Reverse Phase Protein Array Profiling Identifies Recurrent Protein Expression Patterns of DNA Damage-Related Proteins across Acute and Chronic Leukemia: Samples from Adults and the Children’s Oncology Group
Abstract
1. Introduction
2. Results
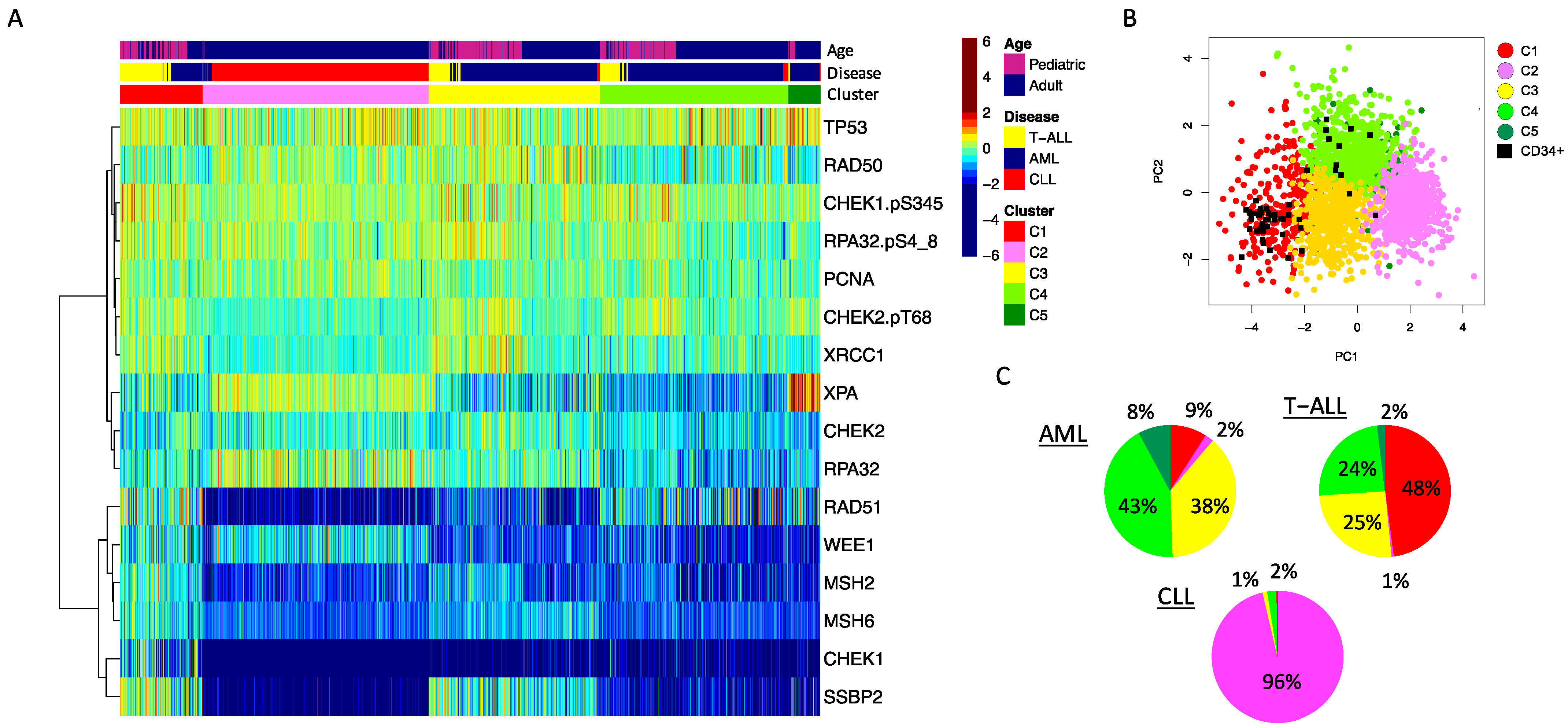
2.1. DDR Protein Expression Patterns Are Differentially Expressed between Acute and Chronic Leukemia Subtypes
2.2. DDR Protein Expression Levels Are Different across Age Groups in Acute Leukemia
2.3. Disease Specific Characteristics Significantly Associated with DDR Protein Expression Patterns
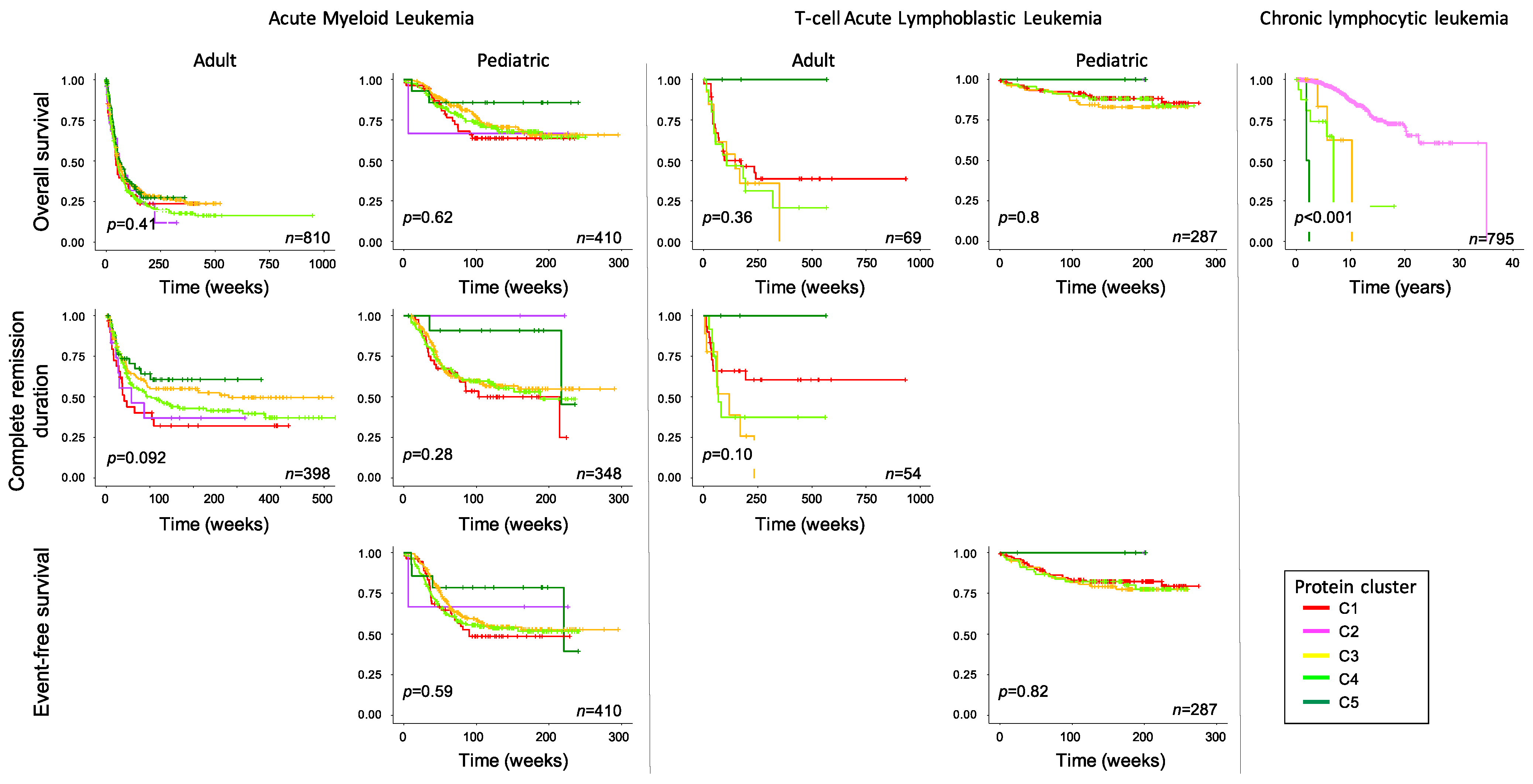
2.4. Expression Patterns Associated with Survival Outcome across Diseases
3. Discussion
4. Materials and Methods
Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasaki, K.; Jabbour, E.; Short, N.J.; Jain, N.; Ravandi, F.; Pui, C.H.; Kantarjian, H. Acute lymphoblastic leukemia: A population-based study of outcome in the United States based on the surveillance, epidemiology, and end results (SEER) database, 1980–2017. Am. J. Hematol. 2021, 96, 650–658. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Alrawashdh, N.; Sweasy, J.; Erstad, B.; McBride, A.; Persky, D.O.; Abraham, I. Survival trends in chronic lymphocytic leukemia across treatment eras: US SEER database analysis (1985–2017). Ann. Hematol. 2021, 100, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.T.; So, C.W.E. DNA damage accumulation and repair defects in acute myeloid leukemia: Implications for pathogenesis, disease progression, and chemotherapy resistance. Chromosoma 2014, 123, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.-W.; Sun, Q.-Y.; Tan, K.-T.; Chien, W.; Mayakonda, A.; Yeoh, A.E.J.; Kawamata, N.; Nagata, Y.; Jin-Fen, X.; Loh, X.-Y.; et al. Mutational Landscape of Pediatric Acute Lymphoblastic Leukemia. Cancer Res. 2017, 77, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.A.; Tausch, E.; Taylor-Weiner, A.N.; Stewart, C.; Reiter, J.G.; Bahlo, J.; Kluth, S.; Bozic, I.; Lawrence, M.; Böttcher, S.; et al. Mutations driving CLL and their evolution in progression and relapse. Nature 2015, 526, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med. 2013, 369, 2059–2074. [Google Scholar]
- Rose, D.; Haferlach, T.; Schnittger, S.; Perglerová, K.; Kern, W.; Haferlach, C. Subtype-specific patterns of molecular mutations in acute myeloid leukemia. Leukemia 2017, 31, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Hoff, F.W.; van Dijk, A.D.; Qiu, Y.; Hu, C.W.; Ries, R.E.; Ligeralde, A.; Jenkins, G.N.; Gerbing, R.B.; Gamis, A.S.; Aplenc, R.; et al. Clinical relevance of proteomic profiling in de novo pediatric acute myeloid leukemia: A Children’s Oncology Group study. Haematologica 2022, 107, 2329–2343. [Google Scholar] [CrossRef] [PubMed]
- Griffen, T.L.; Hoff, F.W.; Qiu, Y.; Lillard, J.W., Jr.; Ferrajoli, A.; Thompson, P.; Toro, E.; Ruiz, K.; Burger, J.; Wierda, W.; et al. Proteomic profiling based classification of CLL provides prognostication for modern therapy and identifies novel therapeutic targets. Blood Cancer J. 2022, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Kornblau, S.M.; Coombes, K.R. Use of reverse phase protein microarrays to study protein expression in leukemia: Technical and methodological lessons learned. Methods Mol. Biol. 2011, 785, 141–155. [Google Scholar] [PubMed]
- Tibes, R.; Qiu, Y.; Lu, Y.; Hennessy, B.; Andreeff, M.; Mills, G.B.; Kornblau, S.M. Reverse phase protein array: Validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol. Cancer Ther. 2006, 5, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.W.; Kornblau, S.M.; Slater, J.H.; Qutub, A.A. Progeny Clustering: A Method to Identify Biological Phenotypes. Sci. Rep. 2015, 5, 12894. [Google Scholar] [CrossRef] [PubMed]
- Teachey, D.T.; Devidas, M.; Wood, B.L.; Chen, Z.; Hayashi, R.J.; Hermiston, M.L.; Annett, R.D.; Archer, J.H.; Asselin, B.L.; August, K.J.; et al. Children’s Oncology Group Trial AALL1231: A Phase III Clinical Trial Testing Bortezomib in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia and Lymphoma. J. Clin. Oncol. 2022, 40, 2106–2118. [Google Scholar] [CrossRef] [PubMed]
- Aplenc, R.; Meshinchi, S.; Sung, L.; Alonzo, T.; Choi, J.; Fisher, B.; Gerbing, R.; Hirsch, B.; Horton, T.; Kahwash, S.; et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: A report from the Children’s Oncology Group. Haematologica 2020, 105, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Akbani, R.; Ng, P.K.; Werner, H.M.; Shahmoradgoli, M.; Zhang, F.; Ju, Z.; Liu, W.; Yang, J.-Y.; Yoshihara, K.; Li, J.; et al. A pan-cancer proteomic perspective on The Cancer Genome Atlas. Nat. Commun. 2014, 5, 3887. [Google Scholar] [CrossRef] [PubMed]
- Hartigan, J.A.; Wong, M.A. Algorithm AS 136: A K-Means Clustering Algorithm. J. Royal Stat. Soc. 1979, 28, 100–108. [Google Scholar] [CrossRef]


| Array Name | Disease | Age Category | # Samples | # Antibodies | # DNADR and DDR Antibody |
|---|---|---|---|---|---|
| AML A3 | AML | Adult | 810 | 412 | 21 |
| TH2 | AML | Pediatric | 500 | 296 | 19 |
| TH3 | T-cell ALL | Adult | 69 | 321 | 20 |
| Pediatric | 292 | 321 | 20 | ||
| CLL | CLL | Adult | 795 | 384 | 24 |
| AML | Total | C1 | C2 | C3 | C4 | C5 | p | |
|---|---|---|---|---|---|---|---|---|
| Number | Count | 100% | 9% | 2% | 38% | 43% | 8% | |
| Gender | Female | 44% | 50% | 43% | 46% | 44% | 36% | 0.321 |
| Age (years old) | Median | 51.59 | 19.06 | 64.22 | 30.46 | 58.75 | 64.95 | p < 0.001 |
| <2 | 5% | 10% | 0% | 1% | 7% | 3% | p < 0.001 | |
| 2–10 | 13% | 14% | 11% | 16% | 11% | 3% | 0.005 | |
| 10–18 | 18% | 25% | 0% | 25% | 12% | 10% | p < 0.001 | |
| 18–30 | 6% | 8% | 14% | 8% | 4% | 0% | 0.001 | |
| 30–60 | 18% | 17% | 14% | 18% | 18% | 25% | 0.404 | |
| 60+ | 41% | 25% | 61% | 32% | 48% | 59% | p < 0.001 | |
| Ethnicity (n = 562) | Hispanic or Latino | 13% | 14% | 7% | 16% | 11% | 7% | 0.859 |
| Central nerve system involvement (n = 1269) | Positive | 16% | 24% | 0% | 19% | 14% | 4% | p < 0.001 |
| Cytogenetics (n = 1202) | t(8;21) | 8% | 1% | 4% | 11% | 7% | 5% | 0.002 |
| Inv16 | 7% | 10% | 0% | 7% | 7% | 1% | 0.043 | |
| Diploid | 30% | 30% | 18% | 38% | 25% | 25% | p < 0.001 | |
| MLL | 9% | 13% | 7% | 6% | 11% | 6% | 0.022 | |
| 8, −5, −7 | 23% | 18% | 25% | 16% | 28% | 30% | p < 0.001 | |
| Other | 16% | 18% | 21% | 17% | 14% | 13% | 0.340 | |
| Risk group (n = 1223) | Favorable | 19% | 18% | 11% | 26% | 16% | 7% | <0.001 |
| Intermediate | 46% | 50% | 39% | 45% | 46% | 44% | 0.814 | |
| Unfavorable | 28% | 24% | 32% | 25% | 31% | 33% | 0.098 | |
| CEBPA mutation (n = 1068) | Mutated | 8% | 6% | 4% | 13% | 5% | 5% | 0.001 |
| NPM1 mutation (n = 1120) | Mutated | 13% | 9% | 11% | 20% | 9% | 4% | p < 0.001 |
| FLT3-ITD mutation (n = 1133) | Mutated | 16% | 20% | 7% | 26% | 9% | 3% | p < 0.001 |
| White blood cell count | >100,000 | 13% | 31% | 8% | 12% | 13% | 1% | p < 0.001 |
| T-ALL | Total | C1 | C2 | C3 | C4 | C5 | p | |
|---|---|---|---|---|---|---|---|---|
| Number | Count | 100% | 48% | 1% | 25% | 24% | 2% | |
| Gender | Female | 23% | 23% | 0% | 17% | 31% | 14% | 0.227 |
| Age (years old at time of diagnosis) | Median | 13 | 7.5 | 11 | 11 | 7 | 0.121 | |
| <2 | 4% | 4% | 0% | 2% | 7% | 0% | 0.173 | |
| 2–10 | 37% | 31% | 100% | 41% | 41% | 57% | ||
| 10–18 | 33% | 35% | 0% | 37% | 29% | 0% | ||
| 18–30 | 15% | 18% | 0% | 9% | 15% | 14% | ||
| 30–60 | 9% | 10% | 0% | 9% | 8% | 14% | ||
| 60+ | 2% | 2% | 0% | 2% | 0% | 14% | ||
| Ethnicity (n = 338) | Hispanic or Latino | 22% | 26% | 0% | 20% | 14% | 43% | 0.050 |
| Race (n = 323) | Black | 9% | 8% | 0% | 13% | 9% | 0% | 0.555 |
| Central nerve system involvement (n = 349) | Positive | 29% | 34% | 0% | 28% | 20% | 29% | 0.153 |
| Early T-cell precursor (n = 342) | Yes | 14% | 13% | 0% | 20% | 8% | 14% | 0.305 |
| Risk group (n = 275) | Standard risk | 24% | 24% | 0% | 22% | 28% | 14% | 0.689 |
| Intermediate risk | 48% | 45% | 100% | 55% | 45% | 43% | ||
| Very high risk | 4% | 2% | 0% | 7% | 6% | 0% | ||
| T-cell receptor rearrangement (n = 312) | Yes | 12% | 10% | 0% | 10% | 17% | 14% | 0.247 |
| White blood cell count (n = 358) | >100,000 | 46% | 51% | 100% | 51% | 28% | 43% | 0.003 |
| CLL | Total | C2 | Not C2 | p | |
|---|---|---|---|---|---|
| Number | Count | 100% | 96% | 4% | |
| Age (years old at time of diagnosis) | Median | 57 | 57 | 58 | 0.121 |
| 30–60 | 57% | 58% | 55% | 0.948 | |
| 60+ | 43% | 42% | 45% | ||
| Gender | Female | 39% | 39% | 38% | 1.000 |
| Race (n = 750) | Black | 4% | 4% | 10% | 0.234 |
| Not Black | 90% | 90% | 86% | ||
| NA | 6% | 6% | 3% | ||
| Binet stage (n = 784) | A | 62% | 62% | 52% | 0.563 |
| B | 9% | 10% | 7% | ||
| C | 28% | 27% | 34% | ||
| IGHV gene mutation status (n = 576) | Mutated | 37% | 38% | 24% | 0.269 |
| Rai stage (n = 784) | 0 | 34% | 34% | 28% | 0.692 |
| I | 29% | 30% | 21% | ||
| II | 6% | 6% | 7% | ||
| III | 17% | 16% | 24% | ||
| IV | 13% | 13% | 14% | ||
| Deletion 11q (n = 711) | Yes | 13% | 13% | 7% | 0.240 |
| Deletion 13q (n = 711) | Yes | 34% | 35% | 17% | 0.027 |
| Trisomy 12 (n = 711) | Yes | 14% | 14% | 17% | 0.413 |
| Deletion 17p (n = 711) | Yes | 9% | 8% | 24% | 0.006 |
| Chromosome 9 (n = 711) | Yes | 2% | 2% | 10% | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoff, F.W.; Griffen, T.L.; Brown, B.D.; Horton, T.M.; Burger, J.; Wierda, W.; Hubner, S.E.; Qiu, Y.; Kornblau, S.M. Reverse Phase Protein Array Profiling Identifies Recurrent Protein Expression Patterns of DNA Damage-Related Proteins across Acute and Chronic Leukemia: Samples from Adults and the Children’s Oncology Group. Int. J. Mol. Sci. 2023, 24, 5460. https://doi.org/10.3390/ijms24065460
Hoff FW, Griffen TL, Brown BD, Horton TM, Burger J, Wierda W, Hubner SE, Qiu Y, Kornblau SM. Reverse Phase Protein Array Profiling Identifies Recurrent Protein Expression Patterns of DNA Damage-Related Proteins across Acute and Chronic Leukemia: Samples from Adults and the Children’s Oncology Group. International Journal of Molecular Sciences. 2023; 24(6):5460. https://doi.org/10.3390/ijms24065460
Chicago/Turabian StyleHoff, Fieke W., Ti’ara L. Griffen, Brandon D. Brown, Terzah M. Horton, Jan Burger, William Wierda, Stefan E. Hubner, Yihua Qiu, and Steven M. Kornblau. 2023. "Reverse Phase Protein Array Profiling Identifies Recurrent Protein Expression Patterns of DNA Damage-Related Proteins across Acute and Chronic Leukemia: Samples from Adults and the Children’s Oncology Group" International Journal of Molecular Sciences 24, no. 6: 5460. https://doi.org/10.3390/ijms24065460
APA StyleHoff, F. W., Griffen, T. L., Brown, B. D., Horton, T. M., Burger, J., Wierda, W., Hubner, S. E., Qiu, Y., & Kornblau, S. M. (2023). Reverse Phase Protein Array Profiling Identifies Recurrent Protein Expression Patterns of DNA Damage-Related Proteins across Acute and Chronic Leukemia: Samples from Adults and the Children’s Oncology Group. International Journal of Molecular Sciences, 24(6), 5460. https://doi.org/10.3390/ijms24065460






