Microparticles in the Development and Improvement of Pharmaceutical Formulations: An Analysis of In Vitro and In Vivo Studies
Abstract
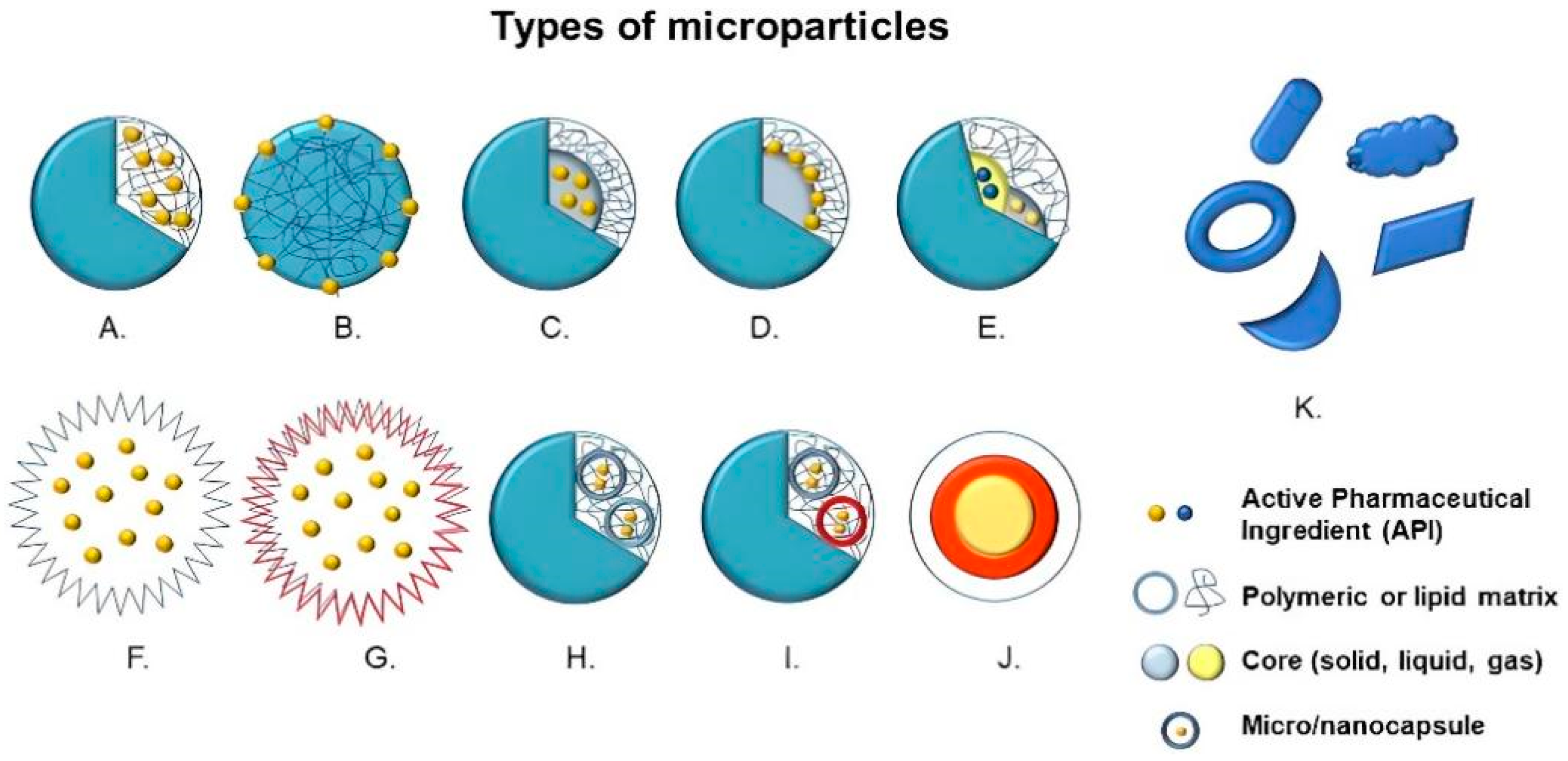
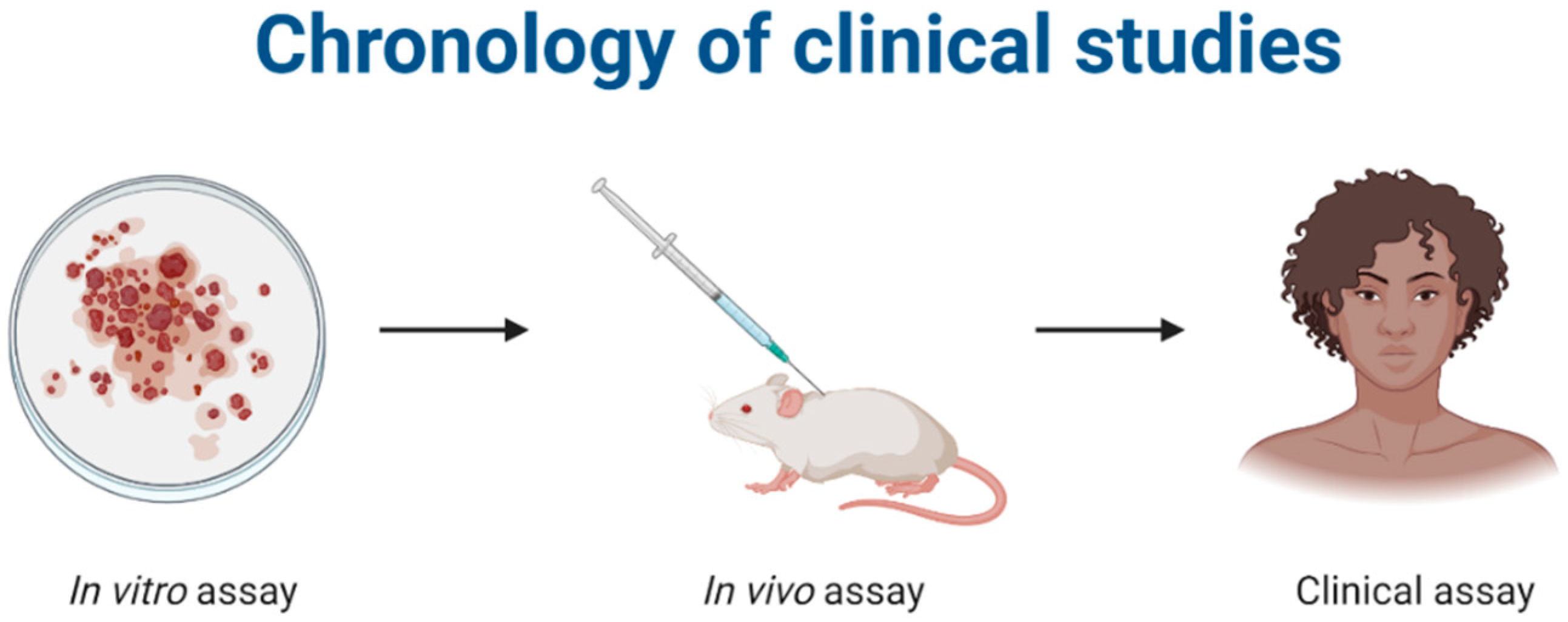
:1. Introduction
2. Methodology
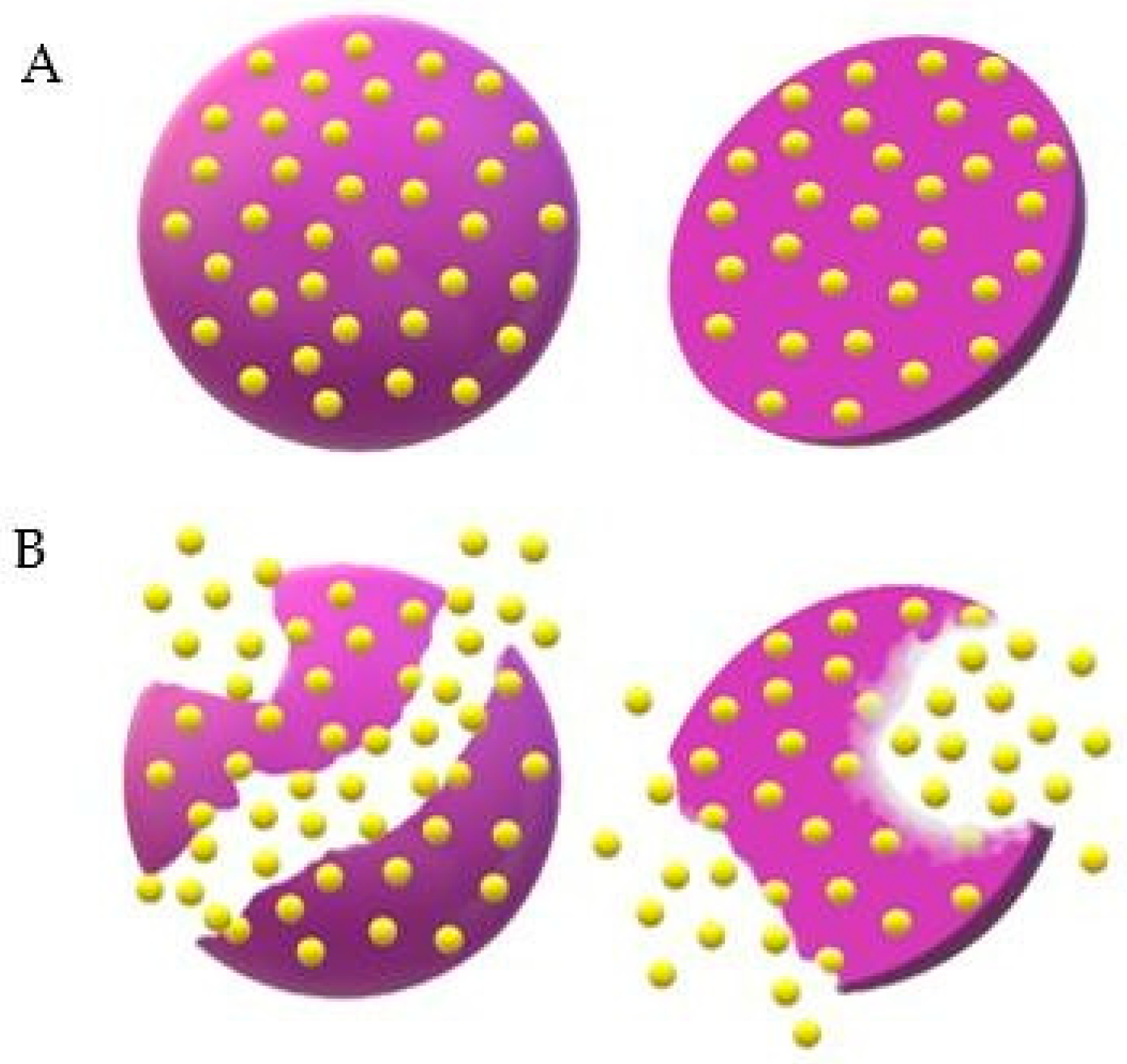
3. Polymeric Microparticles in Biological Tests
3.1. Anticancer Activity
3.1.1. In Vitro Anticancer Activity
3.1.2. In Vivo Anticancer Activity
3.2. Antiparasitic Activity
3.3. Antibacterial Activity
3.3.1. In Vitro Antibacterial Activity
3.3.2. In Vivo Antibacterial Activity
3.4. Antioxidant Activity
3.5. Anti-Inflammatory Activity
3.5.1. In Vitro Anti-Inflammatory Activity
3.5.2. In Vivo Anti-Inflammatory Activity
3.6. Healing Activity
3.6.1. In Vitro Healing Activity
3.6.2. In Vivo Healing Activity
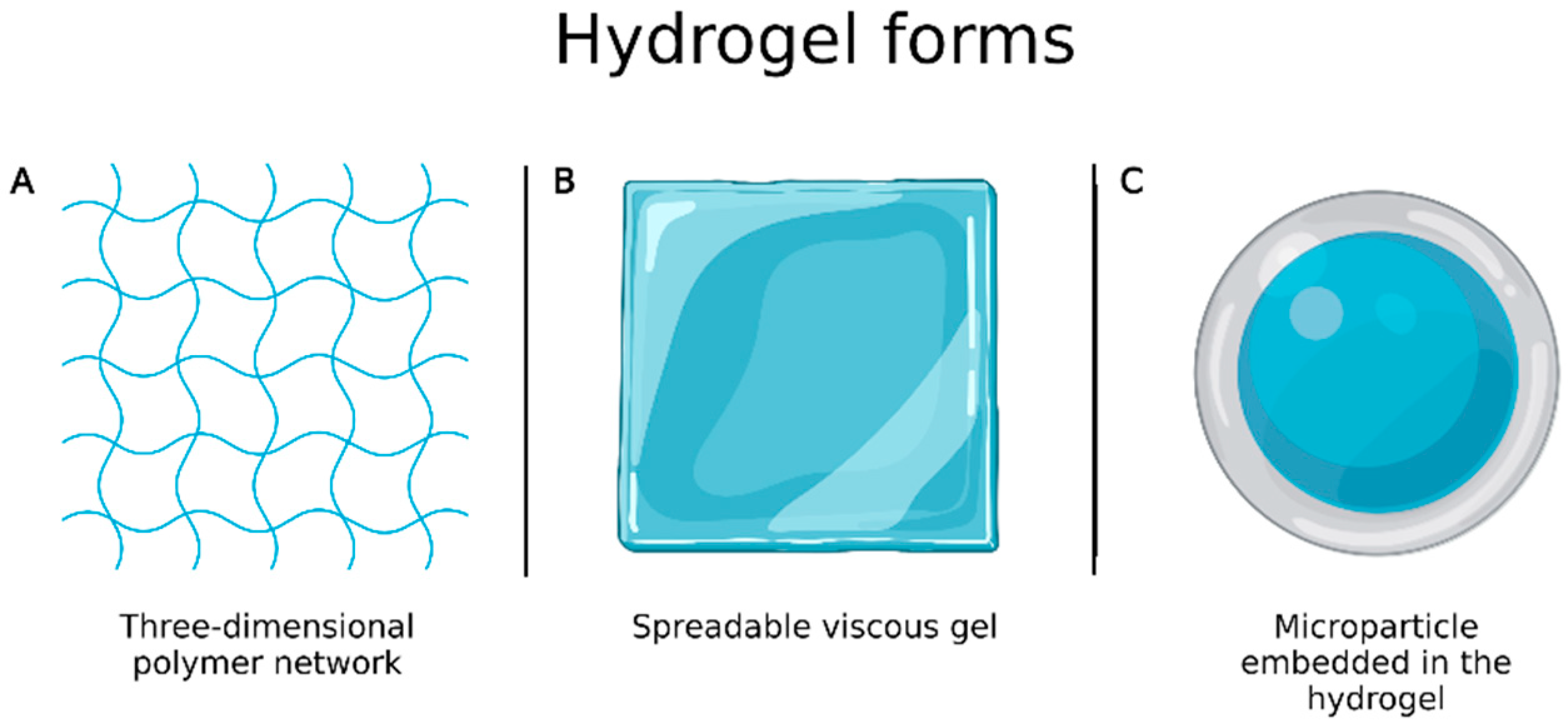
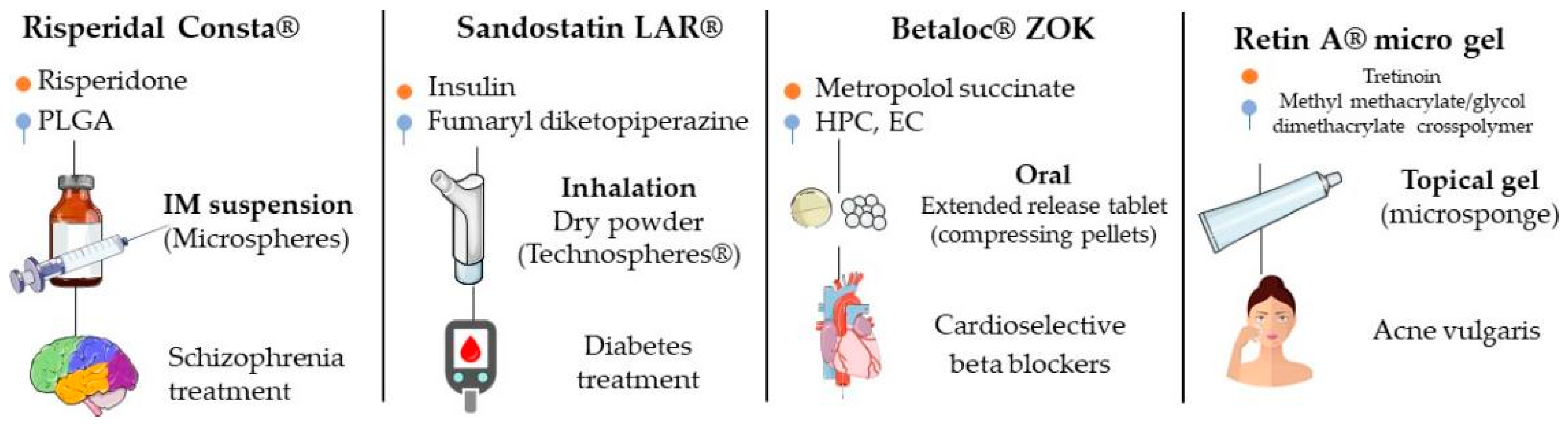
4. Current Market
4.1. DepoDur™
4.2. Afrezza™
4.3. Betaloc™ ZOK
4.4. Lupron Depot™
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent Expansion of Pharmaceutical Nanotechnologies and Targeting Strategies in the Field of Phytopharmaceuticals for the Delivery of Herbal Extracts and Bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.; Marian, E. Sodium Alginate—Natural Microencapsulation Material of Polymeric Microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and Its Biomedical Applications in Health and Diseases: Special Focus on Drug Delivery. Environ. Sci. Pollut. Res. 2020, 27, 19151–19168. [Google Scholar] [CrossRef] [PubMed]
- Otto, D.P.; Otto, A.; De Villiers, M.M. Differences in Physicochemical Properties to Consider in the Design, Evaluation and Choice between Microparticles and Nanoparticles for Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Sulistiawati; Dwipayanti, K.S.; Azhar, M.; Rahman, L.; Pakki, E.; Himawan, A.; Dian Permana, A. Enhanced Skin Localization of Metronidazole Using Solid Lipid Microparticles Incorporated into Polymeric Hydrogels for Potential Improved of Rosacea Treatment: An Ex Vivo Proof of Concept Investigation. Int. J. Pharm. 2022, 628, 122327. [Google Scholar] [CrossRef]
- Gokce, E.H.; Tuncay Tanrıverdi, S.; Eroglu, I.; Tsapis, N.; Gokce, G.; Tekmen, I.; Fattal, E.; Ozer, O. Wound Healing Effects of Collagen-Laminin Dermal Matrix Impregnated with Resveratrol Loaded Hyaluronic Acid-DPPC Microparticles in Diabetic Rats. Eur. J. Pharm. Biopharm. 2017, 119, 17–27. [Google Scholar] [CrossRef]
- Fröhlich, E.; Salar-Behzadi, S. Toxicological Assessment of Inhaled Nanoparticles: Role of in Vivo, Ex Vivo, in Vitro, and in Silico Studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef]
- Campos, E.; Branquinho, J.; Carreira, A.S.; Carvalho, A.; Coimbra, P.; Ferreira, P.; Gil, M.H. Designing Polymeric Microparticles for Biomedical and Industrial Applications. Eur. Polym. J. 2013, 49, 2005–2021. [Google Scholar] [CrossRef]
- Sharma, V.; Vijay, J.; Ganesh, M.R.; Sundaramurthy, A. Multilayer Capsules Encapsulating Nimbin and Doxorubicin for Cancer Chemo-Photothermal Therapy. Int. J. Pharm. 2020, 582, 119350. [Google Scholar] [CrossRef]
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester Based Polymeric Nano and Microparticles for Pharmaceutical Purposes: A Review on Formulation Approaches. J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Hazra, M.; Dasgupta Mandal, D.; Mandal, T.; Bhuniya, S.; Ghosh, M. Designing Polymeric Microparticulate Drug Delivery System for Hydrophobic Drug Quercetin. Saudi Pharm. J. 2015, 23, 429–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serda, R.E.; Gu, J.; Bhavane, R.C.; Liu, X.W.; Chiappini, C.; Decuzzi, P.; Ferrari, M. The Association of Silicon Microparticles with Endothelial Cells in Drug Delivery to the Vasculature. Biomaterials 2009, 30, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Reolon, J.B.; Brustolin, M.; Accarini, T.; Viçozzi, G.P.; Sari, M.H.M.; Bender, E.A.; Haas, S.E.; Brum, M.C.S.; Gündel, A.; Colomé, L.M. Co-Encapsulation of Acyclovir and Curcumin into Microparticles Improves the Physicochemical Characteristics and Potentiates in Vitro Antiviral Action: Influence of the Polymeric Composition. Eur. J. Pharm. Sci. 2019, 131, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.X.; Chen, W.T.; Lien, C.H.; Yang, H.Y.; Chen, K.H.; Wei, Y.F.; Wang, M.H.; Ko, I.T.; Tseng, F.G.; Yin, H.S. Physical, Chemical, and Biological Properties of Chitosan-Coated Alginate Microparticles Loaded with Porcine Interleukin-1β: A Potential Protein Adjuvant Delivery System. Int. J. Mol. Sci. 2022, 23, 9959. [Google Scholar] [CrossRef]
- Nidhi; Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N. Microparticles as Controlled Drug Delivery Carrier for the Treatment of Ulcerative Colitis: A Brief Review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef] [Green Version]
- Rajput, M.; Agrawal, P. Microspheres in Cancer Therapy. Indian J. Cancer 2010, 47, 458. [Google Scholar] [CrossRef]
- Costa Duarte, F.Í.; Sabino de Mendonça Costa, A.B.; Vieira Filho, J.F.; Pinto Freite, V.L.; Alves Freire, J.V.; Converti, A.; Ferrari, M.; Barreto Gomes, A.P.; Ostrosky, E.A.; de Neves Lima, Á.A. In Vitro Release Studies of Ferulic Acid in Semi-Solid Formulations with Optimized Synthetic Membrane. J. Drug Deliv. Sci. Technol. 2021, 61, 102106. [Google Scholar] [CrossRef]
- Cavalcanti, G.R.; Duarte, F.I.C.; Converti, A.; de Lima, Á.A.N. Ferulic Acid Activity in Topical Formulations: Technological and Scientific Prospecting. Curr. Pharm. Des. 2021, 27, 2289–2298. [Google Scholar] [CrossRef]
- Johnson, E.M.; Lee, H.; Jayabalan, R.; Suh, J.W. Ferulic Acid Grafted Self-Assembled Fructo-Oligosaccharide Micro Particle for Targeted Delivery to Colon. Carbohydr. Polym. 2020, 247, 116550. [Google Scholar] [CrossRef]
- Çetin Altındal, D.; Gümüşderelioğlu, M. Dual-Functional Melatonin Releasing Device Loaded with PLGA Microparticles and Cyclodextrin Inclusion Complex for Osteosarcoma Therapy. J. Drug Deliv. Sci. Technol. 2019, 52, 586–596. [Google Scholar] [CrossRef]
- Shi, X.; Li, C.; Gao, S.; Zhang, L.; Han, H.; Zhang, J.; Shi, W.; Li, Q. Combination of Doxorubicin-Based Chemotherapy and Polyethylenimine/p53 Gene Therapy for the Treatment of Lung Cancer Using Porous PLGA Microparticles. Colloids Surf. B Biointerfaces 2014, 122, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, M.; Liu, X.; Du, L.; Jin, Y. Inhalable Oridonin-Loaded Poly(Lactic-Co-Glycolic)Acid Large Porous Microparticles for in Situ Treatment of Primary Non-Small Cell Lung Cancer. Acta Pharm. Sin. B 2017, 7, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.B.; Geary, S.M.; Carrillo-Conde, B.R.; Narasimhan, B.; Salem, A.K. Characterizing the Antitumor Response in Mice Treated with Antigen-Loaded Polyanhydride Microparticles. Acta Biomater. 2013, 9, 5583–5589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Chen, H.; Lv, F.; Wang, J.; Zhao, S.; Li, Y.; Xue, X.; Liu, Y.; Wei, G.; Lu, W. Sequential Release of Paclitaxel and Imatinib from Core–Shell Microparticles Prepared by Coaxial Electrospray for Vaginal Therapy of Cervical Cancer. Int. J. Mol. Sci. 2021, 22, 8760. [Google Scholar] [CrossRef]
- Dwivedi, P.; Han, S.; Mangrio, F.; Fan, R.; Dwivedi, M.; Zhu, Z.; Huang, F.; Wu, Q.; Khatik, R.; Cohn, D.E.; et al. Engineered Multifunctional Biodegradable Hybrid Microparticles for Paclitaxel Delivery in Cancer Therapy. Mater. Sci. Eng. C 2019, 102, 113–123. [Google Scholar] [CrossRef]
- Karan, S.; Debnath, S.; Kuotsu, K.; Chatterjee, T.K. In-Vitro and in-Vivo Evaluation of Polymeric Microsphere Formulation for Colon Targeted Delivery of 5-Fluorouracil Using Biocompatible Natural Gum Katira. Int. J. Biol. Macromol. 2020, 158, 922–936. [Google Scholar] [CrossRef]
- Xu, J.; Lu, X.; Zhu, X.; Yang, Y.; Liu, Q.; Zhao, D.; Lu, Y.; Wen, J.; Chen, X.; Li, N. Formulation and Characterization of Spray-Dried Powders Containing Vincristine-Liposomes for Pulmonary Delivery and Its Pharmacokinetic Evaluation From In Vitro and In Vivo. J. Pharm. Sci. 2019, 108, 3348–3358. [Google Scholar] [CrossRef]
- Kim, I.; Byeon, H.J.; Kim, T.H.; Lee, E.S.; Oh, K.T.; Shin, B.S.; Lee, K.C.; Youn, Y.S. Doxorubicin-Loaded Porous PLGA Microparticles with Surface Attached TRAIL for the Inhalation Treatment of Metastatic Lung Cancer. Biomaterials 2013, 34, 6444–6453. [Google Scholar] [CrossRef]
- Madan, J.; Pandey, R.S.; Jain, U.K.; Katare, O.P.; Aneja, R.; Katyal, A. Sterically Stabilized Gelatin Microassemblies of Noscapine Enhance Cytotoxicity, Apoptosis and Drug Delivery in Lung Cancer Cells. Colloids Surf. B Biointerfaces 2013, 107, 235–244. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, D.; Pan, Y.; Qu, W.; Hao, H.; Wang, X.; Liu, Z.; Xie, S. Nanoparticles for Antiparasitic Drug Delivery. Drug Deliv. 2019, 26, 1206–1221. [Google Scholar] [CrossRef] [PubMed]
- Allotey-Babington, G.L.; Amponsah, S.K.; Nettey, T.; Sasu, C.; Nettey, H. Quinine Sulphate Microparticles as Treatment for Leishmaniasis. J. Trop. Med. 2020, 2020, 5278518. [Google Scholar] [CrossRef] [PubMed]
- Collier, M.A.; Peine, K.J.; Gautam, S.; Oghumu, S.; Varikuti, S.; Borteh, H.; Papenfuss, T.L.; Sataoskar, A.R.; Bachelder, E.M.; Ainslie, K.M. Host-Mediated Leishmania Donovani Treatment Using AR-12 Encapsulated in Acetalated Dextran Microparticles. Int. J. Pharm. 2016, 499, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Batista, A.J.; Pacienza-Lima, W.; Ré, M.I.; Rossi-Bergmann, B. Novel and Safe Single-Dose Treatment of Cutaneous Leishmaniasis with Implantable Amphotericin B-Loaded Microparticles. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 148–155. [Google Scholar] [CrossRef]
- de Sousa-Batista, A.J.; Pacienza-Lima, W.; Arruda-Costa, N.; Falcão, C.A.B.; Ré, M.I.; Rossi-Bergmann, B. Depot Subcutaneous Injection with Chalcone CH8-Loaded Poly(Lactic-Co-Glycolic Acid) Microspheres as a Single-Dose Treatment of Cutaneous Leishmaniasis. Antimicrob. Agents Chemother. 2017, 62, e01822–e01917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Batista, A.J.; Arruda-Costa, N.; Escrivani, D.O.; Reynaud, F.; Steel, P.G.; Rossi-Bergmann, B. Single-Dose Treatment for Cutaneous Leishmaniasis with an Easily Synthesized Chalcone Entrapped in Polymeric Microparticles. Parasitology 2020, 147, 1032–1037. [Google Scholar] [CrossRef]
- Duong, A.D.; Sharma, S.; Peine, K.J.; Gupta, G.; Satoskar, A.R.; Bachelder, E.M.; Wyslouzil, B.E.; Ainslie, K.M. Electrospray Encapsulation of Toll-Like Receptor Agonist Resiquimod in Polymer Microparticles for the Treatment of Visceral Leishmaniasis. Mol. Pharm. 2013, 10, 1045–1055. [Google Scholar] [CrossRef]
- Hoseini, M.H.M.; Moradi, M.; Alimohammadian, M.H.; Shahgoli, V.K.; Darabi, H.; Rostami, A. Immunotherapeutic Effects of Chitin in Comparison with Chitosan against Leishmania Major Infection. Parasitol. Int. 2016, 65, 99–104. [Google Scholar] [CrossRef]
- Ospina-Villa, J.D.; Gómez-Hoyos, C.; Zuluaga-Gallego, R.; Triana-Chávez, O. Encapsulation of Proteins from Leishmania Panamensis into PLGA Particles by a Single Emulsion-Solvent Evaporation Method. J. Microbiol. Methods 2019, 162, 1–7. [Google Scholar] [CrossRef]
- Gomes, D.C.O.; da Souza, B.L.S.C.; Schwedersky, R.P.; Covre, L.P.; de Matos Guedes, H.L.; Lopes, U.G.; Ré, M.I.; Rossi-Bergmann, B. Intranasal Immunization with Chitosan Microparticles Enhances LACK-DNA Vaccine Protection and Induces Specific Long-Lasting Immunity against Visceral Leishmaniasis. Microbes Infect. 2022, 24, 104884. [Google Scholar] [CrossRef]
- Mwangi, T.W.; Mohammed, M.; Dayo, H.; Snow, R.W.; Marsh, K. Clinical Algorithms for Malaria Diagnosis Lack Utility among People of Different Age Groups. Trop. Med. Int. Health 2005, 10, 530–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva de Barros, A.O.; Portilho, F.L.; Dos Santos Matos, A.P.; Ricci-Junior, E.; Alencar, L.M.R.; Dos Santos, C.C.; Paumgartten, F.J.R.; Iram, S.H.; Mazier, D.; Franetich, J.F.; et al. Preliminary Studies on Drug Delivery of Polymeric Primaquine Microparticles Using the Liver High Uptake Effect Based on Size of Particles to Improve Malaria Treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112275. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Fazly Bazzaz, B.S. Breakthroughs in Bacterial Resistance Mechanisms and the Potential Ways to Combat Them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Loupias, P.; Dassonville-Klimpt, A.; Sonnet, P. Drug Delivery Systems Designed to Overcome Antimicrobial Resistance. Med. Res. Rev. 2019, 39, 2343–2396. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J. Pharm. Sci. 2015, 104, 872–905. [Google Scholar] [CrossRef]
- Yildiz-Peköz, A.; Akbal, O.; Tekarslan, S.H.; Sagirli, A.O.; Mulazimoglu, L.; Morina, D.; Cevher, E. Preparation and Characterization of Doripenem-Loaded Microparticles for Pulmonary Delivery. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 347–357. [Google Scholar] [CrossRef]
- Cunha, L.; Rodrigues, S.; Rosa da Costa, A.M.; Faleiro, L.; Buttini, F.; Grenha, A. Inhalable Chitosan Microparticles for Simultaneous Delivery of Isoniazid and Rifabutin in Lung Tuberculosis Treatment. Drug Dev. Ind. Pharm. 2019, 45, 1313–1320. [Google Scholar] [CrossRef]
- Maghrebi, S.; Joyce, P.; Jambhrunkar, M.; Thomas, N.; Prestidge, C.A. Poly(Lactic-Co-Glycolic) Acid-Lipid Hybrid Microparticles Enhance the Intracellular Uptake and Antibacterial Activity of Rifampicin. ACS Appl. Mater. Interfaces 2020, 12, 8030–8039. [Google Scholar] [CrossRef]
- Ali Said, F.; Bousserrhine, N.; Alphonse, V.; Michely, L.; Belbekhouche, S. Antibiotic Loading and Development of Antibacterial Capsules by Using Porous CaCO3 Microparticles as Starting Material. Int. J. Pharm. 2020, 579, 119175. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, Y.; Mi, G.; He, D.; Zhang, Y.; Xiong, Y.; Webster, T.J.; Tu, J. Fumaryl Diketopiperazine Based Effervescent Microparticles to Escape Macrophage Phagocytosis for Enhanced Treatment of Pneumonia via Pulmonary Delivery. Biomaterials 2020, 228, 119575. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Y.; Wang, B.; Zou, Q.; Zeng, H.; Chen, X.; Liu, X.; Liu, J.; Yu, S. A Novel Gastroretentive Porous Microparticle for Anti-Helicobacter Pylori Therapy: Preparation, in Vitro and in Vivo Evaluation. Int. J. Pharm. 2014, 463, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Ma, Z.; Kang, M.; Galvão, K.N.; Jeong, K.C. Application of Chitosan Microparticles for Treatment of Metritis and in Vivo Evaluation of Broad Spectrum Antimicrobial Activity in Cow Uteri. Biomaterials 2016, 110, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Cambronero-Rojas, A.; Torres-Vergara, P.; Godoy, R.; Von Plessing, C.; Sepúlveda, J.; Gómez-Gaete, C. Capreomycin Oleate Microparticles for Intramuscular Administration: Preparation, in Vitro Release and Preliminary in Vivo Evaluation. J. Control. Release 2015, 209, 229–237. [Google Scholar] [CrossRef]
- Jeon, S.J.; Oh, M.; Yeo, W.S.; Galvão, K.N.; Jeong, K.C. Underlying Mechanism of Antimicrobial Activity of Chitosan Microparticles and Implications for the Treatment of Infectious Diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef] [PubMed]
- Kucukoglu, V.; Uzuner, H.; Kenar, H.; Karadenizli, A. In Vitro Antibacterial Activity of Ciprofloxacin Loaded Chitosan Microparticles and Their Effects on Human Lung Epithelial Cells. Int. J. Pharm. 2019, 569, 118578. [Google Scholar] [CrossRef] [PubMed]
- Dat, N.M.; Huong, L.M.; Cong, C.Q.; Hai, N.D.; Nam, N.T.H.; Thinh, D.B.; Duy, H.K.; Danh, T.T.; Loi, P.H.H.P.; Phong, M.T.; et al. Green Synthesis of Chitosan-Based Membrane Modified with Uniformly Micro-Sizing Selenium Particles Decorated Graphene Oxide for Antibacterial Application. Int. J. Biol. Macromol. 2022, 220, 348–359. [Google Scholar] [CrossRef]
- Kawakita, E.R.H.; Ré, A.C.S.; Peixoto, M.P.G.; Ferreira, M.P.; Ricomini-Filho, A.P.; Freitas, O.; Aires, C.P. Effect of Chitosan Dispersion and Microparticles on Older Streptococcus Mutans Biofilms. Molecules 2019, 24, 1808. [Google Scholar] [CrossRef] [Green Version]
- Galvão, K.N.; de Oliveira, E.B.; Cunha, F.; Daetz, R.; Jones, K.; Ma, Z.; Jeong, K.C.; Bicalho, R.C.; Higgins, C.H.; Rodrigues, M.X.; et al. Effect of Chitosan Microparticles on the Uterine Microbiome of Dairy Cows with Metritis. Appl. Environ. Microbiol. 2020, 86, e01066–e01120. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Masoud Hosseini, S. Effect of Chitosan Molecular Weight as Micro and Nanoparticles on Antibacterial Activity against Some Soft Rot Pathogenic Bacteria. LWT-Food Sci. Technol. 2016, 71, 347–355. [Google Scholar] [CrossRef]
- Weppelmann, T.A.; Jeong, K.C.; Ali, A. Characterization of the Vibriocidal Activity of Chitosan Microparticles: A Potential Therapeutic Agent for Emerging Multidrug-Resistant Cholera Infections. ACS Appl. Mater. Interfaces 2020, 12, 47278–47288. [Google Scholar] [CrossRef]
- Assadi, Z.; Emtiazi, G.; Zarrabi, A. Novel Synergistic Activities of Tetracycline Copper Oxide Nanoparticles Integrated into Chitosan Micro Particles for Delivery against Multiple Drug Resistant Strains: Generation of Reactive Oxygen Species (ROS) and Cell Death. J. Drug Deliv. Sci. Technol. 2018, 44, 65–70. [Google Scholar] [CrossRef]
- Fan, Y.; Ginn, A.; Ma, Z.; Kang, M.; Jeong, K.C.; Wright, A.C. Application of Chitosan Microparticles for Mitigation of Salmonella in Agricultural Water. J. Appl. Microbiol. 2017, 123, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Hatem, S.; Nasr, M.; Elkheshen, S.A.; Geneidi, A.S. Recent Advances in Antioxidant Cosmeceutical Topical Delivery. Curr. Drug Deliv. 2018, 15, 953–964. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; El-Readi, M.Z.; Alhowail, A.H.; Aldubayan, M.A.; Abdellatif, A.A.H. Formulation of Ethyl Cellulose Microparticles Incorporated Pheophytin a Isolated from Suaeda Vermiculata for Antioxidant and Cytotoxic Activities. Molecules 2019, 24, 1501. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Liu, Y.; Ge, Y.; Zhong, Z.; Wang, Z.; Wu, T.; Zhao, X.; Zu, Y. Solubility, Antioxidation, and Oral Bioavailability Improvement of Mangiferin Microparticles Prepared Using the Supercritical Antisolvent Method. Pharmaceutics 2020, 12, 90. [Google Scholar] [CrossRef] [Green Version]
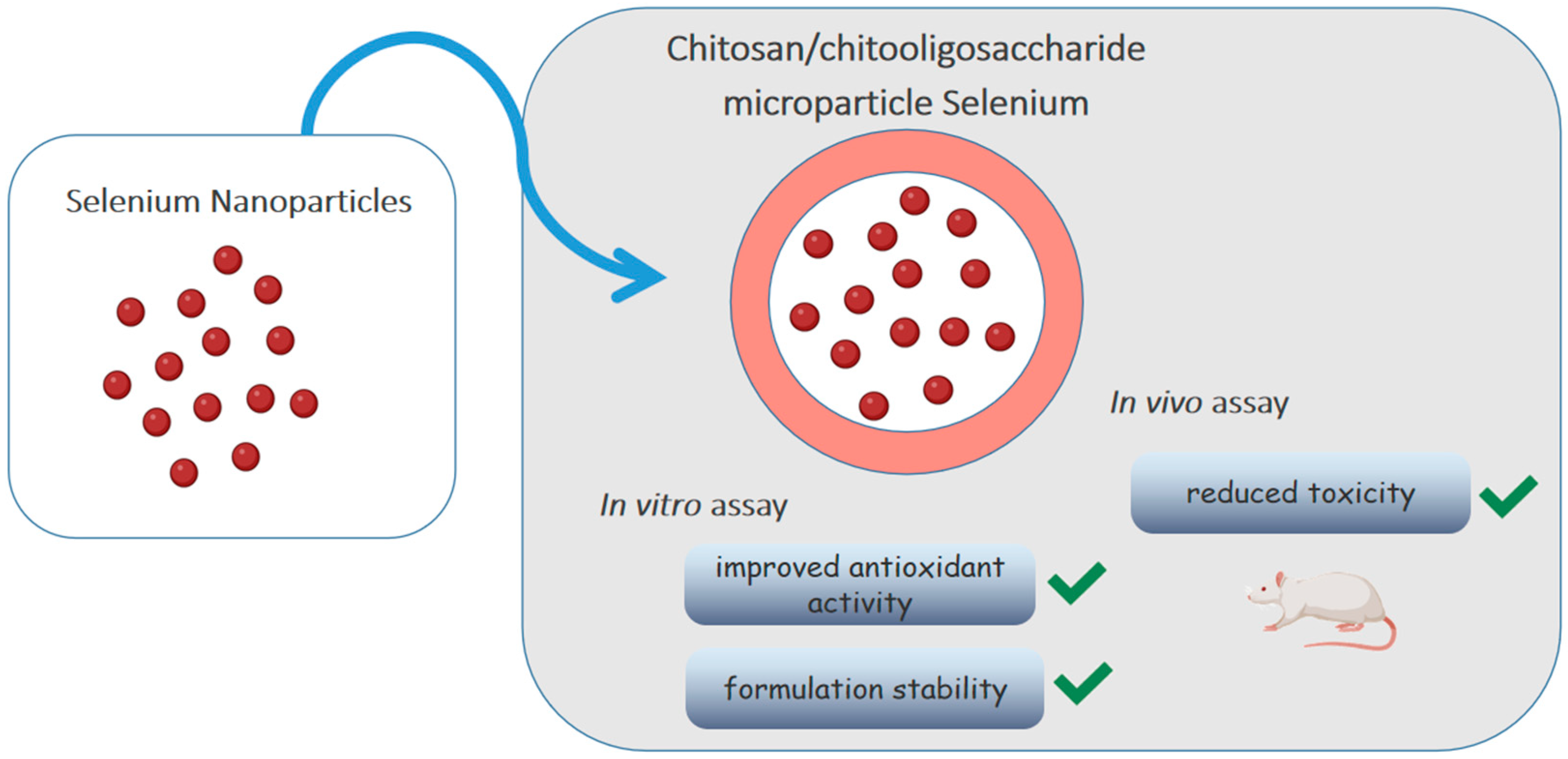
- Bai, K.; Hong, B.; Huang, W.; He, J. Selenium-Nanoparticles-Loaded Chitosan/Chitooligosaccharide Microparticles and Their Antioxidant Potential: A Chemical and in Vivo Investigation. Pharmaceutics 2020, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Singh, A.; Garg, N.; Siril, P.F. Solid Lipid Nanoparticles for the Controlled Delivery of Poorly Water Soluble Non-Steroidal Anti-Inflammatory Drugs. Ultrason. Sonochem. 2018, 40, 686–696. [Google Scholar] [CrossRef]
- Leonard, F.; Srinivasan, S.; Liu, X.; Collnot, E.M.; Ferrari, M.; Lehr, C.M.; Godin, B. Design and in Vitro Characterization of Multistage Silicon-PLGA Budesonide Particles for Inflammatory Bowel Disease. Eur. J. Pharm. Biopharm. 2020, 151, 61–72. [Google Scholar] [CrossRef]
- Afrose, A.; White, E.T.; Howes, T.; George, G.; Rashid, A.; Rintoul, L.; Islam, N. Preparation of Ibuprofen Microparticles by Antisolvent Precipitation Crystallization Technique: Characterization, Formulation, and In Vitro Performance. J. Pharm. Sci. 2018, 107, 3060–3069. [Google Scholar] [CrossRef]
- Ren, F.; Su, J.; Xiong, H.; Tian, Y.; Ren, G.; Jing, Q. Characterization of Ibuprofen Microparticle and Improvement of the Dissolution. Pharm. Dev. Technol. 2017, 22, 63–68. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, D.; Wu, J.; Li, J.; Teng, X.; Gao, X.; Li, R.; Wang, X.; Xia, L.; Xia, Y. The Acitretin and Methotrexate Combination Therapy for Psoriasis Vulgaris Achieves Higher Effectiveness and Less Liver Fibrosis. Pharmacol. Res. 2017, 121, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Mielanczyk, A.; Mrowiec, K.; Kupczak, M.; Mielanczyk, Ł.; Scieglinska, D.; Gogler-Piglowska, A.; Michalski, M.; Gabriel, A.; Neugebauer, D.; Skonieczna, M. Synthesis and in Vitro Cytotoxicity Evaluation of Star-Shaped Polymethacrylic Conjugates with Methotrexate or Acitretin as Potential Antipsoriatic Prodrugs. Eur. J. Pharmacol. 2020, 866, 172804. [Google Scholar] [CrossRef] [PubMed]
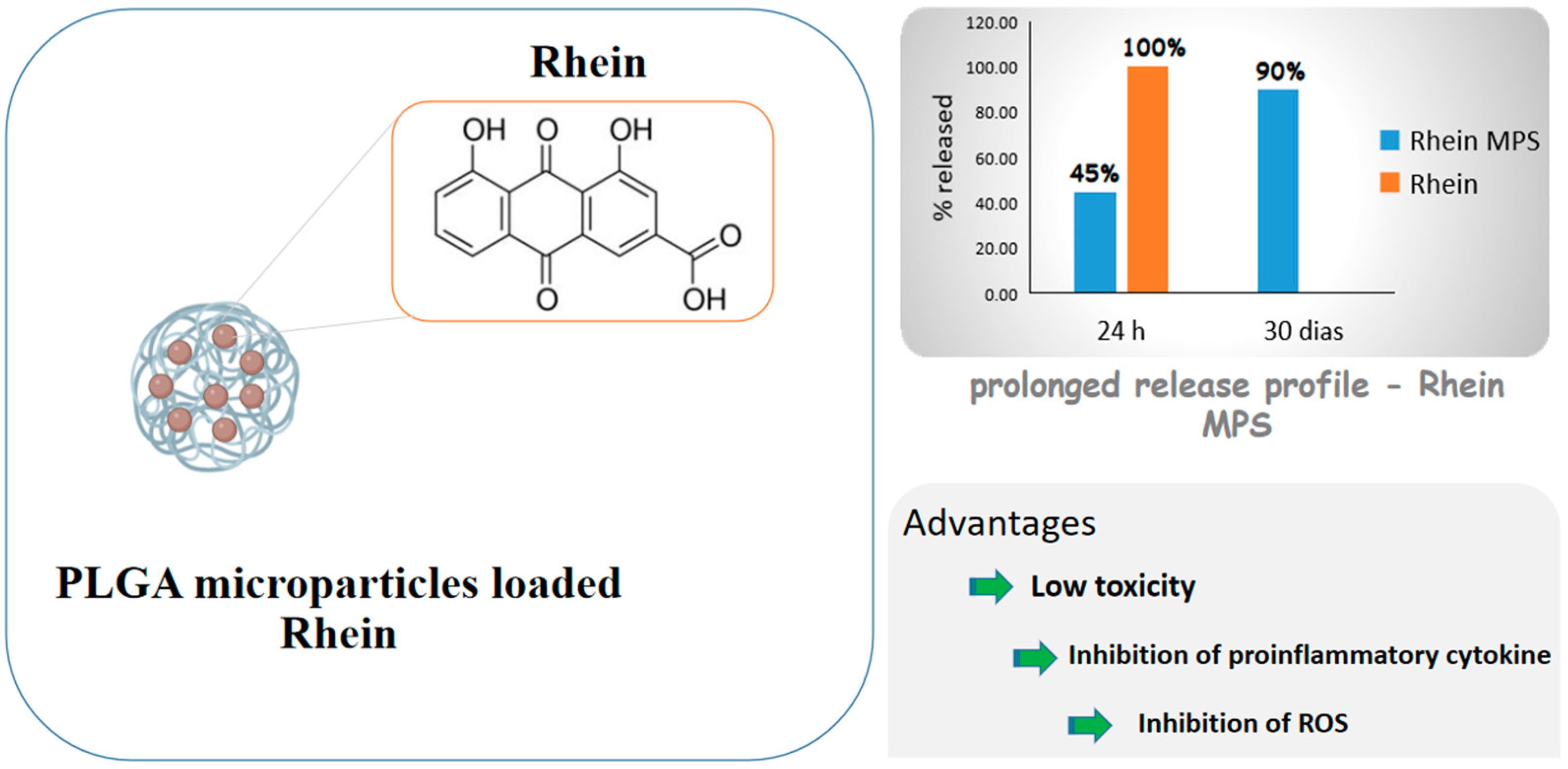
- Gómez-Gaete, C.; Retamal, M.; Chávez, C.; Bustos, P.; Godoy, R.; Torres-Vergara, P. Development, Characterization and In Vitro Evaluation of Biodegradable Rhein-Loaded Microparticles for Treatment of Osteoarthritis. Eur. J. Pharm. Sci. 2017, 96, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-Based Formulations for Resveratrol Delivery: Effects on Resveratrol in Vivo Bioavailability and Bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, I.; Trotta, V.; Lee, W.H.; Loo, C.Y.; Pozzoli, M.; Young, P.M.; Scalia, S.; Traini, D. Resveratrol Solid Lipid Microparticles as Dry Powder Formulation for Nasal Delivery, Characterization and in Vitro Deposition Study. J. Microencapsul. 2016, 33, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Kang, C.; Jung, E.; Yoo, D.; Wu, D.; Lee, D. Porous Antioxidant Polymer Microparticles as Therapeutic Systems for the Airway Inflammatory Diseases. J. Control. Release 2016, 233, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.M.; Elsabahy, M.; Abd-Elkareem, M.; Ibrahim, E.A.; Soliman, G.M. High-Payload Chitosan Microparticles for the Colonic Delivery of Quercetin: Development and in-Vivo Evaluation in a Rabbit Colitis Model. J. Drug Deliv. Sci. Technol. 2020, 58, 101832. [Google Scholar] [CrossRef]
- Palma, E.; Costa, N.; Molinaro, R.; Francardi, M.; Paolino, D.; Cosco, D.; Fresta, M. Improvement of the Therapeutic Treatment of Inflammatory Bowel Diseases Following Rectal Administration of Mesalazine-Loaded Chitosan Microparticles vs Asamax®. Carbohydr. Polym. 2019, 212, 430–438. [Google Scholar] [CrossRef]
- Khattab, A.; Abouhussein, D.M.N.; Mohammad, F.E. Development of Injectable Tenoxicam in Situ Forming Microparticles Based on Sesame Oil and Poly-DL-Lactide: Characterization, Efficacy and Acute Toxicity. J. Drug Deliv. Sci. Technol. 2019, 51, 682–694. [Google Scholar] [CrossRef]
- Sangsuwan, R.; Yik, J.H.N.; Owen, M.; Liu, G.; Haudenschild, D.R.; Lewis, J.S. Intra-Articular Injection of Flavopiridol-Loaded Microparticles for Treatment of Post-Traumatic Osteoarthritis. Acta Biomater. 2022, 149, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.E.A.S.; Soares, J.M.D.; Silva, H.A.L.; de Wanderley, C.W.S.; Moura, C.A.; de Oliveira-Junior, R.G.; de Oliveira, A.P.; Rolim, L.A.; Costa, E.V.; da Almeida, J.R.G.S.; et al. Inhibitory Effects of Morus nigra L. (Moraceae) against Local Paw Edema and Mechanical Hypernociception Induced by Bothrops jararacussu Snake Venom in Mice. Biomed. Pharmacother. 2019, 111, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Nosenko, M.A.; Moysenovich, A.M.; Zvartsev, R.V.; Arkhipova, A.Y.; Zhdanova, A.S.; Agapov, I.I.; Vasilieva, T.V.; Bogush, V.G.; Debabov, V.G.; Nedospasov, S.A.; et al. Novel Biodegradable Polymeric Microparticles Facilitate Scarless Wound Healing by Promoting Re-Epithelialization and Inhibiting Fibrosis. Front. Immunol. 2018, 9, 2851. [Google Scholar] [CrossRef]
- Pagano, C.; Perioli, L.; Baiocchi, C.; Bartoccini, A.; Beccari, T.; Blasi, F.; Calarco, P.; Ceccarini, M.R.; Cossignani, L.; di Michele, A.; et al. Preparation and Characterization of Polymeric Microparticles Loaded with Moringa Oleifera Leaf Extract for Exuding Wound Treatment. Int. J. Pharm. 2020, 587, 119700. [Google Scholar] [CrossRef]
- Zarubova, J.; Hasani-Sadrabadi, M.M.; Bacakova, L.; Li, S. Nano-in-Micro Dual Delivery Platform for Chronic Wound Healing Applications. Micromachines 2020, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Eroğlu, İ.; Gökçe, E.H.; Tsapis, N.; Tanrıverdi, S.T.; Gökçe, G.; Fattal, E.; Özer, Ö. Evaluation of Characteristics and in Vitro Antioxidant Properties of RSV Loaded Hyaluronic Acid–DPPC Microparticles as a Wound Healing System. Colloids Surf. B Biointerfaces 2015, 126, 50–57. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, S.; Dhiman, A. Design of Antibiotic Containing Hydrogel Wound Dressings: Biomedical Properties and Histological Study of Wound Healing. Int. J. Pharm. 2013, 457, 82–91. [Google Scholar] [CrossRef]
- Ribeiro, M.P.; Morgado, P.I.; Miguel, S.P.; Coutinho, P.; Correia, I.J. Dextran-Based Hydrogel Containing Chitosan Microparticles Loaded with Growth Factors to Be Used in Wound Healing. Mater. Sci. Eng. C 2013, 33, 2958–2966. [Google Scholar] [CrossRef]
- Pereira, G.G.; Santos-Oliveira, R.; Albernaz, M.S.; Canema, D.; Weismüller, G.; Barros, E.B.; Magalhães, L.; Lima-Ribeiro, M.H.M.; Pohlmann, A.R.; Guterres, S.S. Microparticles of Aloe Vera/Vitamin E/Chitosan: Microscopic, a Nuclear Imaging and an in Vivo Test Analysis for Burn Treatment. Eur. J. Pharm. Biopharm. 2014, 86, 292–300. [Google Scholar] [CrossRef]
- Reis, M.B.; Pereira, P.A.T.; Caetano, G.F.; Leite, M.N.; Galvão, A.F.; Paula-Silva, F.W.G.; Frade, M.A.C.; Faccioli, L.H. Lipoxin A4 Encapsulated in PLGA Microparticles Accelerates Wound Healing of Skin Ulcers. PLoS ONE 2017, 12, e0182381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasasvini, S.; Anusa, R.S.; VedhaHari, B.N.; Prabhu, P.C.; RamyaDevi, D. Topical Hydrogel Matrix Loaded with Simvastatin Microparticles for Enhanced Wound Healing Activity. Mater. Sci. Eng. C 2017, 72, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Microspheres Market Size, Share & Trends Analysis Report by Type (Hollow, Solid), by Material (Glass, Polymer, Ceramic, Fly ash, Metallic) by Application (Construction Composites, Paints & Coatings, Healthcare, Cosmetics, Oil & Gas, Automotive) and Segment Forecasts, 2018–2025|Grand View Research. Available online: https://www.grandviewresearch.com/industry-analysis/microspheres-industry (accessed on 20 March 2022).
- Mantripragada, S. A Lipid Based Depot (DepoFoam® Technology) for Sustained Release Drug Delivery. Prog. Lipid Res. 2002, 41, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.; Roland, L.M.; Chu, L.F.; Campitelli, V.A.; Riley, E.T. Single-Dose, Extended-Release Epidural Morphine (DepoDurTM) Compared to Conventional Epidural Morphine for Post-Cesarean Pain. Anesth. Analg. 2007, 105, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bode, B.W.; McGill, J.B.; Lorber, D.L.; Gross, J.L.; Chang, P.C.; Bregman, D.B. Inhaled Technosphere Insulin Compared with Injected Prandial Insulin in Type 1 Diabetes: A Randomized 24-Week Trial. Diabetes Care 2015, 38, 2266–2273. [Google Scholar] [CrossRef] [Green Version]
- Neumiller, J.J.; Campbell, R.K.; Wood, L.D. A Review of Inhaled Technosphere Insulin. Ann. Pharmacother. 2010, 44, 1231–1239. [Google Scholar] [CrossRef]
- Rave, K.; Heise, T.; Pfützner, A.; Boss, A.H. Coverage of Postprandial Blood Glucose Excursions with Inhaled Technosphere Insulin in Comparison to Subcutaneously Injected Regular Human Insulin in Subjects with Type 2 Diabetes. Diabetes Care 2007, 30, 2307–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenstock, J.; Bergenstal, R.; Defronzo, R.A.; Hirsch, I.B.; Klonoff, D.; Boss, A.H.; Kramer, D.; Petrucci, R.; Yu, W.; Levy, B. Efficacy and Safety of Technosphere Inhaled Insulin Compared With Technosphere Powder Placebo in Insulin-Naive Type 2 Diabetes Suboptimally Controlled With Oral Agents. Diabetes Care 2008, 31, 2177–2182. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, J.; Lorber, D.L.; Petrucci, R.E.; Howard, C.P.; Shearer, D.M.; Bilheimer, D.W.; Chang, P.-C.; Richardson, P.C. Basal/Bolus with Prandial Inhaled Technosphere (R) Insulin (TI) Plus Insulin Glargine QD vs Biaspart 70/30 Insulin BID in T2DM Inadequately Controlled on Insulin with/without Oral Agents. In Proceedings of the Diabetes; American Diabetes Association: Alexandria, VA, USA, 2009; Volume 58, p. A124. [Google Scholar]
- Peyrot, M.; Rubin, R.R. Patient-Reported Outcomes in Adults with Type 2 Diabetes Using Mealtime Inhaled Technosphere Insulin and Basal Insulin versus Premixed Insulin. Diabetes Technol. Ther. 2011, 13, 1201–1206. [Google Scholar] [CrossRef]
- Egstrup, K.; Gundersen, T.; Härkönen, R.; Karlsson, E.; Lundgren, B. The Antianginal Efficacy and Tolerability of Controlled-Release Metoprolol Once Daily: A Comparison with Conventional Metoprolol Tablets Twice Daily. Eur. J. Clin. Pharmacol. 1988, 33, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Oosterhuis, B.; Jonkman, J.H.G.; Kerkhof, F.A. Pharmacokinetic and Pharmacodynamic Comparison of a New Controlled-Release Formulation of Metoprolol with a Traditional Slow-Release Formulation. Eur. J. Clin. Pharmacol. 1988, 33, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Marberger, M.; Kaisary, A.V.; Shore, N.D.; Karlin, G.S.; Savulsky, C.; Mis, R.; Leuratti, C.; Germa, J.R. Effectiveness, Pharmacokinetics, and Safety of a New Sustained-Release Leuprolide Acetate 3.75-Mg Depot Formulation for Testosterone Suppression in Patients with Prostate Cancer: A Phase III, Open-Label, International Multicenter Study. Clin. Ther. 2010, 32, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Perez-Marreno, R.; Chu, F.M.; Gleason, D.; Loizides, E.; Wachs, B.; Tyler, R.C. A Six-Month, Open-Label Study Assessing a New Formulation of Leuprolide 7.5 Mg for Suppression of Testosterone in Patients with Prostate Cancer. Clin. Ther. 2002, 24, 1902–1914. [Google Scholar] [CrossRef] [PubMed]

| Pathogen | Active Principle | Type of Study | Particle Size (µm) | Ref. |
|---|---|---|---|---|
| Escherichia coli O157:H7 EDL933 (ATCC48935), Intrauterine pathogenic E. coli, Salmonella enterica CDC3041-1, and Klebsiella pneumoniae | Chitosan | In vitro and in vivo | 0.6 ± 0.076 | [54] |
| E. coli, Pseudomonas aeruginosa and Staphylococcus aureus | Ciprofloxacin and chitosan | In vitro | 0.712 ± 122 0.720 ± 153 | [55] |
| S. aureus, E. coli, P. aeruginosa and Bacillus subtilis | Selenium | In vitro | 0.592 ± 0.057 | [56] |
| Streptococcus mutans | Chitosan | In vitro | 5.61 | [57] |
| Fusobacterium necrophorum and Bacteroides pyogenes | Ceftiofur | In vitro, in vivo and ex vivo | - | [58] |
| Pseudomonas fluorescens, Erwinia carotovora and E. coli | Chitosan | In vitro | 0.06 ± 5.48; 0.078 ± 6.77; 0.105 ± 8.58 | [59] |
| Vibrio cholerae | Chitosan | In vitro | 0.6 ± 0.076 | [60] |
| Multi-drug resistant (MDR) coagulase-negative Staphylococcus, MDR Pseudomonas p41, MDR Pseudomonas p21, S. aureus ATCC 6538 and P. aeruginosa ATCC 9027 | Copper oxide, Tetracycline and Chitosan | In vitro | 1 | [61] |
| Salmonella enterica | Chitosan | In vitro | - | [62] |
| Product | Active Principle | Matrix | Advantages | Ref. |
|---|---|---|---|---|
| DepoDur™ | Morphine | Cholesterol, DOPC, DPPG, tricaprylin, triolein | Need for lower dose to obtain the therapeutic effect; less adverse effects. | [94,95] |
| Afrezza™ | Insulin | Fumaryl diketopiperazine | Noninvasive administration; better blood glucose control. | [99,101] |
| Betaloc™ ZOK | Metoprolol succinate | Hydroxypropyl cellulose | Less adverse effects; maintenance of drug plasma concentration for longer time. | [102,103] |
| Lupron Depot™ | Leuprolide acetate | PLGA | Need for lower dose to obtain the therapeutic effect; prolonged release. | [104,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, R.Y.P.; de Menezes, D.L.B.; Oliveira, V.d.S.; Converti, A.; de Lima, Á.A.N. Microparticles in the Development and Improvement of Pharmaceutical Formulations: An Analysis of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 5441. https://doi.org/10.3390/ijms24065441
da Silva RYP, de Menezes DLB, Oliveira VdS, Converti A, de Lima ÁAN. Microparticles in the Development and Improvement of Pharmaceutical Formulations: An Analysis of In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2023; 24(6):5441. https://doi.org/10.3390/ijms24065441
Chicago/Turabian Styleda Silva, Rita Y. P., Danielle L. B. de Menezes, Verônica da S. Oliveira, Attilio Converti, and Ádley A. N. de Lima. 2023. "Microparticles in the Development and Improvement of Pharmaceutical Formulations: An Analysis of In Vitro and In Vivo Studies" International Journal of Molecular Sciences 24, no. 6: 5441. https://doi.org/10.3390/ijms24065441
APA Styleda Silva, R. Y. P., de Menezes, D. L. B., Oliveira, V. d. S., Converti, A., & de Lima, Á. A. N. (2023). Microparticles in the Development and Improvement of Pharmaceutical Formulations: An Analysis of In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 24(6), 5441. https://doi.org/10.3390/ijms24065441








