossRef]
- Ferrari, C.; Balandras, F.; Guedon, E.; Olmos, E.; Chevalot, I.; Marc, A. Limiting cell aggregation during mesenchymal stem cell expansion on microcarriers. Biotechnol. Prog. 2012, 28, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Biernaskie, J.; Midha, R.; Kallos, M.S. Control of dissolved oxygen significantly increases the yield of skin-derived Schwann cells during expansion in stirred suspension bioreactors. Eng. Rep. 2021, 3, e12421. [Google Scholar] [CrossRef]
- Mawji, I.; Roberts, E.L.; Dang, T.; Abraham, B.; Kallos, M.S. Challenges and opportunities in downstream separation processes for mesenchymal stromal cells cultured in microcarrier-based stirred suspension bioreactors. Biotechnol. Bioeng. 2022, 119, 3062–3078. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, H.; Alhosseini, S.N.; Tay, A.; Chan, P.P.; Oh, S.K.W.; Warkiani, M.E. Large-scale production of stem cells utilizing microcarriers: A biomaterials engineering perspective from academic research to commercialized products. Biomaterials 2018, 181, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.; Peixoto, C.; Silva, M.M.; Carrondo, M.J.; Serra, M.; Alves, P.M. Filtration methodologies for the clarification and concentration of human mesenchymal stem cells. J. Membr. Sci. 2015, 478, 117–129. [Google Scholar] [CrossRef]
- Heathman, T.R.J.; Glyn, V.A.M.; Picken, A.; Rafiq, Q.A.; Coopman, K.; Nienow, A.W.; Kara, B.; Hewitt, C.J. Expansion, harvest and cryopreservation of human mesenchymal stem cells in a serum-free microcarrier process. Biotechnol. Bioeng. 2015, 112, 1696–1707. [Google Scholar] [CrossRef]
- Jossen, V.; Bos, C.V.D.; Eibl, R.; Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl. Microbiol. Biotechnol. 2018, 102, 3981–3994. [Google Scholar] [CrossRef]
- Li, A.; Kusuma, G.D.; Driscoll, D.; Smith, N.; Wall, D.M.; Levine, B.L.; James, D.; Lim, R. Advances in automated cell washing and concentration. Cytotherapy 2021, 23, 774–786. [Google Scholar] [CrossRef]
- Anderson, K.D.; Guest, J.D.; Dietrich, W.D.; Bunge, M.B.; Curiel, R.; Dididze, M.; Green, B.A.; Khan, A.; Pearse, D.D.; Saraf-Lavi, E.; et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma 2017, 34, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Petrova, E. Injured Nerve Regeneration using Cell-Based Therapies: Current Challenges. Acta Nat. 2015, 7, 38–47. [Google Scholar] [CrossRef]
- Gant, K.L.; Guest, J.D.; Palermo, A.E.; Vedantam, A.; Jimsheleishvili, G.; Bunge, M.B.; Brooks, A.E.; Anderson, K.D.; Thomas, C.K.; Santamaria, A.J.; et al. Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury. J. Neurotrauma 2022, 39, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Gu, G.; Cong, M.; Du, M.; Wang, W.; Shen, M.; Zhang, Q.; Shi, H.; Gu, X.; Ding, F. Repair of peripheral nerve defects by nerve grafts incorporated with extracellular vesicles from skin-derived precursor Schwann cells. Acta Biomater. 2021, 134, 190–203. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, J.; Xue, C.; Wang, Y.; Wang, S.; Bao, S.; Chen, R.; Li, Y.; Gu, Y. Skin derived precursor Schwann cell-generated acellular matrix modified chitosan/silk scaffolds for bridging rat sciatic nerve gap. Neurosci. Res. 2018, 135, 21–31. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, T.; Abraham, B.; Chu, T.-H.; Biernaskie, J.; Midha, R.; Kallos, M.S. A Multi-Stage Bioprocess for the Expansion of Rodent Skin-Derived Schwann Cells in Computer-Controlled Bioreactors. Int. J. Mol. Sci. 2023, 24, 5152. https://doi.org/10.3390/ijms24065152
Walsh T, Abraham B, Chu T-H, Biernaskie J, Midha R, Kallos MS. A Multi-Stage Bioprocess for the Expansion of Rodent Skin-Derived Schwann Cells in Computer-Controlled Bioreactors. International Journal of Molecular Sciences. 2023; 24(6):5152. https://doi.org/10.3390/ijms24065152
Chicago/Turabian StyleWalsh, Tylor, Brett Abraham, Tak-Ho Chu, Jeff Biernaskie, Rajiv Midha, and Michael S. Kallos. 2023. "A Multi-Stage Bioprocess for the Expansion of Rodent Skin-Derived Schwann Cells in Computer-Controlled Bioreactors" International Journal of Molecular Sciences 24, no. 6: 5152. https://doi.org/10.3390/ijms24065152
APA StyleWalsh, T., Abraham, B., Chu, T.-H., Biernaskie, J., Midha, R., & Kallos, M. S. (2023). A Multi-Stage Bioprocess for the Expansion of Rodent Skin-Derived Schwann Cells in Computer-Controlled Bioreactors. International Journal of Molecular Sciences, 24(6), 5152. https://doi.org/10.3390/ijms24065152




