Sessile Trichomes Play Major Roles in Prey Digestion and Absorption, While Stalked Trichomes Function in Prey Predation in Byblis guehoi
Abstract
:1. Introduction
2. Results
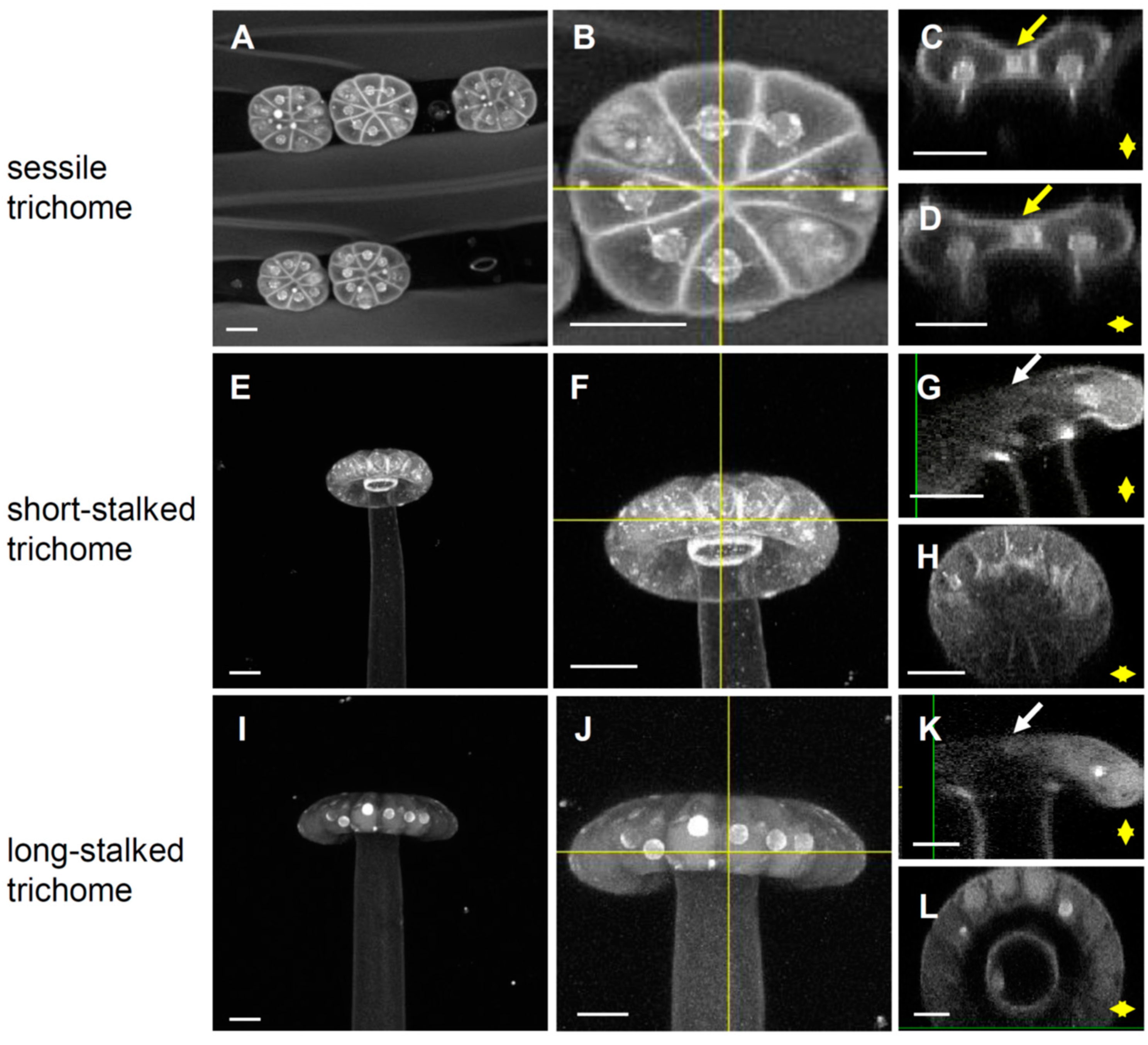
2.1. Byblis Leaves Harbor Three Kinds of Glandular Trichomes
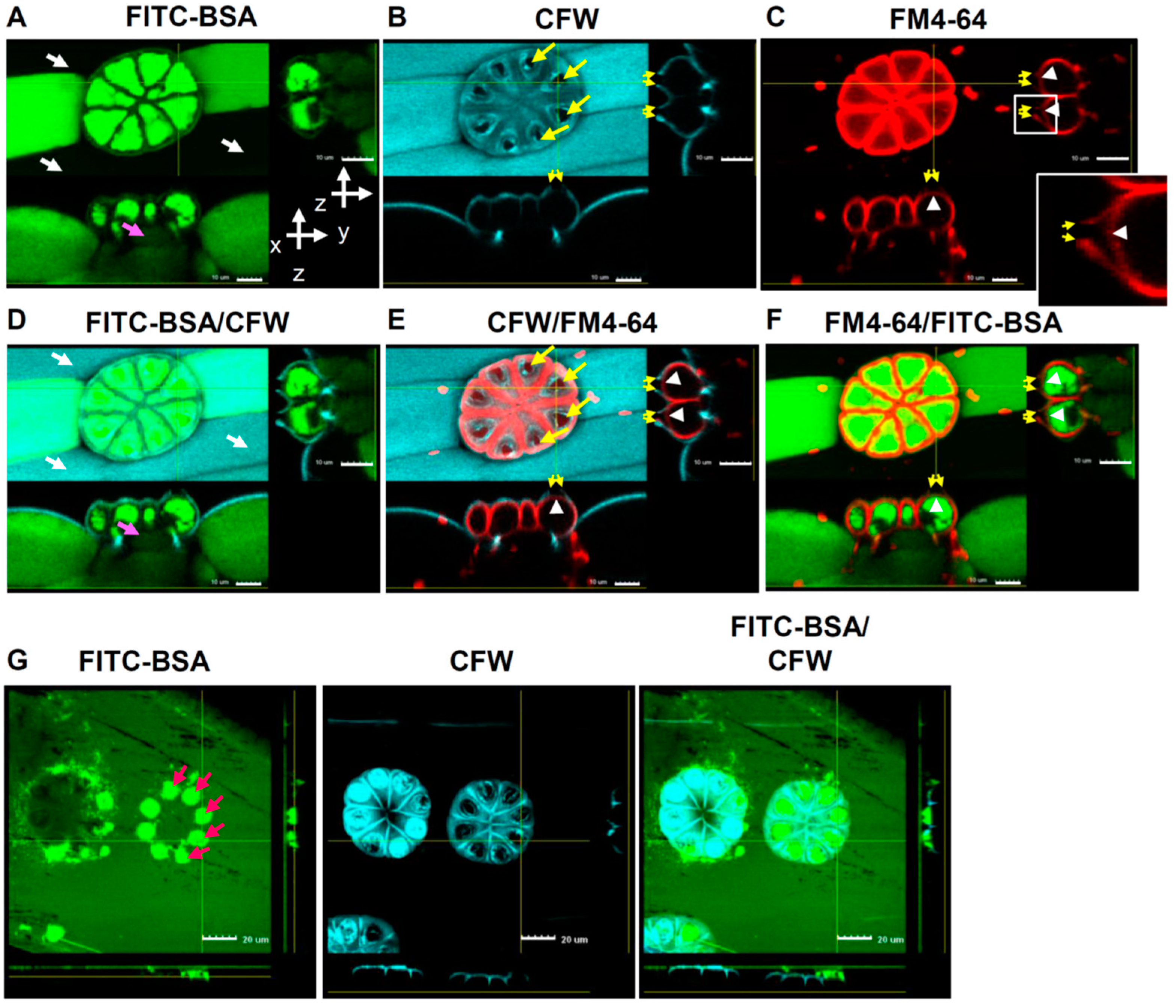
2.2. Close-Up Observations of the Glandular Trichomes
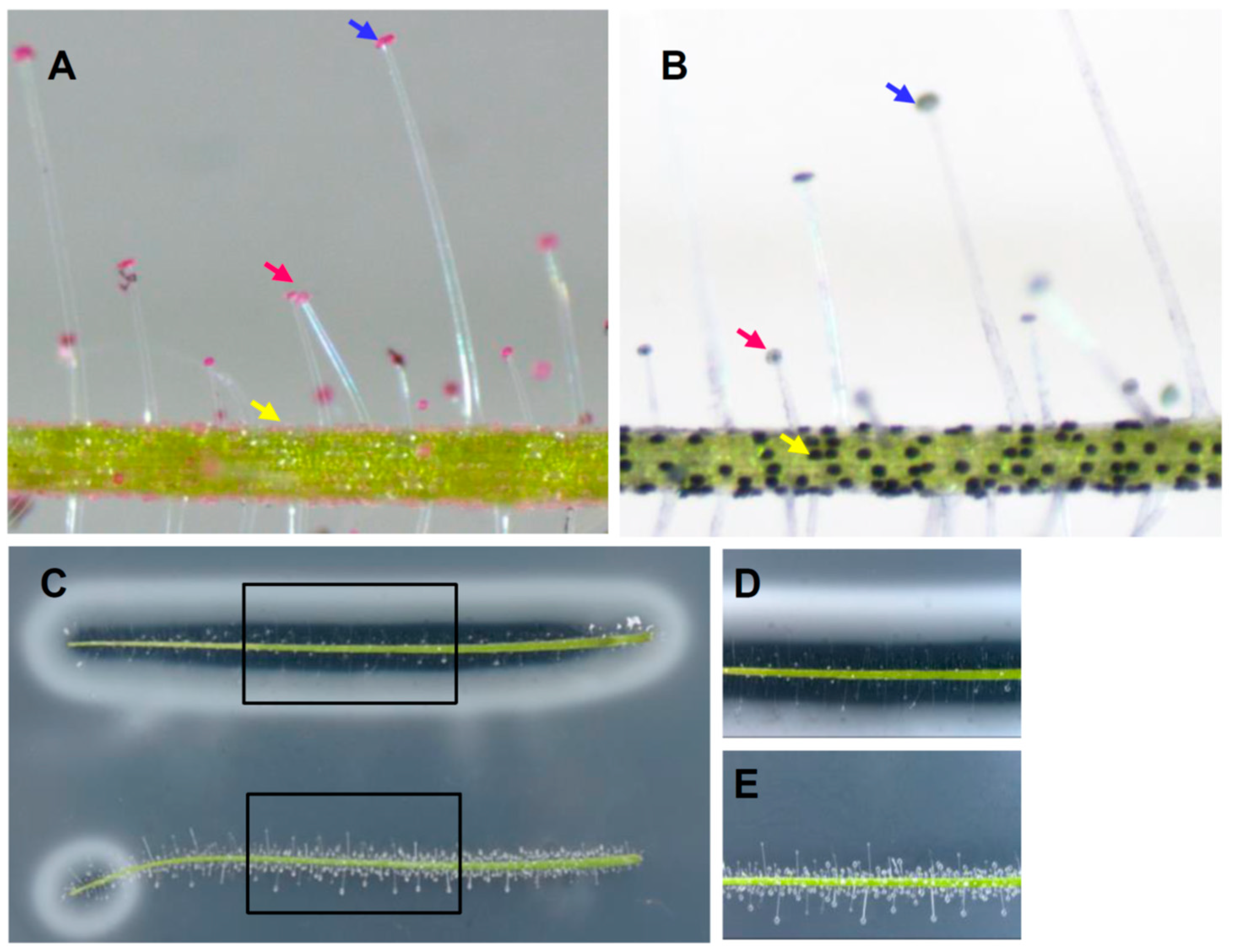
2.3. Differential Roles of Glandular Trichomes Are Suggested by the Different Compounds Secreted
2.4. Differential Roles of Glandular Trichomes Are Suggested by the Different Rates of Nutrient Uptake
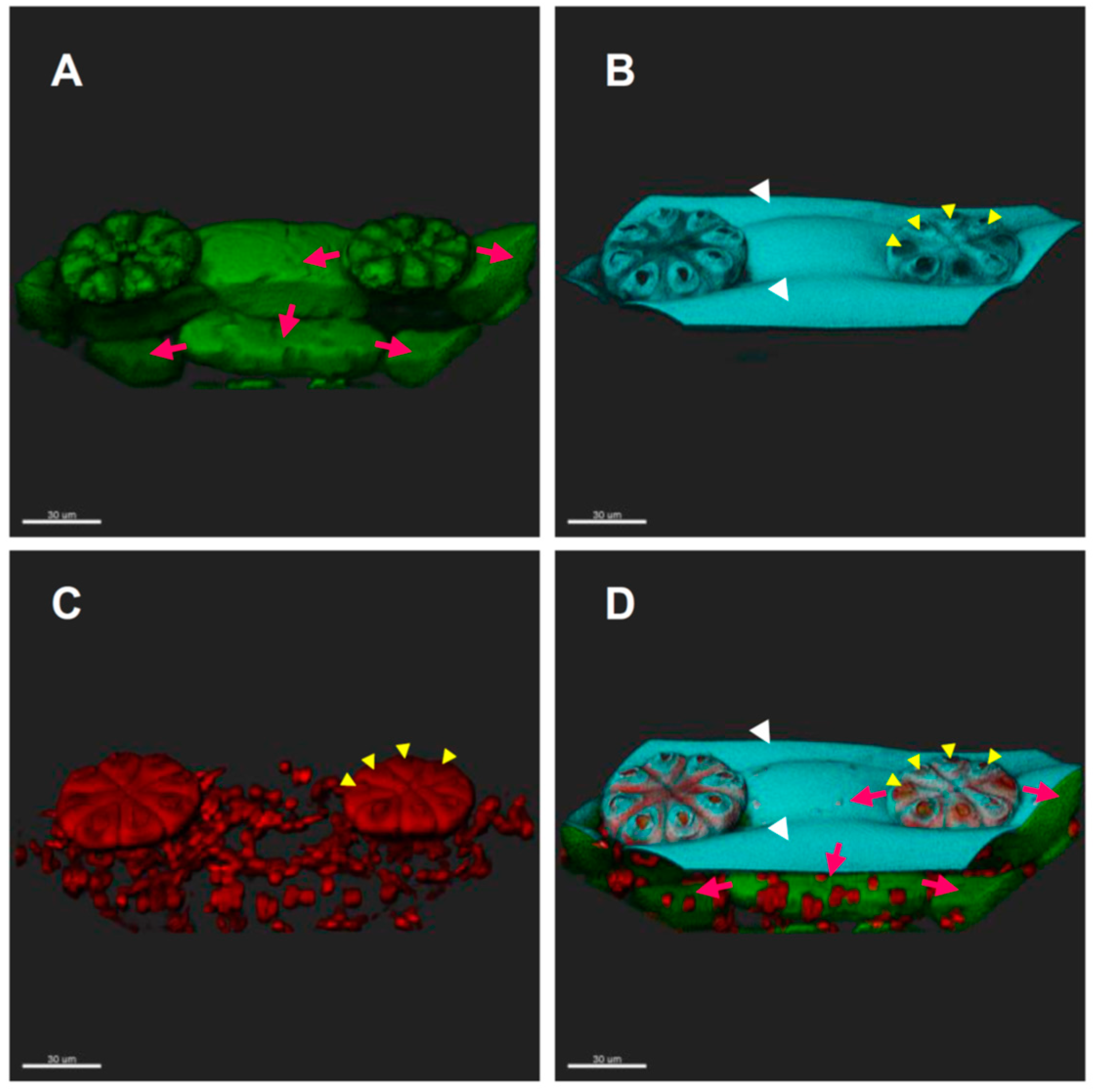
2.5. Route of Intercellular Transport of Nutrients after Uptake by Sessile Trichomes
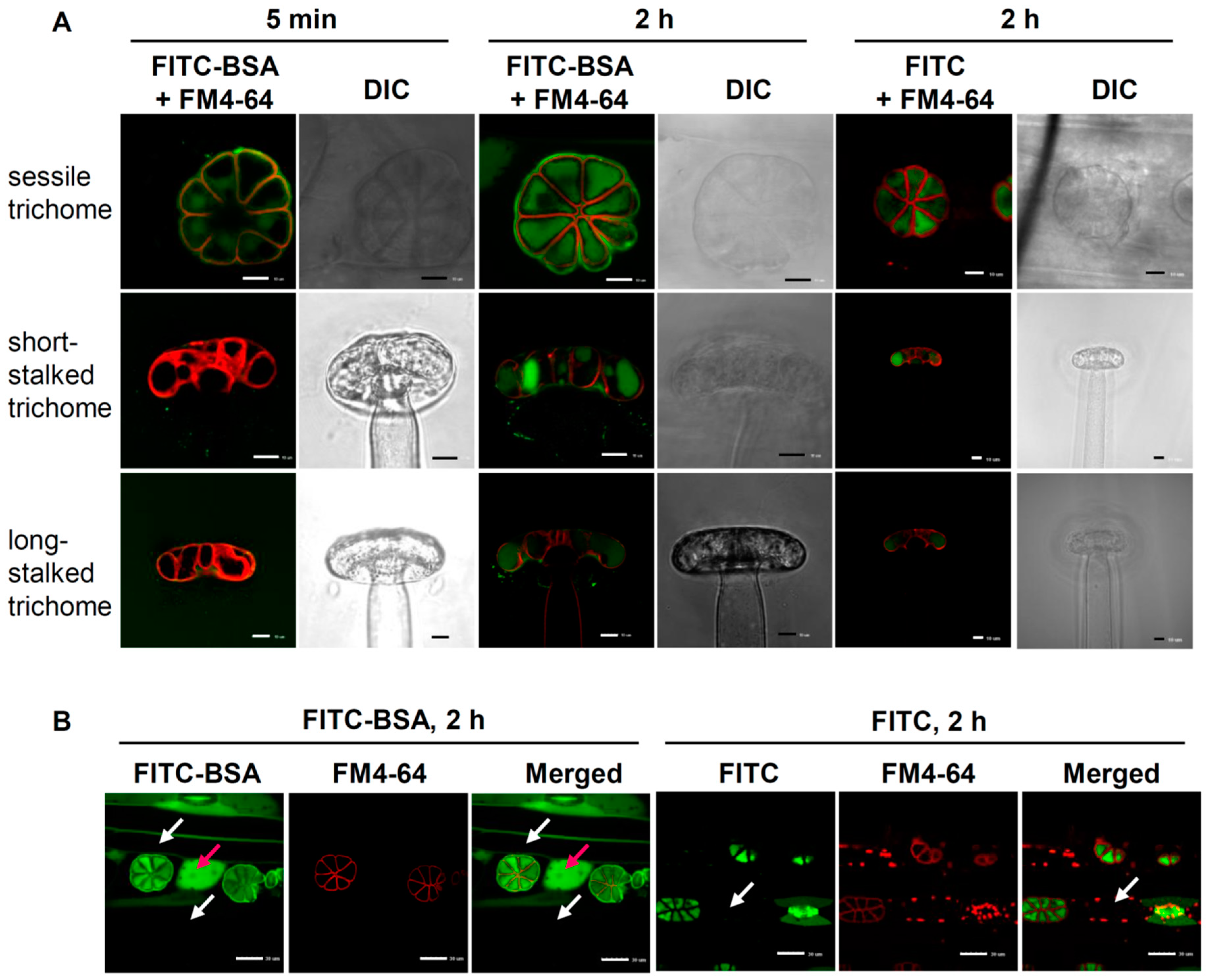
2.6. Pores on the Sessile Trichomes Allow the Entrance of FITC-BSA
3. Discussion
3.1. Different Glandular Trichomes Play Different Roles in B. guehoi and Other Carnivorous Plants
3.2. Incorporation of FITC-BSA into Sessile Cells Is Mediated by Endocytosis
3.3. Nutrients Are Absorbed via Pores Located on the Sessile Trichomes of B. guehoi
3.4. Suspected Polar Distributions of Plasmodesmata in the “Trichome ROW” of Epidermal Cells
4. Materials and Methods
4.1. Source of B. guehoi and Plant Cultivation
4.2. Measurement of Lengths and Densities of Glandular Trichomes
4.3. Histochemical Staining and Light Microscopy
4.4. Confocal Laser Scanning Microscopy and Image Acquisition
4.5. Enzyme Activity Assays
4.6. Nutrient Uptake Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Darwin, C.; Darwin, F.; Murray, J. Insectivorous Plants; John Murray: London, UK, 1875. [Google Scholar]
- Lloyd, F.E. The Carnivorous Plants; Ronald Press: New York, NY, USA, 1942. [Google Scholar]
- Juniper, B.E.; Robins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: Cambridge, MA, USA, 1989. [Google Scholar]
- Ellison, A.M.; Adamec, L. Carnivorous Plants: Physiology, Ecology, and Evolution; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Givnish, T.J. New evidence on the origin of carnivorous plants. Proc. Natl. Acad. Sci. USA 2015, 112, 10–11. [Google Scholar] [CrossRef] [Green Version]
- Oropeza-Aburto, A.; Cervantes-Perez, S.A.; Albert, V.A.; Herrera-Estrella, L. Agrobacterium tumefaciens mediated transformation of the aquatic carnivorous plant Utricularia gibba. Plant Methods 2020, 16, 50. [Google Scholar] [CrossRef] [Green Version]
- Renner, T.; Specht, C.D. Inside the trap: Gland morphologies, digestive enzymes, and the evolution of plant carnivory in the Caryophyllales. Curr. Opin. Plant Biol. 2013, 16, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Callow, J.A.; Hallahan, D.L.; Gray, J.C. Plant Trichomes; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Heil, M. Nectar: Generation, regulation and ecological functions. Trends Plant Sci. 2011, 16, 191–200. [Google Scholar] [CrossRef]
- Mehltreter, K.; Tenhaken, R.; Jansen, S. Nectaries in ferns: Their taxonomic distribution, structure, function, and sugar composition. Am. J. Bot. 2022, 109, 46–57. [Google Scholar] [CrossRef]
- Schuurink, R.; Tissier, A. Glandular trichomes: Micro-organs with model status? New Phytol. 2020, 225, 2251–2266. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, R.W.; Wagner, G.J. Phylloplane proteins: Emerging defenses at the aerial frontline? Trends Plant Sci. 2007, 12, 51–56. [Google Scholar] [CrossRef]
- Lin, Q.; Ane, C.; Givnish, T.J.; Graham, S.W. A new carnivorous plant lineage (Triantha) with a unique sticky-inflorescence trap. Proc. Natl. Acad. Sci. USA 2021, 118, e2022724118. [Google Scholar] [CrossRef]
- Plachno, B.J.; Adamec, L.; Huet, H. Mineral nutrient uptake from prey and glandular phosphatase activity as a dual test of carnivory in semi-desert plants with glandular leaves suspected of carnivory. Ann. Bot. 2009, 104, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Plachno, B.J.; Adamec, L.; Lichtscheidl, I.K.; Peroutka, M.; Adlassnig, W.; Vrba, J. Fluorescence labelling of phosphatase activity in digestive glands of carnivorous plants. Plant Biol. 2006, 8, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, K.; Fang, X.; Alvarez-Ponce, D.; Cai, H.; Carretero-Paulet, L.; Chen, C.; Chang, T.H.; Farr, K.M.; Fujita, T.; Hiwatashi, Y.; et al. Genome of the pitcher plant Cephalotus reveals genetic changes associated with carnivory. Nat. Ecol. Evol. 2017, 1, 59. [Google Scholar] [CrossRef] [Green Version]
- Hedrich, R.; Fukushima, K. On the Origin of Carnivory: Molecular Physiology and Evolution of Plants on an Animal Diet. Annu. Rev. Plant Biol. 2021, 72, 133–153. [Google Scholar] [CrossRef]
- Paszota, P.; Escalante-Perez, M.; Thomsen, L.R.; Risor, M.W.; Dembski, A.; Sanglas, L.; Nielsen, T.A.; Karring, H.; Thogersen, I.B.; Hedrich, R.; et al. Secreted major Venus flytrap chitinase enables digestion of Arthropod prey. Biochim. Biophys. Acta 2014, 1844, 374–383. [Google Scholar] [CrossRef]
- Ravee, R.; Mohd Salleh, F.; Goh, H.H. Discovery of digestive enzymes in carnivorous plants with focus on proteases. PeerJ 2018, 6, e4914. [Google Scholar] [CrossRef] [Green Version]
- Fenner, C.A. Beitrag zur Kenntnis der Anatomie, Entwickelungsgeschichte und Biologie der Laubblatter und Drusen einiger Insectivoren. Flora 1904, 93, 335–434. [Google Scholar]
- Bruce, A. N, On the activity of the glands of Byblis gigantea. Notes Roy. Bot. Garden Edin. 1905, 16, 9–14. [Google Scholar]
- Adlassnig W, Peroutka M, Lang I, and Lichtscheidl IK, Glands of carnivorous plants as a model system in cell biological research. Acta Bot. Gall. 2005, 152, 111–124. [CrossRef] [Green Version]
- Adlassnig, W.; Lendl, T.; Koller-Peroutka, M.; Lang, I. Deadly Glue—Adhesive Traps of Carnivorous Plants. In Biological Adhesive Systems; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Allan, G. Evidence of motile traps in Byblis. Carniv. Plant Newsl. 2019, 48, 51–63. [Google Scholar] [CrossRef]
- Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Koshkin, E.; Lendl, T.; Lichtscheidl, I.K. Endocytotic uptake of nutrients in carnivorous plants. Plant J. 2012, 71, 303–313. [Google Scholar] [CrossRef]
- Lichtscheidl, I.; Lancelle, S.; Weidinger, M.; Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Krammer, S.; Hepler, P.K. Gland cell responses to feeding in Drosera capensis, a carnivorous plant. Protoplasma 2021, 258, 1291–1306. [Google Scholar] [CrossRef]
- Freund, M.; Graus, D.; Fleischmann, A.; Gilbert, K.J.; Lin, Q.; Renner, T.; Stigloher, C.; Albert, V.A.; Hedrich, R.; Fukushima, K. The digestive systems of carnivorous plants. Plant Physiol. 2022, 190, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, S.; Knorr, N.; Ruppert, S.; Speck, T. Chemonastic Stalked Glands in the Carnivorous Rainbow Plant Byblis gigantea LINDL. (Byblidaceae, Lamiales). Int. J. Mol. Sci. 2022, 23, 11514. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Gorb, E.; Gorb, S. Hierarchical organisation of the trap in the protocarnivorous plant Roridula gorgonias (Roridulaceae). J. Exp. Biol. 2009, 212, 3184–3191. [Google Scholar] [CrossRef] [Green Version]
- van Gisbergen, P.A.; Esseling-Ozdoba, A.; Vos, J.W. Microinjecting FM4-64 validates it as a marker of the endocytic pathway in plants. J. Microsc. 2008, 231, 284–290. [Google Scholar] [CrossRef]
- Koller-Peroutka, M.; Krammer, S.; Pavlik, A.; Edlinger, M.; Lang, I.; Adlassnig, W. Endocytosis and Digestion in Carnivorous Pitcher Plants of the Family Sarraceniaceae. Plants 2019, 8, 367. [Google Scholar] [CrossRef] [Green Version]
- Płachno, B.J.; Kozieradzka-Kiszkurno, M.; Swiatek, P. Functional ultrastructure of Genlisea (Lentibulariaceae) digestive hairs. Ann. Bot. 2007, 100, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Herth, W.; Schnepf, E. The fluorochrome, calcofluor white, binds oriented to structural polysaccharide fibrils. Protoplasma 1980, 105, 129–133. [Google Scholar] [CrossRef]
- Bidhendi, A.J.; Chebli, Y.; Geitmann, A. Fluorescence visualization of cellulose and pectin in the primary plant cell wall. J. Microsc. 2020, 278, 164–181. [Google Scholar] [CrossRef]
- Lee, D.K.; Sieburth, L.E. Plasmodesmata formation: Poking holes in walls with ise. Curr. Biol. 2010, 20, R488–R490. [Google Scholar] [CrossRef] [Green Version]
- Sager, R.E.; Lee, J.Y. Plasmodesmata at a glance. J. Cell Sci. 2018, 131, jcs209346. [Google Scholar] [CrossRef] [Green Version]
- Colombo, P.M.; Rascio, N. Ruthenium red staining for electron microscopy of plant material. J. Ultrastruct. Res. 1977, 60, 135–139. [Google Scholar] [CrossRef]
- Chen, W.H.; Hsu, W.H.; Hsu, H.F.; Yang, C.H. A tetraspanin gene regulating auxin response and affecting orchid perianth size and various plant developmental processes. Plant Direct 2019, 3, e00157. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.-F.; Chen, W.-H.; Shen, Y.-H.; Hsu, W.-H.; Mao, W.-T.; Yang, C.-H. Multifunctional evolution of B and AGL6 MADS box genes in orchids. Nat. Commun. 2021, 12, 902. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Chen, A.; Leu, W.-M. Sessile Trichomes Play Major Roles in Prey Digestion and Absorption, While Stalked Trichomes Function in Prey Predation in Byblis guehoi. Int. J. Mol. Sci. 2023, 24, 5305. https://doi.org/10.3390/ijms24065305
Li Y-X, Chen A, Leu W-M. Sessile Trichomes Play Major Roles in Prey Digestion and Absorption, While Stalked Trichomes Function in Prey Predation in Byblis guehoi. International Journal of Molecular Sciences. 2023; 24(6):5305. https://doi.org/10.3390/ijms24065305
Chicago/Turabian StyleLi, You-Xian, Alvin Chen, and Wei-Ming Leu. 2023. "Sessile Trichomes Play Major Roles in Prey Digestion and Absorption, While Stalked Trichomes Function in Prey Predation in Byblis guehoi" International Journal of Molecular Sciences 24, no. 6: 5305. https://doi.org/10.3390/ijms24065305
APA StyleLi, Y.-X., Chen, A., & Leu, W.-M. (2023). Sessile Trichomes Play Major Roles in Prey Digestion and Absorption, While Stalked Trichomes Function in Prey Predation in Byblis guehoi. International Journal of Molecular Sciences, 24(6), 5305. https://doi.org/10.3390/ijms24065305








