The Effect of Belantamab Mafodotin on Primary Myeloma–Stroma Co-Cultures: Asymmetrical Mitochondrial Transfer between Myeloma Cells and Autologous Bone Marrow Stromal Cells
Abstract
1. Introduction
2. Results
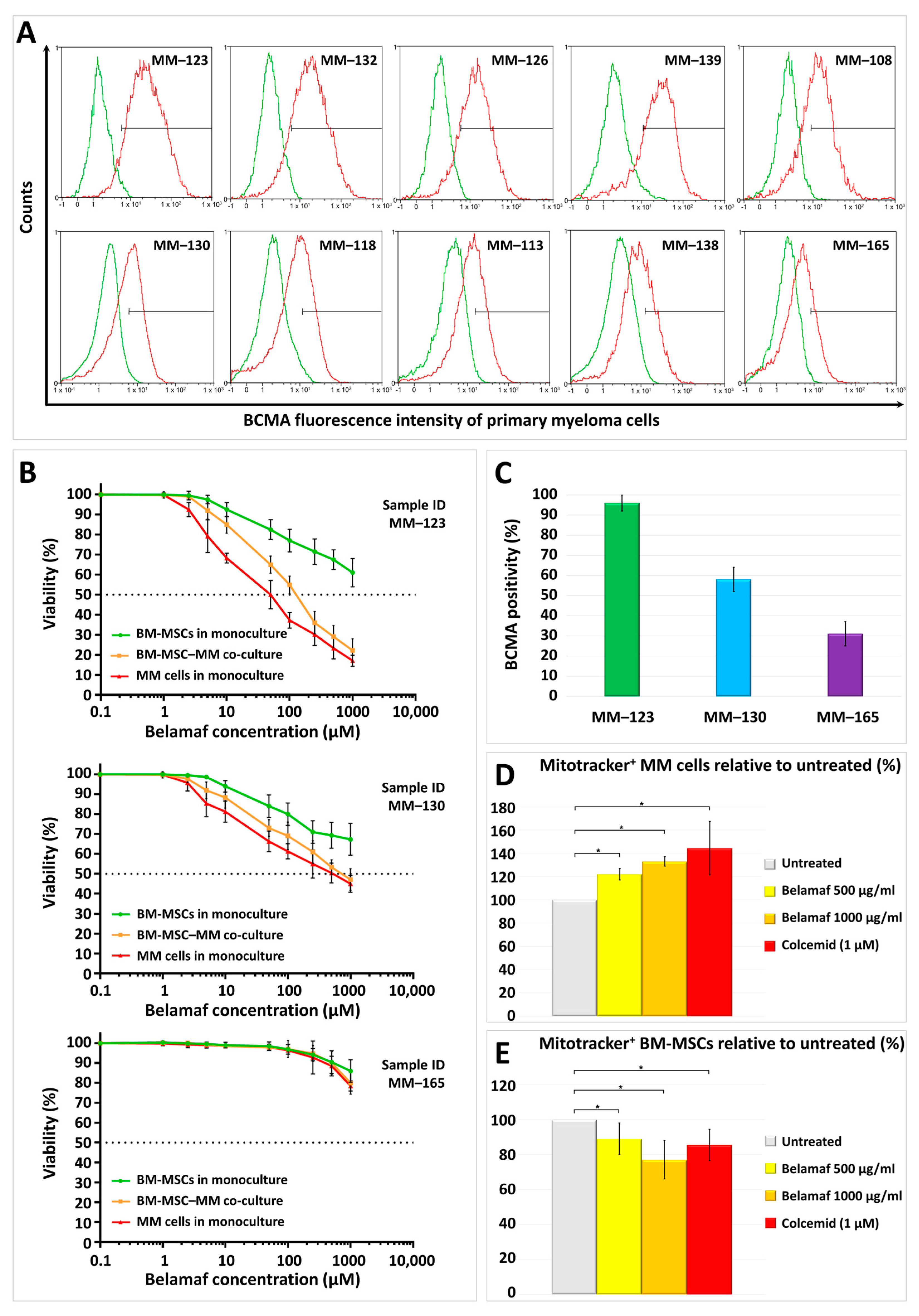
2.1. BCMA Positivity of Myeloma Cells in Primary BM-MSC–MM Co-Cultures Derived from Different Multiple Myeloma Patients
2.2. Cytotoxic Effects of Belantamab Mafodotin on Primary BM-MSC or Myeloma Monocultures, or Their Co-Cultures
2.3. Mitochondrial Transfer between BM-MSCs and Malignant Plasma Cells in the Presence of Higher Doses of Belantamab Mafodotin
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Flow Cytometry
4.3. In Vitro Cytotoxicity Assay
4.4. Mitochondrial Transfer Assay
4.5. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van de Donk, N.; Pawlyn, C.; Yong, K.L. Multiple myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Moreau, P. How I treat myeloma with new agents. Blood 2017, 130, 1507–1513. [Google Scholar] [CrossRef]
- Bal, S.; Giri, S.; Godby, K.N.; Costa, L.J. New regimens and directions in the management of newly diagnosed multiple myeloma. Am. J. Hematol. 2021, 96, 367–378. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Lakshman, A.; Kumar, S.K. Chimeric antigen receptor T-cells, bispecific antibodies, and antibody- drug conjugates for multiple myeloma: An update. Am. J. Hematol. 2022, 97, 99–118. [Google Scholar] [CrossRef]
- Davis, J.A.; Shockley, A.; Hashmi, H. The emergence of b-cell maturation antigen (BCMA) targeting immunotherapy in multiple myeloma. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2022, 28, 960–968. [Google Scholar] [CrossRef]
- Cho, S.F.; Anderson, K.C.; Tai, Y.T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front. Immunol. 2018, 9, 1821. [Google Scholar] [CrossRef]
- Markham, A. Belantamab Mafodotin: First Approval. Drugs 2020, 80, 1607–1613. [Google Scholar] [CrossRef]
- Becnel, M.R.; Lee, H.C. The role of belantamab mafodotin for patients with relapsed and/or refractory multiple myeloma. Ther. Adv. Hematol. 2020, 11, 2040620720979813. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.T.; Mayes, P.A.; Acharya, C.; Zhong, M.Y.; Cea, M.; Cagnetta, A.; Craigen, J.; Yates, J.; Gliddon, L.; Fieles, W.; et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood 2014, 123, 3128–3138. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Anderson, L.D., Jr.; Sutherland, H.J.; Yong, K.; et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018, 19, 1641–1653. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Sborov, D.; Suvannasankha, A.; et al. Longer term outcomes with single-agent belantamab mafodotin in patients with relapsed or refractory multiple myeloma: 13-month follow-up from the pivotal DREAMM-2 study. Cancer 2021, 127, 4198–4212. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Weisel, K.; van de Donk, N.W.; Routledge, D.; Otero, P.R.; Song, K.; Quach, H.; Callander, N.; Minnema, M.C.; Trudel, S.; et al. Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design. Future Oncol. 2021, 17, 1987–2003. [Google Scholar] [CrossRef]
- Hultcrantz, M.; Kleinman, D.; Ghataorhe, P.; McKeown, A.; He, W.; Ling, T.; Jewell, R.C.; Brunner, J.; Byrne, J.; Eliason, L.; et al. Exploring Alternative Dosing Regimens of Single-Agent Belantamab Mafodotin on Safety and Efficacy in Patients with Relapsed or Refractory Multiple Myeloma: DREAMM-14. Blood 2021, 138, 1645. [Google Scholar] [CrossRef]
- Weisel, K.; Hopkins, T.G.; Fecteau, D.; Bao, W.; Quigley, C.; Jewell, R.C.; Nichols, M.; Opalinska, J. Dreamm-3: A Phase 3, Open-Label, Randomized Study to Evaluate the Efficacy and Safety of Belantamab Mafodotin (GSK2857916) Monotherapy Compared with Pomalidomide Plus Low-Dose Dexamethasone (Pom/Dex) in Participants with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2019, 134, 1900. [Google Scholar] [CrossRef]
- Popat, R.; Nooka, A.; Stockerl-Goldstein, K.; Abonour, R.; Ramaekers, R.; Khot, A.; Forbes, A.; Lee, C.; Augustson, B.; Spencer, A.; et al. DREAMM-6: Safety, Tolerability and Clinical Activity of Belantamab Mafodotin (Belamaf) in Combination with Bortezomib/Dexamethasone (BorDex) in Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 19–20. [Google Scholar] [CrossRef]
- Rifkin, R.M.; Boyd, K.; Grosicki, S.; Kim, K.; Di Raimondo, F.; Dimopoulos, M.A.; Weisel, K.; Arnulf, B.; Hajek, R.; Hungria, V.T.M.; et al. DREAMM-7: A Phase III Study of the Efficacy and Safety of Belantamab Mafodotin (Belamaf) with Bortezomib, and Dexamethasone (B-Vd) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 53–54. [Google Scholar] [CrossRef]
- Trudel, S.; Davis, R.; Lewis, N.M.; Bakshi, K.K.; Chopra, B.; Montes de Oca, R.; Ferron-Brady, G.; Eliason, L.; Kremer, B.E.; Gupta, I.; et al. DREAMM-8: A Phase III Study of the Efficacy and Safety of Belantamab Mafodotin with Pomalidomide and Dexamethasone (B-Pd) Vs Pomalidomide Plus Bortezomib and Dexamethasone (PVd) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 4. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Alonso Alonso, A.; Quach, H.; Koh, Y.; Guenther, A.; Min, C.-K.; Zhou, X.L.; Kaisermann, M.; Mis, L.M.; Williams, D.; et al. DREAMM-9: Phase I Study of Belantamab Mafodotin Plus Standard of Care in Patients with Transplant-Ineligible Newly Diagnosed Multiple Myeloma. Blood 2021, 138, 2738. [Google Scholar] [CrossRef]
- Callander, N.S.; Ribrag, V.; Richardson, P.G.; Nooka, A.K.; Song, K.; Uttervall, K.; Minnema, M.C.; Weisel, K.; Quach, H.; Min, C.-K.; et al. DREAMM-5 Study: Investigating the Synergetic Effects of Belantamab Mafodotin Plus Inducible T-Cell Co-Stimulator Agonist (aICOS) Combination Therapy in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2021, 138, 897. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, R.; Alavi, A.S.; Vitali, N.; Bhattacharya, S.; Blackwell, C.; Patel, K.; Seestaller-Wehr, L.; Kaczynski, H.; Shi, H.; Dobrzynski, E.; et al. Belantamab Mafodotin (GSK2857916) Drives Immunogenic Cell Death and Immune-mediated Antitumor Responses In Vivo. Mol. Cancer Ther. 2021, 20, 1941–1955. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Iida, S.; Shitara, K. Non-fucosylated therapeutic antibodies as next-generation therapeutic antibodies. Expert Opin. Biol. Ther. 2006, 6, 1161–1173. [Google Scholar] [CrossRef]
- Offidani, M.; Corvatta, L.; More, S.; Olivieri, A. Belantamab Mafodotin for the Treatment of Multiple Myeloma: An Overview of the Clinical Efficacy and Safety. Drug Des. Dev. Ther. 2021, 15, 2401–2415. [Google Scholar] [CrossRef]
- Matula, Z.; Mikala, G.; Lukacsi, S.; Matko, J.; Kovacs, T.; Monostori, E.; Uher, F.; Valyi-Nagy, I. Stromal Cells Serve Drug Resistance for Multiple Myeloma via Mitochondrial Transfer: A Study on Primary Myeloma and Stromal Cells. Cancers 2021, 13, 3461. [Google Scholar] [CrossRef]
- Joseph, N.S.; Kaufman, J.L.; Dhodapkar, M.V.; Hofmeister, C.C.; Almaula, D.K.; Heffner, L.T.; Gupta, V.A.; Boise, L.H.; Lonial, S.; Nooka, A.K. Long-Term Follow-Up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2020, 38, 1928–1937. [Google Scholar] [CrossRef]
- Boudreault, J.S.; Touzeau, C.; Moreau, P. Triplet combinations in relapsed/refractory myeloma: Update on recent phase 3 trials. Expert Rev. Hematol. 2017, 10, 207–215. [Google Scholar] [CrossRef]
- Su, C.T.; Ye, J.C. Emerging therapies for relapsed/refractory multiple myeloma: CAR-T and beyond. J. Hematol. Oncol. 2021, 14, 115. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, M.; Julian, K.; McClune, B.; Sborov, D.W. Toxicity management strategies for next-generation novel therapeutics in multiple myeloma. Ther. Adv. Hematol. 2022, 13, 20406207221100659. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13, 125. [Google Scholar] [CrossRef]
- Nobari, S.T.; Nojadeh, J.N.; Talebi, M. B-cell maturation antigen targeting strategies in multiple myeloma treatment, advantages and disadvantages. J. Transl. Med. 2022, 20, 82. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, G.; Bergeron, C.; Guedry, R.; Cucarola, J.; Kaye, A.M.; Cornett, E.M.; Kaye, A.D.; Varrassi, G.; Viswanath, O.; Urits, I. Belantamab Mafodotin to Treat Multiple Myeloma: A Comprehensive Review of Disease, Drug Efficacy and Side Effects. Curr. Oncol. 2021, 28, 640–660. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Nooka, A.; Quach, H.; Trudel, S.; Routledge, D.; Song, K.; Nahi, H.; Paul, S.; Khan, J.; Brouch, M.; et al. Dreamm-5 Platform Trial: Belantamab Mafodotin (Belamaf) in Combination with Four Different Novel Agents in Patients with Relapsed/Refractory Multiple Myeloma (RRMM). Blood 2020, 136, 1–2. [Google Scholar] [CrossRef]
- Paul, B.; Rodriguez, C.; Usmani, S.Z. BCMA-Targeted Biologic Therapies: The Next Standard of Care in Multiple Myeloma Therapy. Drugs 2022, 82, 613–631. [Google Scholar] [CrossRef]
- Manier, S.; Ingegnere, T.; Escure, G.; Prodhomme, C.; Nudel, M.; Mitra, S.; Facon, T. Current state and next-generation CAR-T cells in multiple myeloma. Blood Rev. 2022, 54, 100929. [Google Scholar] [CrossRef]
- Nishida, H. Rapid Progress in Immunotherapies for Multiple Myeloma: An Updated Comprehensive Review. Cancers 2021, 13, 2712. [Google Scholar] [CrossRef]
- Bu, D.X.; Singh, R.; Choi, E.E.; Ruella, M.; Nunez-Cruz, S.; Mansfield, K.G.; Bennett, P.; Barton, N.; Wu, Q.; Zhang, J.; et al. Pre-clinical validation of B cell maturation antigen (BCMA) as a target for T cell immunotherapy of multiple myeloma. Oncotarget 2018, 9, 25764–25780. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; Glassford, J.; Percy, L.; Munson, P.; Marafioti, T.; Rodriguez-Justo, M.; Yong, K. APRIL promotes cell-cycle progression in primary multiple myeloma cells: Influence of D-type cyclin group and translocation status. Blood 2011, 117, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Bounds, D.; Paterson, J.; Herledan, G.; Sully, K.; Seestaller-Wehr, L.M.; Fieles, W.E.; Tunstead, J.; McCahon, L.; Germaschewski, F.M.; et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br. J. Haematol. 2016, 174, 911–922. [Google Scholar] [CrossRef]
- Bluhm, J.; Kieback, E.; Marino, S.F.; Oden, F.; Westermann, J.; Chmielewski, M.; Abken, H.; Uckert, W.; Hopken, U.E.; Rehm, A. CAR T Cells with Enhanced Sensitivity to B Cell Maturation Antigen for the Targeting of B Cell Non-Hodgkin’s Lymphoma and Multiple Myeloma. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1906–1920. [Google Scholar] [CrossRef]
- Dogan, A.; Siegel, D.; Tran, N.; Fu, A.; Fowler, J.; Belani, R.; Landgren, O. B-cell maturation antigen expression across hematologic cancers: A systematic literature review. Blood Cancer J. 2020, 10, 73. [Google Scholar] [CrossRef]
- Samur, M.K.; Fulciniti, M.; Aktas Samur, A.; Bazarbachi, A.H.; Tai, Y.T.; Prabhala, R.; Alonso, A.; Sperling, A.S.; Campbell, T.; Petrocca, F.; et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 2021, 12, 868. [Google Scholar] [CrossRef]
- Zaccard, C.R.; Rinaldo, C.R.; Mailliard, R.B. Linked in: Immunologic membrane nanotube networks. J. Leukoc. Biol. 2016, 100, 81–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matula, Z.; Uher, F.; Vályi-Nagy, I.; Mikala, G. The Effect of Belantamab Mafodotin on Primary Myeloma–Stroma Co-Cultures: Asymmetrical Mitochondrial Transfer between Myeloma Cells and Autologous Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2023, 24, 5303. https://doi.org/10.3390/ijms24065303
Matula Z, Uher F, Vályi-Nagy I, Mikala G. The Effect of Belantamab Mafodotin on Primary Myeloma–Stroma Co-Cultures: Asymmetrical Mitochondrial Transfer between Myeloma Cells and Autologous Bone Marrow Stromal Cells. International Journal of Molecular Sciences. 2023; 24(6):5303. https://doi.org/10.3390/ijms24065303
Chicago/Turabian StyleMatula, Zsolt, Ferenc Uher, István Vályi-Nagy, and Gábor Mikala. 2023. "The Effect of Belantamab Mafodotin on Primary Myeloma–Stroma Co-Cultures: Asymmetrical Mitochondrial Transfer between Myeloma Cells and Autologous Bone Marrow Stromal Cells" International Journal of Molecular Sciences 24, no. 6: 5303. https://doi.org/10.3390/ijms24065303
APA StyleMatula, Z., Uher, F., Vályi-Nagy, I., & Mikala, G. (2023). The Effect of Belantamab Mafodotin on Primary Myeloma–Stroma Co-Cultures: Asymmetrical Mitochondrial Transfer between Myeloma Cells and Autologous Bone Marrow Stromal Cells. International Journal of Molecular Sciences, 24(6), 5303. https://doi.org/10.3390/ijms24065303






