Identification of CD114 Membrane Receptors as a Molecular Target in Medulloblastomas
Abstract
:1. Introduction
Objectives
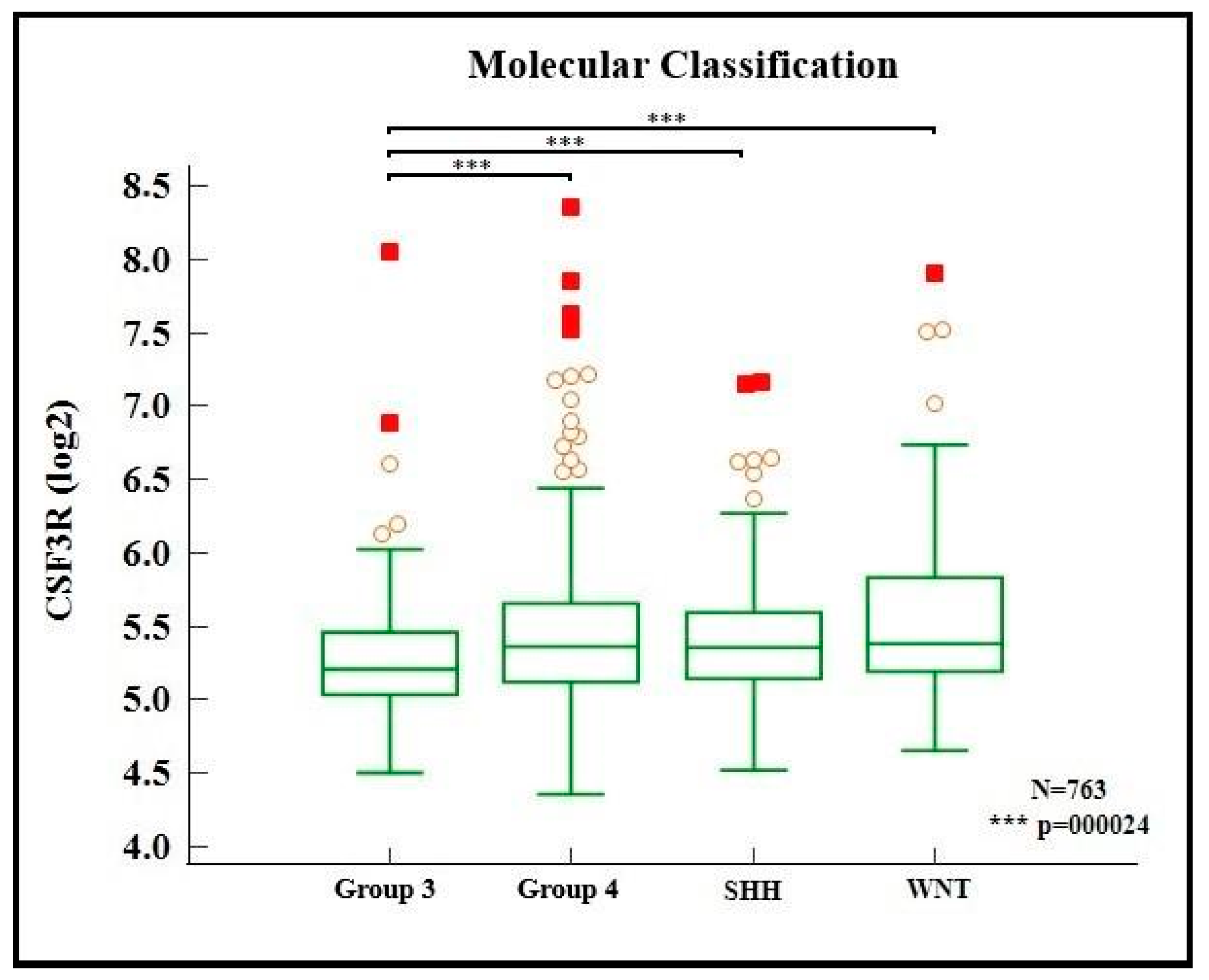
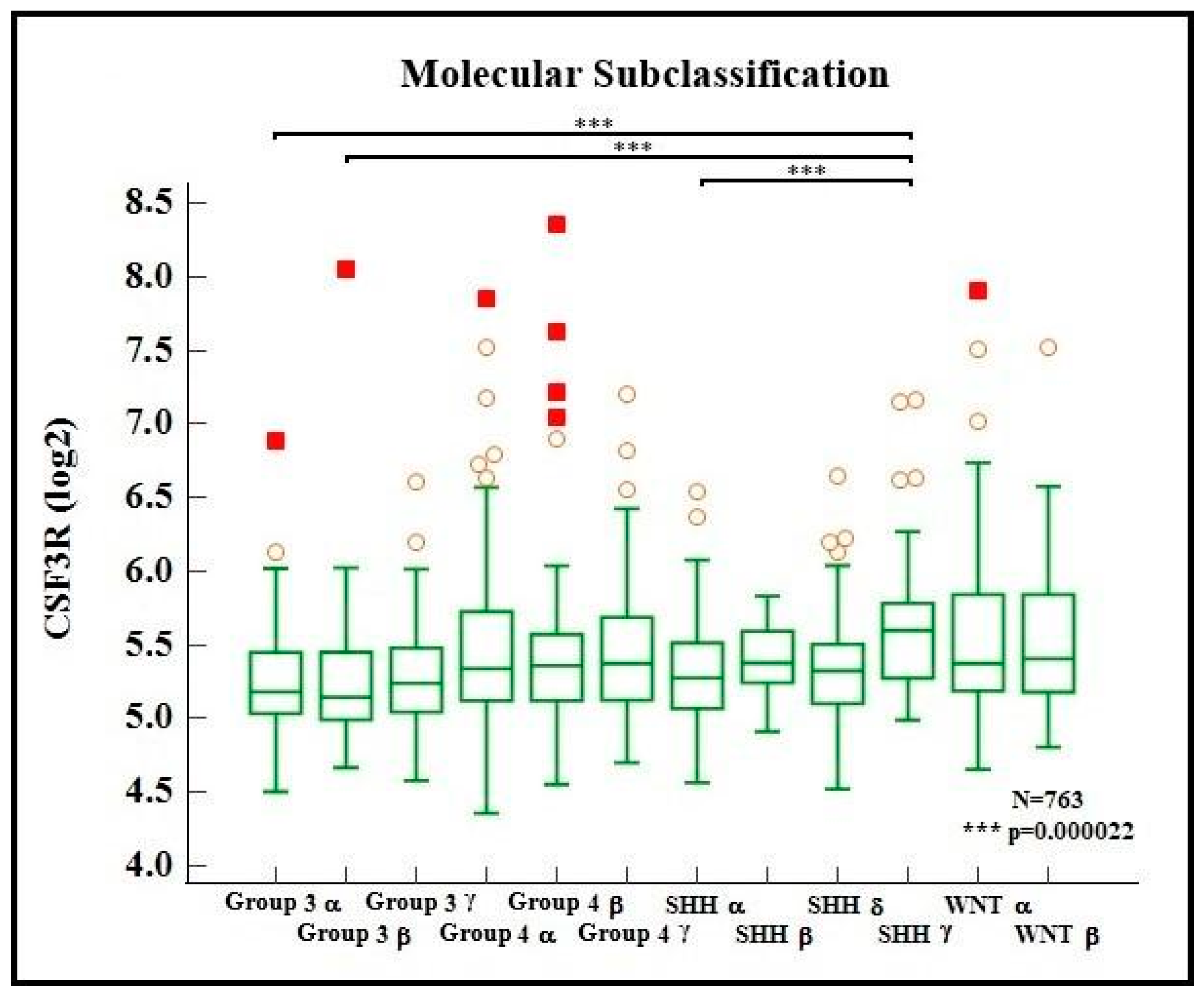
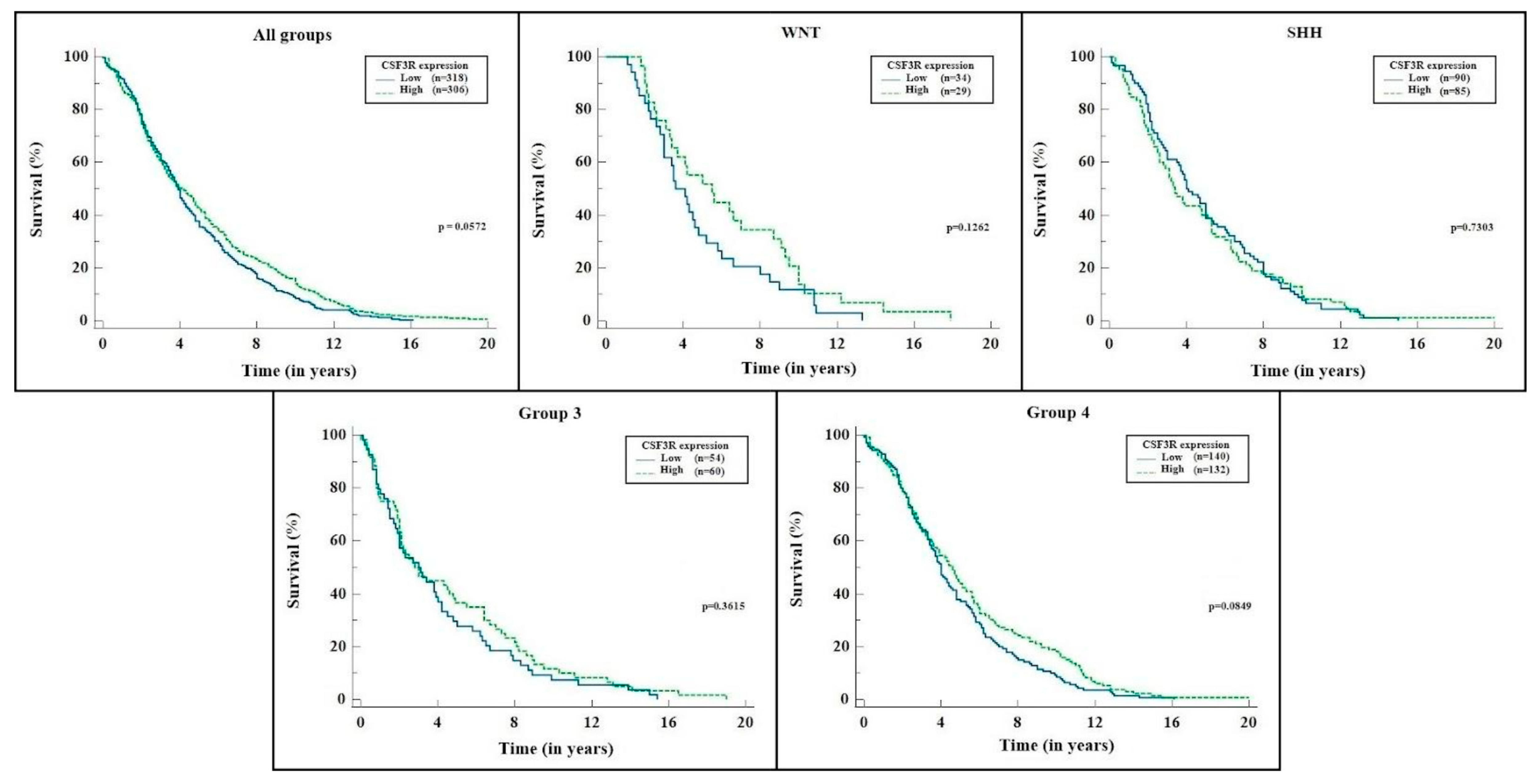
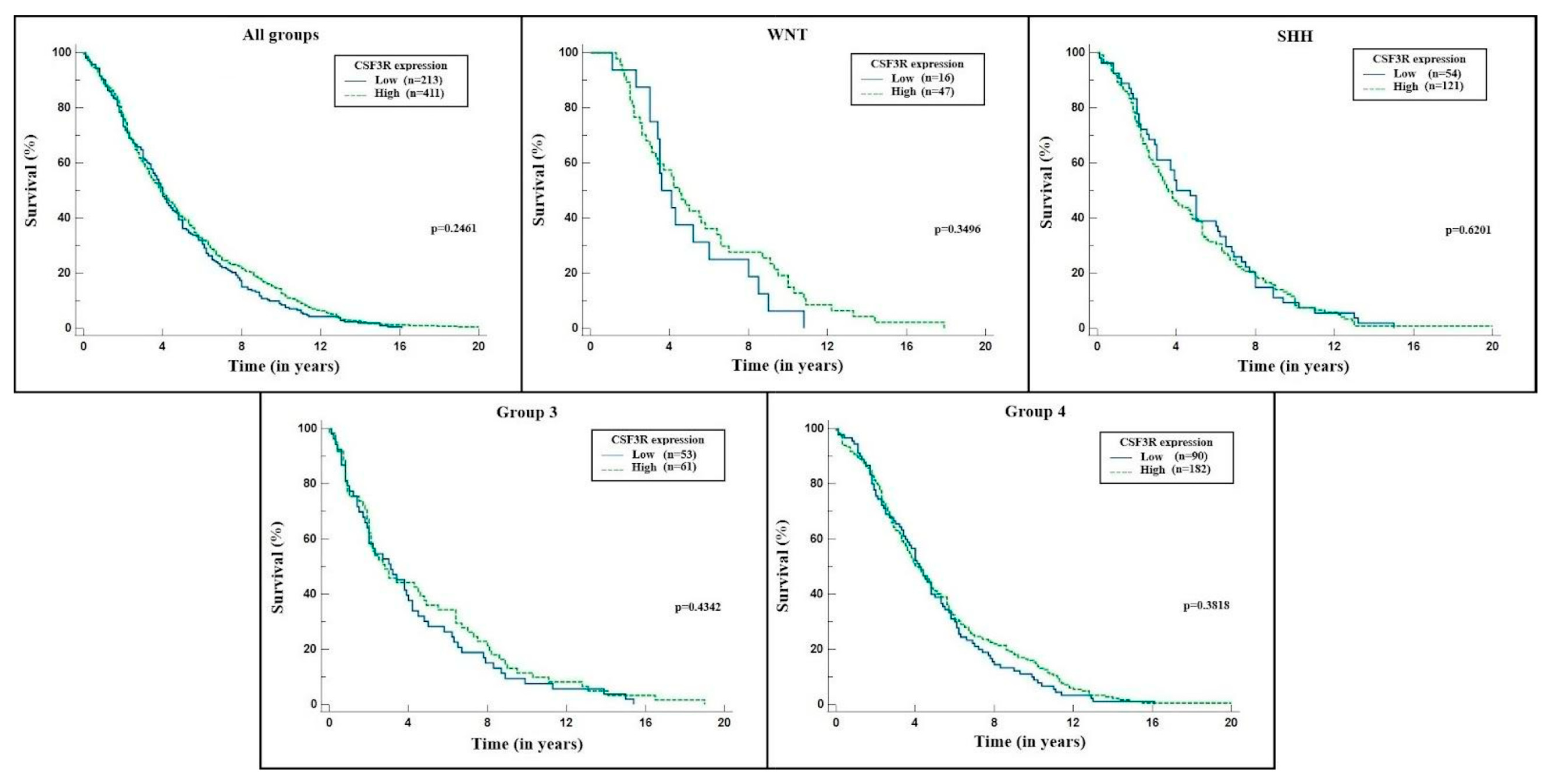
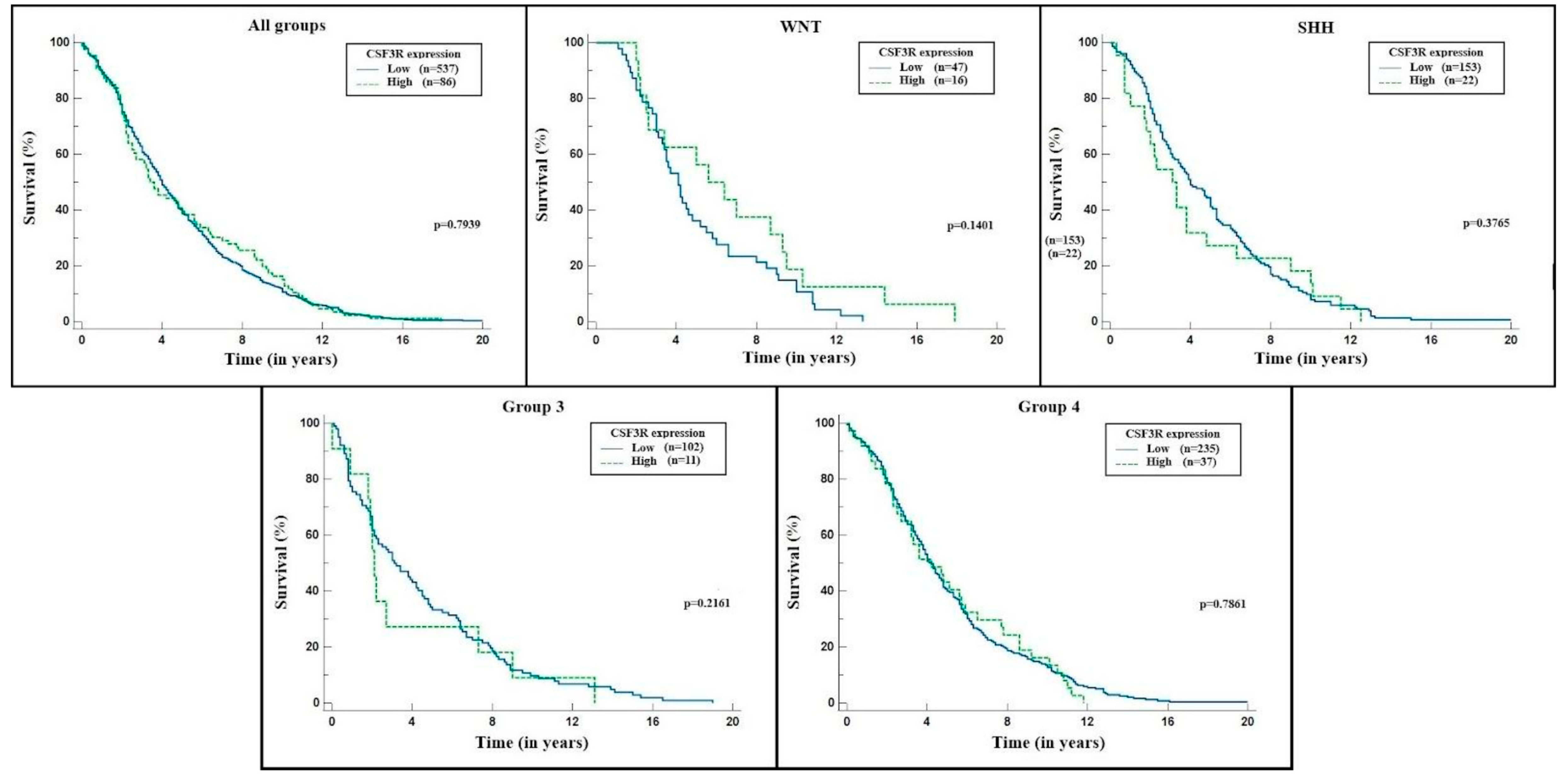
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Selection and Data Collection
4.2. Measurement of Gene Expression
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Archer, T.C.; Mahoney, E.L.; Pomeroy, S.L. Medulloblastoma: Molecular classification-based personal therapeutics. Neurotherapeutics 2017, 14, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, A.; Rizzolo, D. Understanding medulloblastoma. J. Am. Acad. PAs 2017, 30, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Millard, N.E.; De Braganca, K.C. Medulloblastoma. J. Child Neurol. 2016, 31, 1341–1353. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, A.; Guha, S. Granulocyte colony-stimulating factor/granulocyte colony-stimulating factor receptor biological axis promotes survival and growth of bladder cancer cells. Urology 2007, 69, 1210–1215. [Google Scholar] [CrossRef]
- Hirai, K.; Kumakiri, M.; Fujieda, S.; Sunaga, H.; Lao, L.-M.; Imamura, Y.; Ueda, K.; Fukuda, M. Expression of granulocyte colony-stimulating factor and its receptor in epithelial skin tumors. J. Dermatol. Sci. 2001, 25, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Savarese, T.M.; Mitchell, K.; McQuain, C.; Campbell, C.L.; Guardiani, R.; Wuu, J.; Ollari, C.; Reale, F.; Nelson, B.E.; Chen, A.; et al. Coexpression of granulocyte colony stimulating factor and its receptor in primary ovarian carcinomas. Cancer Lett. 2001, 162, 105–115. [Google Scholar] [CrossRef]
- Hsu, D.M.; Agarwal, S.; Benham, A.; Coarfa, C.; Trahan, D.N.; Chen, Z.; Stowers, P.N.; Courtney, A.N.; Lakoma, A.; Barbieri, E.; et al. G-CSF Receptor Positive Neuroblastoma Subpopulations Are Enriched in Chemotherapy-Resistant or Relapsed Tumors and Are Highly Tumorigenic Defining Tumorigenic Cells in Neuroblastoma. Cancer Res. 2013, 73, 4134–4146. [Google Scholar] [CrossRef] [Green Version]
- Cavalli, F.M.G.; Remke, M.; Rampasek, L.; Peacock, J.; Shih, D.J.; Luu, B.; Garzia, L.; Torchia, J.; Nor, C.; Morrissy, A.S.; et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 2017, 31, 737–754. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.R.; Huo, Y.; Liu, A.; Lesperance, J.; Garancher, A.; Wechsler-Reya, R.J.; Zage, P.E. Characterization of G-CSF receptor expression in medulloblastoma. Neuro Oncol. Adv. 2020, 2, vdaa062. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.; Tsherniak, A.; Tamayo, P.; Santagata, S.; Ligon, A.; Greulich, H.; Berhoukim, R.; Amani, V.; Goumnerova, L.; Eberhart, C.G.; et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J. Clin. Oncol. 2011, 29, 1424. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Northcott, P.A.; Korshunov, A.; Remke, M.; Cho, Y.-J.; Clifford, S.C.; Eberhart, C.G.; Parsons, D.W.; Rutkowski, S.; Gajjar, A.; et al. Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol. 2012, 123, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakura, F.; Nishiya, K.; Wentzel, A.S.; Hino, E.; Miyamae, J.; Okano, M.; Wiegertjes, G.F.; Moritomo, T. Paralogs of common carp granulocyte colony-stimulating factor (G-CSF) have different functions regarding development, trafficking and activation of neutrophils. Front. Immunol. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Zage, P.E.; Whittle, S.B.; Shohet, J.M. CD114: A new member of the neural Crest-Derived cancer stem cell marker family. J. Cell. Biochem. 2017, 118, 221–231. [Google Scholar] [CrossRef]
- Kumar, J.; Fraser, F.W.; Riley, C.; Ahmed, N.; McCulloch, D.R.; Ward, A.C. Granulocyte colony-stimulating factor receptor signalling via Janus kinase 2/signal transducer and activator of transcription 3 in ovarian cancer. Br. J. Cancer 2014, 110, 133–145. [Google Scholar] [CrossRef]
- Gnanaraj, J.; Parnes, A.; Francis, C.W.; Go, R.S.; Takemoto, C.M.; Hashmi, S.K. Approach to pancytopenia: Diagnostic algorithm for clinical hematologists. Blood Rev. 2018, 32, 361–367. [Google Scholar] [CrossRef]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef] [Green Version]
- Staar, S.; Rudat, V.; Stuetzer, H.; Dietz, A.; Volling, P.; Schroeder, M.; Flentje, M.; Eckel, H.E.; Mueller, R.-P. Intensified hyperfractionated accelerated radiotherapy limits the additional benefit of simultaneous chemotherapy—Results of a multicentric randomized German trial in advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 1161–1171. [Google Scholar] [CrossRef]
- Russell, H.; Shohet, J.M. G-CSF counteracts chemotherapy toxicity in neuroblastoma. Nature Reviews. Clin. Oncol. 2011, 8, 6–8. [Google Scholar] [CrossRef]
- Nazio, F.; Po, A.; Abballe, L.; Ballabio, C.; Camassei, F.D.; Bordi, M.; Camera, A.; Caruso, S.; Caruana, I.; Pezzullo, M.; et al. Targeting cancer stem cells in medulloblastoma by inhibiting AMBRA1 dual function in autophagy and STAT3 signalling. Acta Neuropathol. 2021, 142, 537–564. [Google Scholar] [CrossRef]
- Paul, M.R.; Zage, P.E. Overview and recent advances in the targeting of medulloblastoma cancer stem cells. Expert Rev. Anticancer Ther. 2021, 21, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Walter, A.; Kobert, N.; Mihic-Probst, D.; Zipser, M.; Belloni, B.; Seifert, B.; Moch, H.; Dummer, R.; Broek, M.V.D.; et al. Human CD271-Positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth human melanoma contains CD271-positive melanoma stem cells. Cancer Res. 2011, 71, 3098–3109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of cells initiating human melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, C.; Valduga, J.; Chateau, A.; Richard, M.; Pellegrini-Moïse, N.; Barberi-Heyob, M.; Chastagner, P.; Boura, C. Stimulation of medulloblastoma stem cells differentiation by a peptidomimetic targeting neuropilin-1. Oncotarget 2018, 9, 15312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vo, D.T.; Subramaniam, D.; Remke, M.; Burton, T.L.; Uren, P.J.; Gelfond, J.A.; Abreu, R.D.S.; Burns, S.C.; Qiao, M.; Suresh, U.; et al. The RNA-binding protein Musashi1 affects medulloblastoma growth via a network of cancer-related genes and is an indicator of poor prognosis. Am. J. Pathol. 2012, 181, 1762–1772. [Google Scholar] [CrossRef] [Green Version]
- Kahn, S.A.; Wang, X.; Nitta, R.T.; Gholamin, S.; Theruvath, J.; Hutter, G.; Azad, T.D.; Wadi, L.; Bolin, S.; Ramaswamy, V.; et al. Notch1 regulates the initiation of metastasis and self-renewal of Group 3 medulloblastoma. Nat. Commun. 2018, 9, 4121. [Google Scholar] [CrossRef] [Green Version]
- Roussel, M.F.; Robinson, G.W. Role of MYC in medulloblastoma. Cold Spring Harb. Perspect. Med. 2013, 3, a014308. [Google Scholar] [CrossRef] [Green Version]
- Skoda, J.; Nunukova, A.; Loja, T.; Zambo, I.; Neradil, J.; Mudry, P.; Zitterbart, K.; Hermanova, M.; Hampl, A.; Sterba, J.; et al. Cancer stem cell markers in pediatric sarcomas: Sox2 is associated with tumorigenicity in immunodeficient mice. Tumor Biol. 2016, 37, 9535–9548. [Google Scholar] [CrossRef]
- Manoranjan, B.; Venugopal, C.; McFarlane, N.; Doble, B.; Dunn, S.E.; Scheinemann, K.; Singh, S.K. Medulloblastoma stem cells: Modeling tumor heterogeneity. Cancer Lett. 2013, 338, 23–31. [Google Scholar] [CrossRef]
| Variable | WNT | SHH | Group 3 | Group 4 | Total Sample | |
|---|---|---|---|---|---|---|
| Age (years) | 10.8 (8.0–16.0) | 8.8 (2.1–21.5) | 5.1 (3.1–8.0) | 8.0 (5.8–11.0) | 8.0 (4.8–12.0) | |
| Gender | Male | 29 | 128 | 99 | 216 | 472 |
| Female | 35 | 82 | 38 | 92 | 247 | |
| Gender ratio | 0.8 | 1.6 | 2.6 | 2.3 | 1.9 | |
| Survival (years) | 4.2 (2.7–8.3) | 3.9 (2.1–7.0) | 3.0 (1.4–6.7) | 4.2 (2.3–6.8) | 4.0 (2.1–6.9) | |
| Variable | WNT | SHH | Group 3 | Group 4 | Total Sample |
|---|---|---|---|---|---|
| Classical | 40 (10.3%) | 78 (20.2%) | 68 (17.6%) | 201 (51.9%) | 387 (100%) |
| Desmoplastic | 5 (4.6%) | 73 (67%) | 8 (7.3%) | 23 (21.1%) | 109 (100%) |
| MBEN | 0 (0%) | 10 (55.6%) | 2 (11.1%) | 6 (33.3%) | 18 (100%) |
| LCA | 5 (7%) | 20 (28%) | 25 (35%) | 22 (30%) | 72 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, J.M.; Reis Ramos, J.I.; Teixeira e Sousa, I.; Bighetti-Trevisan, R.L.; Ribas Filho, J.M.; Isolan, G.R. Identification of CD114 Membrane Receptors as a Molecular Target in Medulloblastomas. Int. J. Mol. Sci. 2023, 24, 5331. https://doi.org/10.3390/ijms24065331
Monteiro JM, Reis Ramos JI, Teixeira e Sousa I, Bighetti-Trevisan RL, Ribas Filho JM, Isolan GR. Identification of CD114 Membrane Receptors as a Molecular Target in Medulloblastomas. International Journal of Molecular Sciences. 2023; 24(6):5331. https://doi.org/10.3390/ijms24065331
Chicago/Turabian StyleMonteiro, Jander Moreira, Jaqueline Isadora Reis Ramos, Ian Teixeira e Sousa, Rayana Longo Bighetti-Trevisan, Jurandir Marcondes Ribas Filho, and Gustavo Rassier Isolan. 2023. "Identification of CD114 Membrane Receptors as a Molecular Target in Medulloblastomas" International Journal of Molecular Sciences 24, no. 6: 5331. https://doi.org/10.3390/ijms24065331





