Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance
Abstract
1. Introduction
2. Bryophytes and Heavy Metals
2.1. Metal Tolerance and Accumulation of Heavy Metals in Bryophytes
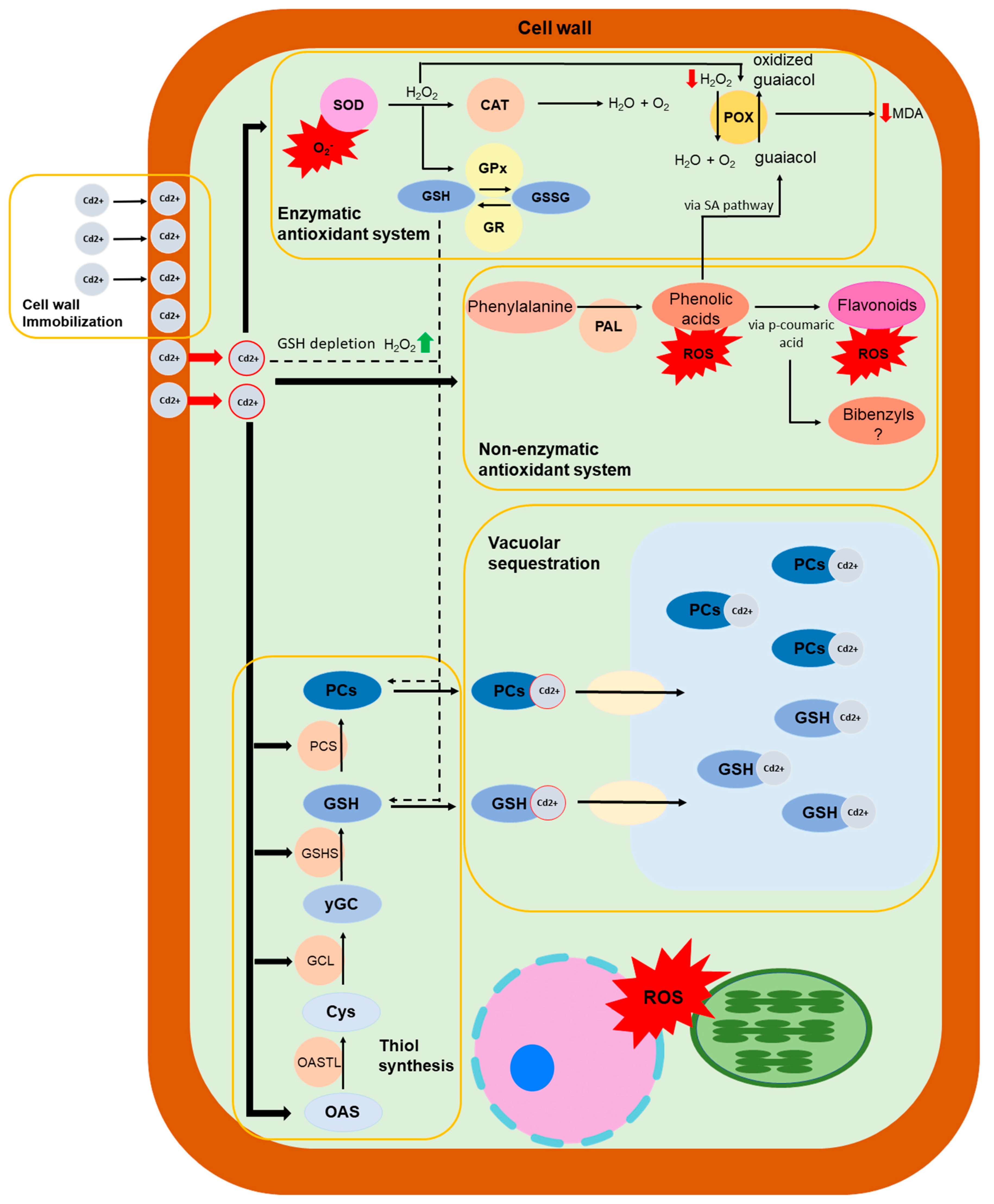
2.2. Bryophytes’ Defences against Heavy Metals
2.3. Non-Enzymatic and Enzymatic Antioxidant Systems in Bryophytes
2.4. Non-Protein Thiol Compounds in Heavy Metal Stress
2.5. Cysteine Biosynthesis
2.6. Glutathione
| Organism | Heavy Metal | [mM] | Vivo | Vitro | Glutathione | Phytochelatins | Ref. |
|---|---|---|---|---|---|---|---|
| Lunularia cruciata | Cd | 0.0008 mM | X | - | - | [36] | |
| 0.004 mM | - | ||||||
| 0.002 mM | - | ||||||
| Lunularia cruciata | Cd | 0.036 mM | X | X | 14.4 ± 3.2 nmol g−1 fw | 11.2 ± 0.7 nmol g−1 fw | [14] |
| Fe | 0.1 mM | - | - | ||||
| Zn | 0.1 mM | X | - | - | |||
| Lunularia cruciata | Al, As, Cd, Cu, Cr, Fe, Pb, Mn, V, Hg, Ni | different concentrations in the three sites studied | X | 0.2 ± 0.02 nmol g−1 fw | 3.7 ± 0.1 nmol g−1 fw | [63] | |
| Sphagnum palustre L. | Cd | 0.036 mM | X | 34.2 ± 2.1 nmol g−1 fw | 13.3 ± 0.8 nmol g−1 fw | [125] | |
| Polytrichastrum formosum | 367.7 ± 25.6 nmol g−1 fw | N.D | |||||
| Hypnum cupressiforme | 123.6 ± 28.1 nmol g−1 fw | N.D | |||||
| Fontinalis antipyretica | 148.0 ± 19.1 nmol g−1 fw | N.D | |||||
| Leptodictyum riparium | Cd | 0.036 mM | X | - | 50.89 ± 3.88 pmol PCn g−1 fw min−1 (PCs activity) | [22] | |
| 0.36 mM | X | - | 96.83 ± 5.77 pmol PCn g−1 fw min−1 (PCs activity) | ||||
| 0.1 mM | X | - | 90.77 ± 6.57 pmol PCn g−1 fw min−1 (PCs activity) | ||||
| Marchantia polymorpha L. | Cd | 50 mM | X | - | - | [126] | |
| Zn | 0.2 mM | X | - | - | |||
| Nitella mucronate | Cd | 0.018 mM | X | - | 22.6 ± 4.5 nmol g−1 fw | [127] | |
| Cd | 0.036 mM | X | - | 33.8 ± 9.0 nmol g−1 fw | |||
| Fe(II) | 0.0075 mM | X | - | 18.5 ± 1.3 nmol g−1 fw | |||
| Fe(II) | 0.03 mM | X | - | 12.7 ± 1.6 nmol g−1 fw | |||
| Fe(III) | 0.0075 mM | X | - | 19.6 ± 1.0 nmol g−1 fw | |||
| Fe(III) | 0.03 mM | X | - | 40.1 ± 6.2 nmol g−1 fw | |||
| Zn | 0.030 mM | X | - | 7.1 ± 3.7 nmol g−1 fw | |||
| Fontinalis antipyretica | Cr | From 6.25 × 10−5 to 50 mM | X | 6.25 × 10−2 mM | - | [90] | |
| Fontinalis antipyretica | Cd | 0.1 mM | X | 1.95 mM | - | [128] | |
| Fontinalis dalecarlica | Cd | 0.1 mM | X | 0.42 mM | - | ||
| Taxithelium nepalense | Cr | 0.1 mM | X | - | - | [38] | |
| Cr | 1 mM | X | - | - | |||
| Pb | 0.1 mM | X | - | - | |||
| Pb | 1 mM | X | - | - | |||
| Polytrichum comune | Cr | 0, 0.01 mM | X | - | - | [129] | |
| Cr | 0.1 mM | X | - | - | |||
| Cr | 1 mM | X | - | - | |||
| Cu | 0, 0.01 mM | X | - | - | |||
| Cu | 0.1 mM | X | - | - | |||
| Cu | 1 mM | X | - | - | |||
| Zn | 0, 0.01 mM | X | - | - | |||
| Zn | 0.1 mM | X | - | - | |||
| Zn | 1 mM | X | - | - | |||
| Physcomitrella patens | Cd | 0.005 mM | X | 226.2 ± 42.9 nmol g−1 fw (24 h after the treatment) | - | [130] | |
| 286.9 ± 51.7 nmol g−1 fw (72 h after the treatment) | - | ||||||
| 369.3 ± 44.1 nmol g−1 fw (120 h after the treatment) | - | ||||||
| Cd | 0.01 mM | X | 240.6 ± 10.6 nmol g−1 fw (24 h after the treatment) | - | |||
| 354.5 ± 27.0 nmol g−1 fw (72 h after the treatment) | - | ||||||
| 425.5 ± 1.3 nmol g−1 fw (120 h after the treatment) | - |
2.7. Phytochelatins
2.8. Glutathione and Phytochelatins in Bryophytes
2.9. Metallothioneins in Metal Detoxification
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duffus, J.H. Heavy Metal—A Meaningless Term? Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Adnan, M.; Xiao, B.; Xiao, P.; Zhao, P.; Li, R.; Bibi, S. Research Progress on Heavy Metals Pollution in the Soil of Smelting Sites in China. Toxics 2022, 10, 231. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; pp. 133–164. ISBN 978-3-7643-8340-4. [Google Scholar]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ. -Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Stafilov, T.; Šajn, R.; Boev, B.; Cvetković, J.; Mukaetov, D.; Andreevski, M.; Lepitkova, S. Distribution of Some Elements in Surface Soil over the Kavadarci Region, Republic of Macedonia. Environ. Earth Sci. 2010, 61, 1515. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Cheng, K.; Tian, H.Z.; Dunhua, Z.; Lu, L.; Wang, Y.; Chen, J.; Liu, X.; Jia, W.; Huang, Z. Atmospheric Emission Inventory of Cadmium from Anthropogenic Sources. Int. J. Environ. Sci. Technol. 2014, 11, 605–616. [Google Scholar] [CrossRef]
- Shrivastava, M.; Khandelwal, A.; Srivastava, S. Heavy Metal Hyperaccumulator Plants: The Resource to Understand the Extreme Adaptations of Plants Towards Heavy Metals. In Plant-Metal Interactions; Srivastava, S., Srivastava, A.K., Suprasanna, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 79–97. ISBN 978-3-030-20732-8. [Google Scholar]
- Goyer, R. Issue Paper on the Human Health Effects of Metals; US Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- Banfalvi, G. Cellular Effects of Heavy Metals; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Basile, A.; Cogoni, A.E.; Bassi, P.; Fabrizi, E.; Sorbo, S.; Giordano, S.; Cobianchi, R.C. Accumulation of Pb and Zn in Gametophytes and Sporophytes of the Moss Funaria Hygrometrica (Funariales). Ann. Bot. 2001, 87, 537–543. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Cobianchi, R.C.; Trinchella, F.; Capasso, C.; Carginale, V. Toxicity, Accumulation, and Removal of Heavy Metals by Three Aquatic Macrophytes. Int. J. Phytoremediat. 2012, 14, 374–387. [Google Scholar] [CrossRef]
- Reski, R. Development, Genetics and Molecular Biology of Mosses. Bot. Acta 1998, 111, 1–15. [Google Scholar] [CrossRef]
- Degola, F.; De Benedictis, M.; Petraglia, A.; Massimi, A.; Fattorini, L.; Sorbo, S.; Basile, A.; Sanità di Toppi, L. A Cd/Fe/Zn-Responsive Phytochelatin Synthase Is Constitutively Present in the Ancient Liverwort Lunularia Cruciata (L.) Dumort. Plant Cell Physiol. 2014, 55, 1884–1891. [Google Scholar] [CrossRef]
- Sassmann, S.; Wernitznig, S.; Lichtscheidl, I.K.; Lang, I. Comparing Copper Resistance in Two Bryophytes: Mielichhoferia Elongata Hornsch. versus Physcomitrella Patens Hedw. Protoplasma 2010, 246, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Stanković, J.D.; Sabovljević, A.D.; Sabovljević, M.S. Bryophytes and Heavy Metals: A Review. Acta Bot. Croat. 2018, 77, 109–118. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Rabęda, I.; Basińska, A.; Lewandowski, M.; Mellerowicz, E.J.; Napieralska, A.; Samardakiewicz, S.; Woźny, A. Pectinous Cell Wall Thickenings Formation—A Common Defense Strategy of Plants to Cope with Pb. Environ. Pollut. 2016, 214, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Bruns, I.N.A.; Sutter, K.; Menge, S.; Neumann, D.; Krauss, G.-J. Cadmium Lets Increase the Glutathione Pool in Bryophytes. J. Plant Physiol. 2001, 158, 79–89. [Google Scholar] [CrossRef]
- Fasani, E.; Li, M.; Varotto, C.; Furini, A.; DalCorso, G. Metal Detoxification in Land Plants: From Bryophytes to Vascular Plants. STATE of the Art and Opportunities. Plants 2022, 11, 237. [Google Scholar] [CrossRef]
- Leinenweber, G.; Stegen, S.; Diaz-Palma, P. Increase of Total Glutathione as a Response to Cd Induced Stress in a Chilean Endemic Bryophytes (Thuidium Sp.). J. Chil. Chem. Soc. 2009, 54, 401–404. [Google Scholar] [CrossRef]
- Bellini, E.; Maresca, V.; Betti, C.; Castiglione, M.R.; Fontanini, D.; Capocchi, A.; Sorce, C.; Borsò, M.; Bruno, L.; Sorbo, S.; et al. The Moss Leptodictyum Riparium Counteracts Severe Cadmium Stress by Activation of Glutathione Transferase and Phytochelatin Synthase, but Slightly by Phytochelatins. Int. J. Mol. Sci. 2020, 21, 1583. [Google Scholar] [CrossRef]
- Bargagli, R. Trace Elements in Terrestrial Plants: An Ecophysiological Approach to Biomonitoring and Biorecovery; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-540-64551-1. [Google Scholar]
- Macnair, M.R.; Tilstone, G.H.; Smith, S.E. The Genetics of Metal Tolerance and Accumulation in Higher Plants; CRC Press: Boca Raton, FL, USA, 2020; pp. 235–250. ISBN 978-0-367-80314-8. [Google Scholar]
- Ernst, W.H.O. Evolution of Metal Tolerance in Higher Plants. For. Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Basile, A.; Sorbo, S.; Bassi, P.; Napolitano, E.; Cogoni, A.E.; Cobianchi, R.C. Effects of Heavy Metals on Protonemal Development and Ultrastructure in Populations of the Moss Funaria Hygrometrica Hedw. (Bryophyta) from a Mine and an Unpolluted Site. Fresenius Environ. Bull. 2008, 17, 1956–1963. [Google Scholar]
- Jules, E.S.; Shaw, A.J. Adaptation to Metal-Contaminated Soils in Populations of the Moss, Ceratodon Purpureus: Vegetative Growth and Reproductive Expression. Am. J. Bot. 1994, 81, 791–797. [Google Scholar] [CrossRef]
- Boyd, R.S.; Martens, S.N. Nickel Hyperaccumulation by Thlaspi Montanum Var. Montanum (Brassicaceae): A Constitutive Trait. Am. J. Bot. 1998, 85, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Meharg, A.A. Integrated Tolerance Mechanisms: Constitutive and Adaptive Plant Responses to Elevated Metal Concentrations in the Environment. Plant Cell Environ. 1994, 17, 989–993. [Google Scholar] [CrossRef]
- Briggs, D. Population Differentiation in Marchantia Polymorpha L. in Various Lead Pollution Levels. Nature 1972, 238, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Brown, D.H. Cadmium Tolerance in a Metal-Contaminated Population of the Grassland Moss Rhytidiadelphus Squarrosus. Ann. Bot. 1995, 75, 21–29. [Google Scholar] [CrossRef]
- Brown, D.H.; House, K.L. Evidence of a Copper-Tolerant Ecotype of the Hepatic Solenostoma Crenulatum. Ann. Bot. 1978, 42, 1383–1392. [Google Scholar] [CrossRef]
- Shaw, J. Genetic Variation for Tolerance to Copper and Zinc within and among Populations of the Moss, Funaria Hygrometrica Hedw. New Phytol. 1988, 109, 211–222. [Google Scholar] [CrossRef]
- Boquete, M.T.; Spagnuolo, V.; Fernández, J.Á.; Aboal, J.R.; Imperatore, I.; Giordano, S. Genetic Structuring of the Moss Pseudoscleropodium Purum Sampled at Different Distances from a Pollution Source. Ecotoxicology 2016, 25, 1812–1821. [Google Scholar] [CrossRef]
- Basile, A.; Giordano, S.; Cafiero, G.; Spagnuolo, V.; Castaldo-Cobianchi, R. Tissue and Cell Localization of Experimentally-Supplied Lead in Funaria Hygrometrica Hedw. Using X-Ray SEM and TEM Microanalysis. J. Bryol. 1994, 18, 69–81. [Google Scholar] [CrossRef]
- Carginale, V.; Sorbo, S.; Capasso, C.; Trinchella, F.; Cafiero, G.; Basile, A. Accumulation, Localisation, and Toxic Effects of Cadmium in the Liverwort Lunularia Cruciata. Protoplasma 2004, 223, 53–61. [Google Scholar] [CrossRef]
- Esposito, S.; Sorbo, S.; Conte, B.; Basile, A. Effects of Heavy Metals on Ultrastructure and HSP70S Induction in the Aquatic Moss Leptodictyum Riparium Hedw. Int. J. Phytoremediat. 2012, 14, 443–455. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Toxic Effects, Oxidative Stress and Ultrastructural Changes in Moss Taxithelium Nepalense (Schwaegr.) Broth. under Chromium and Lead Phytotoxicity. Water Air Soil Pollut. 2005, 167, 73–90. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, S.K. Induction of Oxidative Stress and Ultrastructural Changes in Moss Taxithelium Nepalense (Schwaegr.) Broth. under Lead and Arsenic Phytotoxicity. Curr. Sci. 2004, 87, 342–348. [Google Scholar]
- Świsłowski, P.; Rajfur, M.; Wacławek, M. Influence of Heavy Metal Concentration on Chlorophyll Content in Mosses. Ecol. Chem. Eng. S 2020, 27, 591–601. [Google Scholar] [CrossRef]
- Phaenark, C.; Niamsuthi, A.; Paejaroen, P.; Chunchob, S.; Cronberg, N.; Sawangproh, W. Comparative Toxicity of Heavy Metals Cd, Pb, and Zn to Three Acrocarpous Moss Species Using Chlorophyll Contents. Trends Sci. 2023, 20, 4287. [Google Scholar] [CrossRef]
- Shakya, K.; Chettri, M.K.; Sawidis, T. Impact of Heavy Metals (Copper, Zinc, and Lead) on the Chlorophyll Content of Some Mosses. Arch. Environ. Contam. Toxicol. 2008, 54, 412–421. [Google Scholar] [CrossRef]
- Stanković, J.D.; Janković, S.; Lang, I.; Vujičić, M.M.; Sabovljević, M.S.; Sabovljević, A.D. The Toxic Metal Stress in Two Mosses of Different Growth Forms under Axenic and Controlled Conditions. Bot. Serbica 2021, 45, 31–47. [Google Scholar] [CrossRef]
- Tremper, A.H.; Agneta, M.; Burton, S.; Higgs, D.E.B. Field and Laboratory Exposures of Two Moss Species to Low Level Metal Pollution. J. Atmos. Chem. 2004, 49, 111–120. [Google Scholar] [CrossRef]
- Maresca, V.; Bellini, E.; Landi, S.; Capasso, G.; Cianciullo, P.; Carraturo, F.; Pirintsos, S.; Sorbo, S.; Sanità di Toppi, L.; Esposito, S.; et al. Biological Responses to Heavy Metal Stress in the Moss Leptodictyum Riparium (Hedw.) Warnst. Ecotoxicol. Environ. Saf. 2022, 229, 113078. [Google Scholar] [CrossRef]
- Brown, D.H.; Wells, J.M. Physiological Effects of Heavy Metals on the Moss Rhytidiadelphus Squarrosus. Ann. Bot. 1990, 66, 641–647. [Google Scholar] [CrossRef]
- Chen, Y.-E.; Mao, H.-T.; Ma, J.; Wu, N.; Zhang, C.-M.; Su, Y.-Q.; Zhang, Z.-W.; Yuan, M.; Zhang, H.-Y.; Zeng, X.-Y.; et al. Biomonitoring Chromium III or VI Soluble Pollution by Moss Chlorophyll Fluorescence. Chemosphere 2018, 194, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Díaz, S.; Villares, R.; Vázquez, M.D.; Carballeira, A. Physiological Effects of Exposure to Arsenic, Mercury, Antimony and Selenium in the Aquatic Moss Fontinalis Antipyretica Hedw. Water Air Soil Pollut. 2013, 224, 1659. [Google Scholar] [CrossRef]
- Gupta, A.; Chopra, R.N. Effect of Some Heavy Metals on Growth and Archegonial Formation in the Female Clones of Riccia Discolor (Hepaticae, Ricciaceae) Grown in Vitro. Fragm. Florist. Geobot. 1995, 40, 533–544. [Google Scholar]
- Sidhus, M.; Brown, D.H. A New Laboratory Technique for Studying the Effects of Heavy Metals on Bryophyte Growth. Ann. Bot. 1996, 78, 711–717. [Google Scholar] [CrossRef]
- Sassmann, S.; Weidinger, M.; Adlassnig, W.; Hofhansl, F.; Bock, B.; Lang, I. Zinc and Copper Uptake in Physcomitrella Patens: Limitations and Effects on Growth and Morphology. Environ. Exp. Bot. 2015, 118, 12–20. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Lenartowska, M.; Samardakiewicz, S.; Bilski, H.; Woźny, A. Lead Deposited in the Cell Wall of Funaria Hygrometrica Protonemata Is Not Stable—A Remobilization Can Occur. Environ. Pollut. 2010, 158, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Krzesłowska, M. The Cell Wall in Plant Cell Response to Trace Metals: Polysaccharide Remodeling and Its Role in Defense Strategy. Acta Physiol. Plant. 2011, 33, 35–51. [Google Scholar] [CrossRef]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New Insights into Pectin Methylesterase Structure and Function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- BUCK, G.W.; BROWN, D.H. The Effect of Desiccation on Cation Location in Lichens. Ann. Bot. 1979, 44, 265–277. [Google Scholar] [CrossRef]
- Itouga, M.; Hayatsu, M.; Sato, M.; Tsuboi, Y.; Kato, Y.; Toyooka, K.; Suzuki, S.; Nakatsuka, S.; Kawakami, S.; Kikuchi, J.; et al. Protonema of the Moss Funaria Hygrometrica Can Function as a Lead (Pb) Adsorbent. PLoS ONE 2017, 12, e0189726. [Google Scholar] [CrossRef]
- Krzesłowska, M.; Lenartowska, M.; Mellerowicz, E.J.; Samardakiewicz, S.; Woźny, A. Pectinous Cell Wall Thickenings Formation-A Response of Moss Protonemata Cells to Lead. Environ. Exp. Bot. 2009, 65, 119–131. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P.; Hedbavny, J. Comparison of Vascular and Non-Vascular Aquatic Plant as Indicators of Cadmium Toxicity. Chemosphere 2017, 180, 86–92. [Google Scholar] [CrossRef]
- Kováčik, J.; Dresler, S.; Babula, P. Long-Term Impact of Cadmium in Protonema Cultures of Physcomitrella Patens. Ecotoxicol. Environ. Saf. 2020, 193, 110333. [Google Scholar] [CrossRef]
- Dazy, M.; Masfaraud, J.-F.; Férard, J.-F. Induction of Oxidative Stress Biomarkers Associated with Heavy Metal Stress in Fontinalis Antipyretica Hedw. Chemosphere 2009, 75, 297–302. [Google Scholar] [CrossRef]
- Maresca, V.; Fusaro, L.; Sorbo, S.; Siciliano, A.; Loppi, S.; Paoli, L.; Monaci, F.; Karam, E.A.; Piscopo, M.; Guida, M.; et al. Functional and Structural Biomarkers to Monitor Heavy Metal Pollution of One of the Most Contaminated Freshwater Sites in Southern Europe. Ecotoxicol. Environ. Saf. 2018, 163, 665–673. [Google Scholar] [CrossRef]
- Maresca, V.; Sorbo, S.; Loppi, S.; Funaro, F.; Del Prete, D.; Basile, A. Biological Effects from Environmental Pollution by Toxic Metals in the “Land of Fires” (Italy) Assessed Using the Biomonitor Species Lunularia Cruciata L. (Dum). Environ. Pollut. 2020, 265, 115000. [Google Scholar] [CrossRef]
- Maresca, V.; Lettieri, G.; Sorbo, S.; Piscopo, M.; Basile, A. Biological Responses to Cadmium Stress in Liverwort Conocephalum Conicum (Marchantiales). Int. J. Mol. Sci. 2020, 21, 6485. [Google Scholar] [CrossRef]
- Maresca, V.; Salbitani, G.; Moccia, F.; Cianciullo, P.; Carraturo, F.; Sorbo, S.; Insolvibile, M.; Carfagna, S.; Panzella, L.; Basile, A. Antioxidant Response to Heavy Metal Pollution of Regi Lagni Freshwater in Conocephalum Conicum L. (Dum.). Ecotoxicol. Environ. Saf. 2022, 234, 113365. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Conte, B.; Cardi, M.; Esposito, S. Ultrastructural Changes and Heat Shock Proteins 70 Induced by Atmospheric Pollution Are Similar to the Effects Observed under in Vitro Heavy Metals Stress in Conocephalum Conicum (Marchantiales--Bryophyta). Environ. Pollut. 2013, 182, 209–216. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Cardi, M.; Lentini, M.; Castiglia, D.; Cianciullo, P.; Conte, B.; Loppi, S.; Esposito, S. Effects of Heavy Metals on Ultrastructure and Hsp70 Induction in Lemna Minor L. Exposed to Water along the Sarno River, Italy. Ecotoxicol. Environ. Saf. 2015, 114, 93–101. [Google Scholar] [CrossRef]
- Basile, A.; Loppi, S.; Piscopo, M.; Paoli, L.; Vannini, A.; Monaci, F.; Sorbo, S.; Lentini, M.; Esposito, S. The Biological Response Chain to Pollution: A Case Study from the “Italian Triangle of Death” Assessed with the Liverwort Lunularia Cruciata. Environ. Sci. Pollut. Res. 2017, 24, 26185–26193. [Google Scholar] [CrossRef] [PubMed]
- Basile, A.; Sorbo, S.; Lentini, M.; Conte, B.; Esposito, S. Water Pollution Causes Ultrastructural and Functional Damages in Pellia Neesiana (Gottsche) Limpr. J. Trace Elem. Med. Biol. 2017, 43, 80–86. [Google Scholar] [CrossRef]
- Esposito, S.; Loppi, S.; Monaci, F.; Paoli, L.; Vannini, A.; Sorbo, S.; Maresca, V.; Fusaro, L.; Karam, E.A.; Lentini, M.; et al. In-Field and in-Vitro Study of the Moss Leptodictyum Riparium as Bioindicator of Toxic Metal Pollution in the Aquatic Environment: Ultrastructural Damage, Oxidative Stress and HSP70 Induction. PLoS ONE 2018, 13, e0195717. [Google Scholar] [CrossRef] [PubMed]
- Zengin, F.K.; Munzuroglu, O. Effects of Some Heavy Metals on Content of Chlorophyll, Proline and Some Antioxidant Chemicals in Bean [Phaseolus Vulgaris L.] Seedlings. Acta Biol. Cracoviensia. Ser. Bot. 2005, 47, 157–164. [Google Scholar]
- Foyer, C.H.; Noctor, G. Redox Sensing and Signalling Associated with Reactive Oxygen in Chloroplasts, Peroxisomes and Mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Novakovic, M.; Bukvicki, D.; Anchang, K.Y. Bis-Bibenzyls, Bibenzyls, and Terpenoids in 33 Genera of the Marchantiophyta (Liverworts): Structures, Synthesis, and Bioactivity. J. Nat. Prod. 2022, 85, 729–762. [Google Scholar] [CrossRef]
- Cianciullo, P.; Maresca, V.; Sorbo, S.; Basile, A. Antioxidant and Antibacterial Properties of Extracts and Bioactive Compounds in Bryophytes. Appl. Sci. 2022, 12, 160. [Google Scholar] [CrossRef]
- Pryce, R.J. Biosynthesis of Lunularic Acid—A Dihydrostilbene Endogenous Growth Inhibitor of Liverworts. Phytochemistry 1971, 10, 2679–2685. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Liang, C.; Liu, L.; Song, X.; Zhao, Y.; Wang, J.; Niu, J. Characterization of the Key Bibenzyl Synthase in Dendrobium Sinense. Int. J. Mol. Sci. 2022, 23, 6780. [Google Scholar] [CrossRef]
- Sun, S.-Q.; He, M.; Cao, T.; Zhang, Y.-C.; Han, W. Response Mechanisms of Antioxidants in Bryophyte (Hypnum Plumaeforme) under the Stress of Single or Combined Pb and/or Ni. Environ. Monit. Assess. 2009, 149, 291–302. [Google Scholar] [CrossRef]
- Sun, S.-Q.; He, M.; Cao, T.; Yusuyin, Y.; Han, W.; Li, J.-L. Antioxidative Responses Related to H2O2 Depletion in Hypnum Plumaeforme under the Combined Stress Induced by Pb and Ni. Environ. Monit. Assess. 2010, 163, 303–312. [Google Scholar] [CrossRef]
- Scandalios, J.G. Oxidative Stress: Molecular Perception and Transduction of Signals Triggering Antioxidant Gene Defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef]
- Menezes-Benavente, L.; Kernodle, S.P.; Margis-Pinheiro, M.; Scandalios, J.G. Salt-Induced Antioxidant Metabolism Defenses in Maize (Zea Mays L.) Seedlings. Redox Rep. 2004, 9, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxidative Stress Responses—What Have Genome-Scale Studies Taught Us? Genome Biol. 2002, 3, reviews1019.1. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione Peroxidase Family—An Evolutionary Overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Penel, C.; Dunand, C. Performing the Paradoxical: How Plant Peroxidases Modify the Cell Wall. Trends Plant Sci. 2004, 9, 534–540. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and Glutathione: The Heart of the Redox Hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Wood, Z.A.; Schröder, E.; Robin Harris, J.; Poole, L.B. Structure, Mechanism and Regulation of Peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Aydogana, S.; Erdag, B.; Aktas, L. Bioaccumulation and Oxidative Stress Impact of Pb, Ni, Cu, and Cr Heavy Metals in Two Bryophyte Species, Pleurochaete Squarrosa and Timmiella Barbuloides. Turk. J. Bot. 2017, 41, 464–475. [Google Scholar] [CrossRef]
- Schützendübel, A.; Polle, A. Plant Responses to Abiotic Stresses: Heavy Metal-induced Oxidative Stress and Protection by Mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-Q.; Wang, G.-X.; He, M.; Cao, T. Effects of Pb and Ni Stress on Oxidative Stress Parameters in Three Moss Species. Ecotoxicol. Environ. Saf. 2011, 74, 1630–1635. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, M.; Zhang, H.; Zeng, X.; Liu, H.; Du, X. Influences of Cu and Cr Stress on Antioxidant System and Chlorophyll Fluorescence in Terrestrial Moss Taxiphyllum Taxirameum. Fresenius Environ. Bull. 2015, 24, 2211–2219. [Google Scholar]
- Dazy, M.; Béraud, E.; Cotelle, S.; Meux, E.; Masfaraud, J.-F.; Férard, J.-F. Antioxidant Enzyme Activities as Affected by Trivalent and Hexavalent Chromium Species in Fontinalis Antipyretica Hedw. Chemosphere 2008, 73, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.; Hiraishi, N.; Shimokawa, H.; Tamura, Y.; Otsuki, M.; Kasugai, S.; Ohya, K.; Tagami, J. The Inhibition Effect of Non-Protein Thiols on Dentinal Matrix Metalloproteinase Activity and HEMA Cytotoxicity. J. Dent. 2014, 42, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ferrat, L.; Gnassia-Barelli, M.; Pergent-Martini, C.; Roméo, M. Mercury and Non-Protein Thiol Compounds in the Seagrass Posidonia Oceanica. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 134, 147–155. [Google Scholar] [CrossRef]
- Hicks, L.M.; Cahoon, R.E.; Bonner, E.R.; Rivard, R.S.; Sheffield, J.; Jez, J.M. Thiol-Based Regulation of Redox-Active Glutamate-Cysteine Ligase from Arabidopsis Thaliana. Plant Cell 2007, 19, 2653–2661. [Google Scholar] [CrossRef]
- Gekeler, W.; Grill, E.; Winnacker, E.-L.; Zenk, M.H. Survey of the Plant Kingdom for the Ability to Bind Heavy Metals through Phytochelatins. Z. Nat. C 1989, 44, 361–369. [Google Scholar] [CrossRef]
- Weng, B.; Xie, X.; Weiss, D.J.; Liu, J.; Lu, H.; Yan, C. Kandelia Obovata (S., L.) Yong Tolerance Mechanisms to Cadmium: Subcellular Distribution, Chemical Forms and Thiol Pools. Mar. Pollut. Bull. 2012, 64, 2453–2460. [Google Scholar] [CrossRef]
- Viarengo, A.; Nott, J.A. Mechanisms of Heavy Metal Cation Homeostasis in Marine Invertebrates. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1993, 104, 355–372. [Google Scholar] [CrossRef]
- Carfagna, S.; Vona, V.; Salbitani, G.; Sorbo, S.; Lanza, N.; Conte, B.; Di Martino Rigano, V.; Cobianchi, R.C.; Golia, B.; Basile, A. Cysteine Synthesis in Scorpiurum Circinatum as a Suitable Biomarker in Air Pollution Monitoring. Int. J. Environ. Health 2011, 5, 93–105. [Google Scholar] [CrossRef]
- Wirtz, M.; Hell, R. Functional Analysis of the Cysteine Synthase Protein Complex from Plants: Structural, Biochemical and Regulatory Properties. J. Plant Physiol. 2006, 163, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Zagorchev, L.; Seal, C.E.; Kranner, I.; Odjakova, M. A Central Role for Thiols in Plant Tolerance to Abiotic Stress. Int. J. Mol. Sci. 2013, 14, 7405–7432. [Google Scholar] [CrossRef] [PubMed]
- Hell, R.; Wirtz, M. Molecular Biology, Biochemistry and Cellular Physiology of Cysteine Metabolism in Arabidopsis Thaliana. Arab. Book 2011, 9, e0154. [Google Scholar] [CrossRef]
- Harms, K.; Von Ballmoos, P.; Brunold, C.; Höfgen, R.; Hesse, H. Expression of a Bacterial Serine Acetyltransferase in Transgenic Potato Plants Leads to Increased Levels of Cysteine and Glutathione. Plant J. 2000, 22, 335–343. [Google Scholar] [CrossRef]
- Ruiz, J.; Blumwald, E. Salinity-Induced Glutathione Synthesis in Brassica Napus. Planta 2002, 214, 965–969. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in Plants: Biosynthesis and Physiological Role in Environmental Stress Tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and Glutathione Reductase: A Boon in Disguise for Plant Abiotic Stress Defense Operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione--Linking Cell Proliferation to Oxidative Stress. Free. Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Gasperl, A.; Zellnig, G.; Kocsy, G.; Müller, M. Organelle-Specific Localization of Glutathione in Plants Grown under Different Light Intensities and Spectra. Histochem. Cell Biol. 2022, 158, 213–227. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione Is a Key Player in Metal-Induced Oxidative Stress Defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef]
- Carfagna, S.; Salbitani, G.; Innangi, M.; Menale, B.; De Castro, O.; Di Martino, C.; Crawford, T.W. Simultaneous Biochemical and Physiological Responses of the Roots and Leaves of Pancratium Maritimum (Amaryllidaceae) to Mild Salt Stress. Plants 2021, 10, 345. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Vernoux, T.; Wilson, R.C.; Seeley, K.A.; Reichheld, J.-P.; Muroy, S.; Brown, S.; Maughan, S.C.; Cobbett, C.S.; Van Montagu, M.; Inzé, D.; et al. The Root Meristemless1/Cadmium Sensitive2 Gene Defines a Glutathione-Dependent Pathway Involved in Initiation and Maintenance of Cell Division during Postembryonic Root Development. Plant Cell 2000, 12, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Hatano-Iwasaki, A.; Yanagida, M.; Iwabuchi, M. Level of Glutathione Is Regulated by ATP-Dependent Ligation of Glutamate and Cysteine through Photosynthesis in Arabidopsis Thaliana: Mechanism of Strong Interaction of Light Intensity with Flowering. Plant Cell Physiol. 2004, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Perrone, A.; Rosati, L.; Laezza, C.; Carfagna, S. Sulfur Starvation in Extremophilic Microalga Galdieria Sulphuraria: Can Glutathione Contribute to Stress Tolerance? Plants 2022, 11, 481. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between Biosynthesis, Compartmentation and Transport in the Control of Glutathione Homeostasis and Signalling. J. Exp. Bot. 2002, 53, 1283–1304. [Google Scholar] [CrossRef]
- Wachter, A.; Wolf, S.; Steininger, H.; Bogs, J.; Rausch, T. Differential Targeting of GSH1 and GSH2 Is Achieved by Multiple Transcription Initiation: Implications for the Compartmentation of Glutathione Biosynthesis in the Brassicaceae. Plant J. 2005, 41, 15–30. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Singh, S.; Nazar, R.; Khan, N.A. Sulfur Assimilation and Cadmium Tolerance in Plants. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 271–302. ISBN 978-3-540-76326-0. [Google Scholar]
- HongBo, S.; ZongSuo, L.; MingAn, S.; ShiMeng, S.; ZanMin, H. Investigation on Dynamic Changes of Photosynthetic Characteristics of 10 Wheat (Triticum Aestivum L.) Genotypes during Two Vegetative-Growth Stages at Water Deficits. Colloids Surf. B Biointerfaces 2005, 43, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface Between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.H.; Mittova, V.; Kiddle, G.; Heazlewood, J.L.; Bartoli, C.G.; Theodoulou, F.L.; Foyer, C.H. Control of Ascorbate Synthesis by Respiration and Its Implications for Stress Responses. Plant Physiol. 2003, 133, 443–447. [Google Scholar] [CrossRef]
- Shao, H.-B.; Chu, L.-Y.; Lu, Z.-H.; Kang, C.-M. Primary Antioxidant Free Radical Scavenging and Redox Signaling Pathways in Higher Plant Cells. Int. J. Biol. Sci. 2007, 4, 8–14. [Google Scholar] [CrossRef]
- Yazaki, K. ABC Transporters Involved in the Transport of Plant Secondary Metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega Villasante, C.; Escobar, C. Contribution of Glutathione to the Control of Cellular Redox Homeostasis under Toxic Metal and Metalloid Stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [PubMed]
- Nocito, F.F.; Lancilli, C.; Crema, B.; Fourcroy, P.; Davidian, J.-C.; Sacchi, G.A. Heavy Metal Stress and Sulfate Uptake in Maize Roots. Plant Physiol. 2006, 141, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Petraglia, A.; De Benedictis, M.; Degola, F.; Pastore, G.; Calcagno, M.; Ruotolo, R.; Mengoni, A.; Sanità di Toppi, L. The Capability to Synthesize Phytochelatins and the Presence of Constitutive and Functional Phytochelatin Synthases Are Ancestral (Plesiomorphic) Characters for Basal Land Plants. J. Exp. Bot. 2014, 65, 1153–1163. [Google Scholar] [CrossRef]
- Li, M.; Barbaro, E.; Bellini, E.; Saba, A.; Sanità di Toppi, L.; Varotto, C. Ancestral Function of the Phytochelatin Synthase C-Terminal Domain in Inhibition of Heavy Metal-Mediated Enzyme Overactivation. J. Exp. Bot. 2020, 71, 6655–6669. [Google Scholar] [CrossRef]
- Fontanini, D.; Andreucci, A.; Ruffini Castiglione, M.; Basile, A.; Sorbo, S.; Petraglia, A.; Degola, F.; Bellini, E.; Bruno, L.; Varotto, C.; et al. The Phytochelatin Synthase from Nitella Mucronata (Charophyta) Plays a Role in the Homeostatic Control of Iron(II)/(III). Plant Physiol. Biochem. 2018, 127, 88–96. [Google Scholar] [CrossRef]
- Bleuel, C.; Wesenberg, D.; Sutter, K.; Miersch, J.; Braha, B.; Bärlocher, F.; Krauss, G.-J. The Use of the Aquatic Moss Fontinalis Antipyretica L. Ex Hedw. as a Bioindicator for Heavy Metals: 3. Cd2+ Accumulation Capacities and Biochemical Stress Response of Two Fontinalis Species. Sci. Total Environ. 2005, 345, 13–21. [Google Scholar] [CrossRef]
- Panda, S.K.; Choudhury, S. Changes in Nitrate Reductase Activity and Oxidative Stress Response in the Moss Polytrichum Commune Subjected to Chromium, Copper and Zinc Phytotoxicity. Braz. J. Plant Physiol. 2005, 17, 191–197. [Google Scholar] [CrossRef]
- Rother, M.; Krauss, G.-J.; Grass, G.; Wesenberg, D. Sulphate Assimilation under Cd2+ Stress in Physcomitrella Patens—Combined Transcript, Enzyme and Metabolite Profiling. Plant Cell Environ. 2006, 29, 1801–1811. [Google Scholar] [CrossRef]
- Sharma, P.; Bano, A.; Nadda, A.K.; Sharma, S.; Varjani, S.; Singh, S.P. Crosstalk and Gene Expression in Microorganisms under Metals Stress. Arch. Microbiol. 2022, 204, 410. [Google Scholar] [CrossRef] [PubMed]
- Murasugi, A.; Wada, C.; Hayashi, Y. Cadmium-Binding Peptide Induced in Fission Yeast, Schizosaccharomyces Pombe. J. Biochem. 1981, 90, 1561–1565. [Google Scholar] [CrossRef]
- Kondo, N.; Imai, K.; Isobe, M.; Goto, T.; Murasugi, A.; Wada-Nakagawa, C.; Hayashi, Y. Cadystin a and b, Major Unit Peptides Comprising Cadmium Binding Peptides Induced in a Fission Yeast—Separation, Revision of Structures and Synthesis. Tetrahedron Lett. 1984, 25, 3869–3872. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Liang, L.; Chen, C.; Wei, S.; Zhou, Q. Phytochelatin and Oxidative Stress Under Heavy Metal Stress Tolerance in Plants. In Reactive Oxygen Species and Oxidative Damage in Plants Under Stress; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 191–217. ISBN 978-3-319-20421-5. [Google Scholar]
- Grill, E.; Winnacker, E.-L.; Zenk, M.H. Phytochelatins: The Principal Heavy-Metal Complexing Peptides of Higher Plants. Science 1985, 230, 674–677. [Google Scholar] [CrossRef]
- Rea, P.A.; Vatamaniuk, O.K.; Rigden, D.J. Weeds, Worms, and More. Papain’s Long-Lost Cousin, Phytochelatin Synthase. Plant Physiol. 2004, 136, 2463–2474. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L. Role of Phytoremediation Strategies in Removal of Heavy Metals. In Emerging Issues in the Water Environment during Anthropocene: A South East Asian Perspective; Springer: Singapore, 2020; pp. 223–259. ISBN 978-981-329-770-8. [Google Scholar]
- Grill, E.; Löffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the Heavy-Metal-Binding Peptides of Plants, Are Synthesized from Glutathione by a Specific Gamma-Glutamylcysteine Dipeptidyl Transpeptidase (Phytochelatin Synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S. Phytochelatin Biosynthesis and Function in Heavy-Metal Detoxification. Curr. Opin. Plant Biol. 2000, 3, 211–216. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and Metallothioneins: Roles in Heavy Metal Detoxification and Homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Bellini, E.; Betti, C.; Sanità di Toppi, L. Responses to Cadmium in Early-Diverging Streptophytes (Charophytes and Bryophytes): Current Views and Potential Applications. Plants 2021, 10, 770. [Google Scholar] [CrossRef]
- Li, M.; Leso, M.; Buti, M.; Bellini, E.; Bertoldi, D.; Saba, A.; Larcher, R.; Sanità di Toppi, L.; Varotto, C. Phytochelatin Synthase De-Regulation in Marchantia Polymorpha Indicates Cadmium Detoxification as Its Primary Ancestral Function in Land Plants and Provides a Novel Visual Bioindicator for Detection of This Metal. J. Hazard. Mater. 2022, 440, 129844. [Google Scholar] [CrossRef]
- Davidian, J.C.; Grill, D.; De Kok, L.J.; Stulen, I.; Hawkesford, M.J.; Schnug, E.; Rennenberg, H. Sulfur Transport and Assimilation in Plants: Regulation, Interaction, Signaling; Backhuys Publishers: New York, NY, USA, 2003; ISBN 978-90-5782-138-7. [Google Scholar]
- Salbitani, G.; Wirtz, M.; Hell, R.; Carfagna, S. Affinity Purification of O-Acetylserine(Thiol)Lyase from Chlorella Sorokiniana by Recombinant Proteins from Arabidopsis Thaliana. Metabolites 2014, 4, 629–639. [Google Scholar] [CrossRef]
- Pakdee, O.; Songnuan, W.; Panvisavas, N.; Pokethitiyook, P.; Yokthongwattana, K.; Meetam, M. Functional characterization of metallothionein-like genes from Physcomitrella patens: Expression profiling, yeast heterologous expression, and disruption of PpMT1.2a gene. Planta 2019, 250, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, X.; Qian, M.; Zheng, L.; Lian, C.; Xia, Y.; Shen, Z. Copper-induced hydrogen peroxide upregulation of a metallothionein gene, OsMT2c, from Oryza sativa L. confers copper tolerance in Arabidopsis thaliana. J. Hazard. Mater. 2015, 294, 99–108. [Google Scholar] [CrossRef]
- Grennan, A.K. Metallothioneins, a diverse protein family. Plant Physiol. 2011, 155, 1750–1751. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salbitani, G.; Maresca, V.; Cianciullo, P.; Bossa, R.; Carfagna, S.; Basile, A. Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance. Int. J. Mol. Sci. 2023, 24, 5302. https://doi.org/10.3390/ijms24065302
Salbitani G, Maresca V, Cianciullo P, Bossa R, Carfagna S, Basile A. Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance. International Journal of Molecular Sciences. 2023; 24(6):5302. https://doi.org/10.3390/ijms24065302
Chicago/Turabian StyleSalbitani, Giovanna, Viviana Maresca, Piergiorgio Cianciullo, Rosanna Bossa, Simona Carfagna, and Adriana Basile. 2023. "Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance" International Journal of Molecular Sciences 24, no. 6: 5302. https://doi.org/10.3390/ijms24065302
APA StyleSalbitani, G., Maresca, V., Cianciullo, P., Bossa, R., Carfagna, S., & Basile, A. (2023). Non-Protein Thiol Compounds and Antioxidant Responses Involved in Bryophyte Heavy-Metal Tolerance. International Journal of Molecular Sciences, 24(6), 5302. https://doi.org/10.3390/ijms24065302












