The Gilded Clot: Review of Metal-Modulated Platelet Activation, Coagulation, and Fibrinolysis
Abstract
1. Introduction
2. Definitions and Search Strategy
3. Specific Metals and their Effects on Platelet Activation, Coagulation, and Fibrinolysis
3.1. Al—Aluminum (Atomic Number 13, Atomic Weight 26.98)
3.2. Au—Gold (Atomic Number 79, 196.97 Atomic Weight)
3.3. Ba—Barium (Atomic Number 56, Atomic Weight 137.33)
3.4. Be—Beryllium (Atomic Number 4, Atomic Weight 9.01)
3.5. Ca—Calcium (Atomic Number 20, Atomic Weight 40.08)
3.6. Cd—Cadmium (Atomic Number 48, Atomic Weight 112.41)
3.7. Co—Cobalt (Atomic Number 27, Atomic Weight 58.93)
3.8. Cr—Chromium (Atomic Number 24, Atomic Weight 51.99)
3.9. Cs—Cesium (Atomic Number 55, Atomic Weight 132.90)
3.10. Cu—Copper (Atomic Number 29, Atomic Weight 63.54)
3.11. Fe—Iron (Atomic Number 26, Atomic Weight 55.84)
3.12. Ga—Gallium (Atomic Number 31, Atomic Weight 69.72)
3.13. Hg—Mercury (Atomic Number 80, Atomic Weight 200.59)
3.14. Li—Lithium (Atomic Number 3, Atomic Weight 6.94)
3.15. Mg—Magnesium (Atomic Number 12, Atomic Weight 24.30)
3.16. Mn—Manganese (Atomic Number 25, Atomic Weight 54.93)
3.17. Ni—Nickel (Atomic Number 28, Atomic Weight 58.69)
3.18. Pb—Lead (Atomic Number 82, Atomic Weight 207.20)
3.19. Pt—Platinum (Atomic Number 78, Atomic Weight 195.08)
3.20. Rb—Rubidium (Atomic Number 37, Atomic Weight 85.47)
3.21. Ru—Ruthenium (Atomic Number 44, Atomic Weight 101.07)
3.22. Sc—Scandium (Atomic Number 21, Atomic Weight 44.96)
3.23. Sr—Strontium (Atomic Number 38, Atomic Weight 87.62)
3.24. V—Vanadium (Atomic Number 23, Atomic Weight 50.94)
3.25. Zn—Zinc (Atomic Number 30, Atomic Weight 65.38)
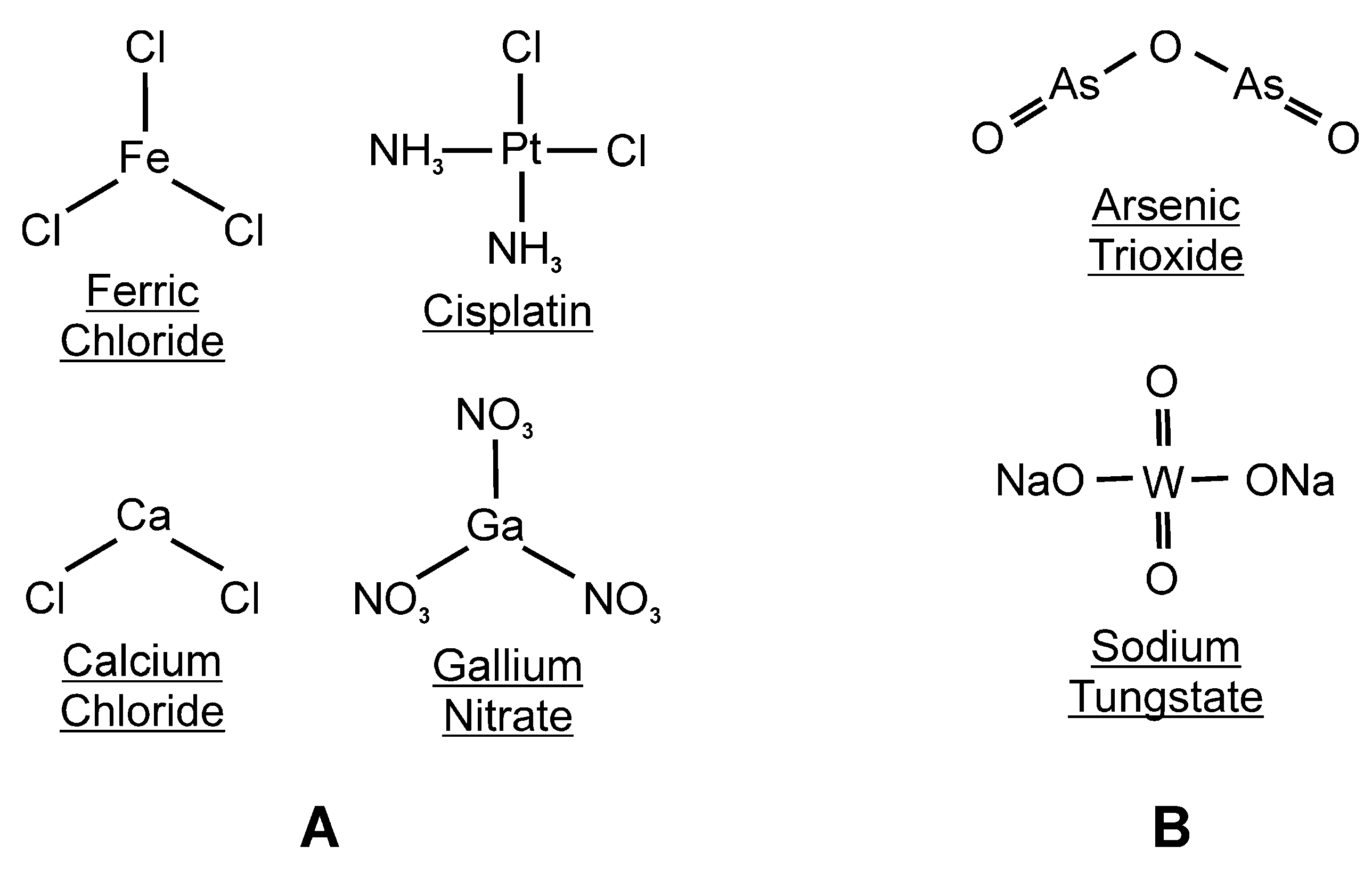
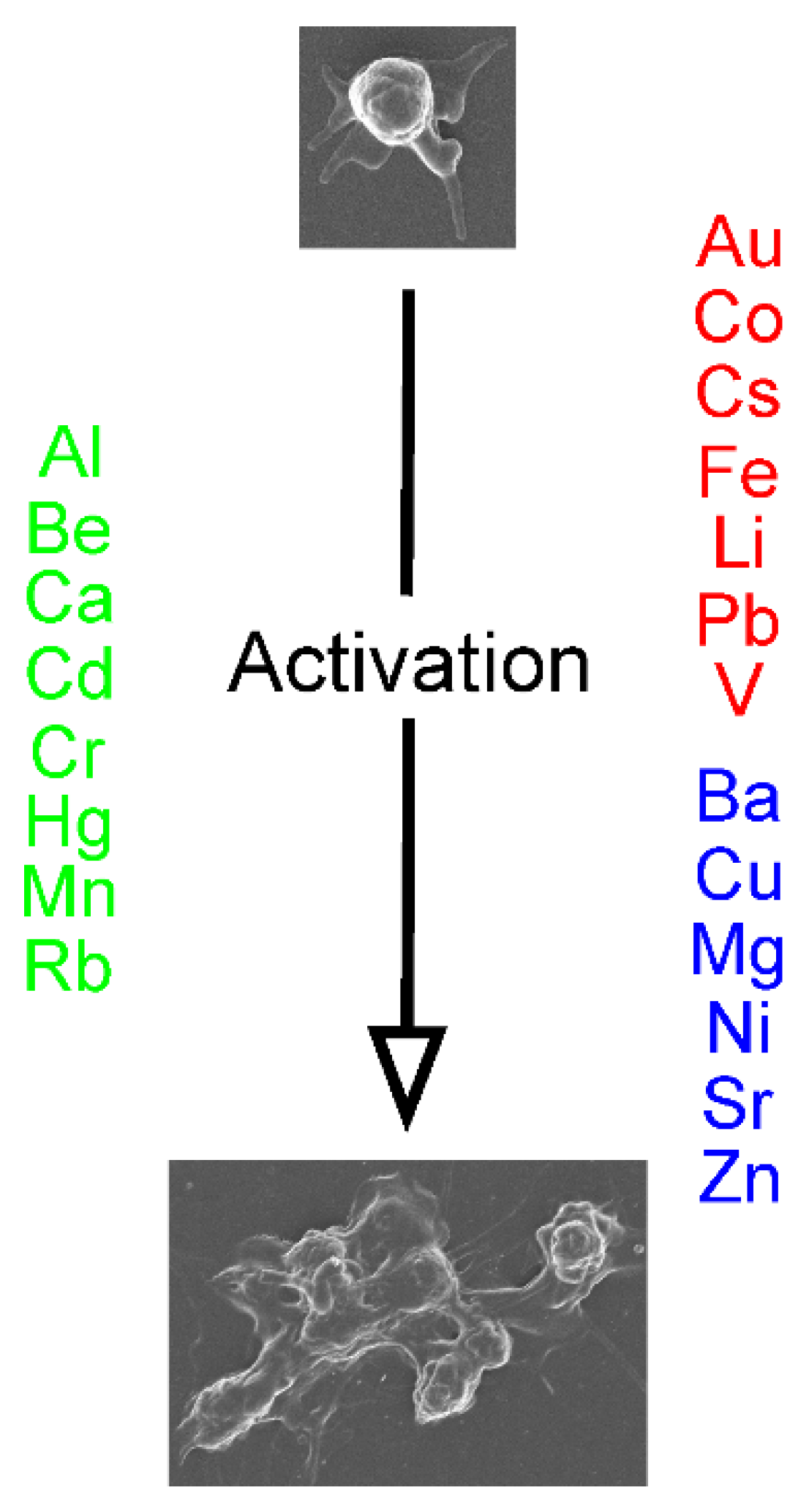
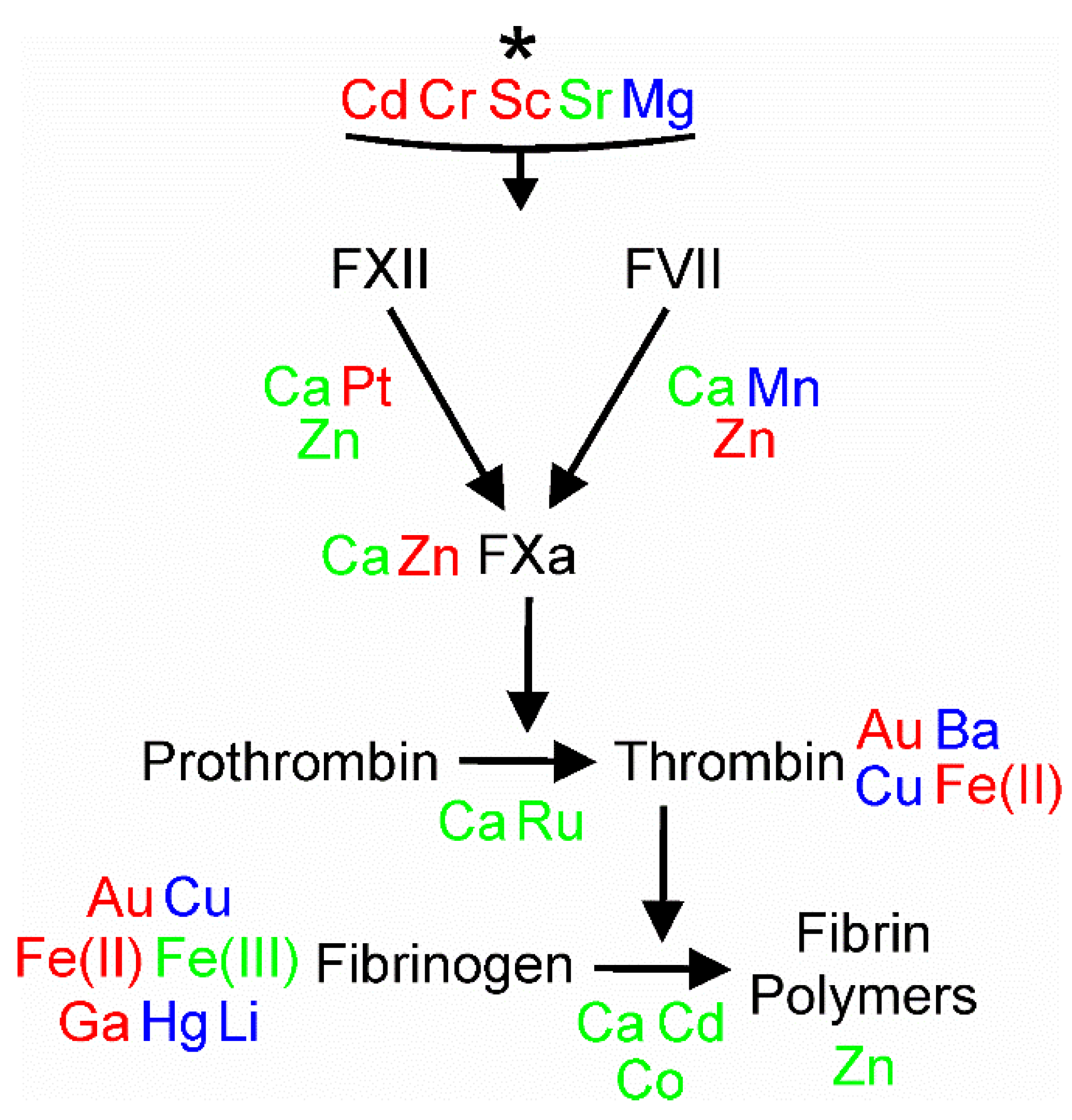
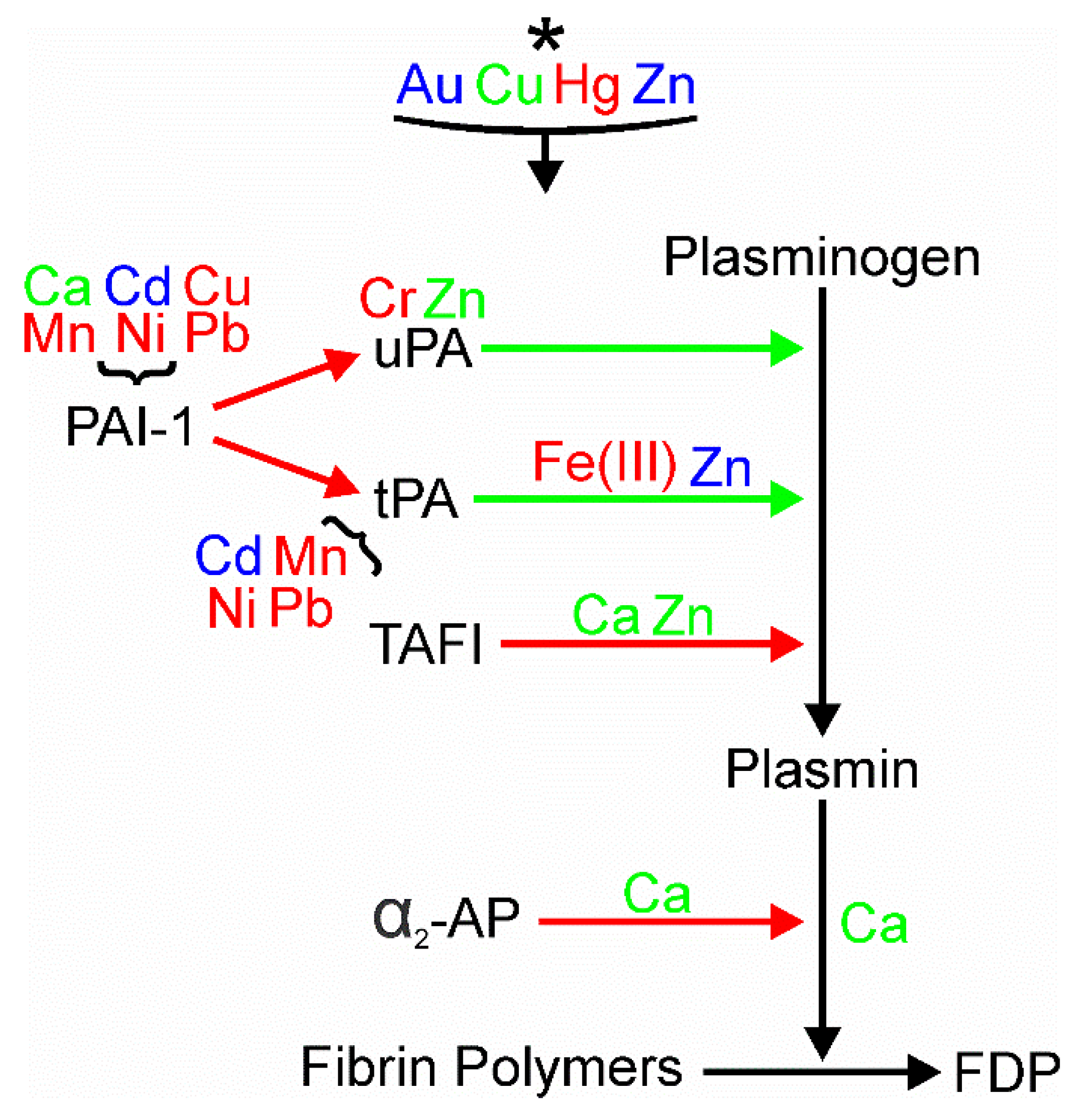
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neiva, T.J.; Fries, D.M.; Monteiro, H.P.; D’Amico, E.A.; Chamone, D.A. Aluminum induces lipid peroxidation and aggregation of human blood platelets. Braz. J. Med. Biol. Res. 1997, 30, 599–604. [Google Scholar] [CrossRef]
- Abou-Shady, E.A.; Farrag, H.E.; el-Damarawy, N.A.; Mohamed, F.A.; Kamel, A.M.; Massoud, A.A. In vitro effects of trace elements on blood clotting and platelet function. A—Iron, copper, and gold. J. Egypt. Public Health Assoc. 1991, 66, 21–48. [Google Scholar] [PubMed]
- Kean, W.F.; Lock, C.J.; Somers, D.; Rischke, J.; Nablo, L.; Kassam, Y.B.; Hogan, M.G.; Buchanan, W.W.; Howard-Lock, H.E. Action of gold sodium thiomalate on experimental thrombosis in vivo. J. Pharm. Sci. 1991, 80, 113–118. [Google Scholar] [CrossRef]
- Andersen, R.B.; Winther, O. Blood-fibrinolysis during chrysotherapy. Acta Rheumatol. Scand. 1968, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Blache, D.; Ciavatti, M.; Ojeda, C. Platelet aggregation and endogenous 5-HT secretion in presence of Ca2+, Sr2+ and Ba2+. Effects of calcium antagonists. Thromb. Res. 1987, 46, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.B. Role of alkaline earth metal ions in the activation of thromboplastin system. Indian J. Biochem. 1971, 8, 191–193. [Google Scholar] [PubMed]
- Suzuki, K.; Hashimoto, S. The influences of divalent metal ions on fibrin monomer polymerization. Biochim. Biophys. Acta 1976, 439, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Togna, G.; Togna, A.R.; Russo, P.; Caprino, L. Toxicological effects of beryllium on platelets and vascular endothelium. Toxicol. Appl. Pharmacol. 1997, 144, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- Davlouros, P.; Xanthopoulou, I.; Mparampoutis, N.; Giannopoulos, G.; Deftereos, S.; Alexopoulos, D. Role of Calcium in Platelet Activation: Novel Insights and Pharmacological Implications. Med. Chem. 2016, 12, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Stenflo, J.; Stenberg, Y.; Muranyi, A. Calcium-binding EGF-like modules in coagulation proteinases: Function of the calcium ion in module interactions. Biochim. Biophys. Acta 2000, 1477, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Butenas, S.; Mann, K.G. Blood coagulation. Biochemistry 2002, 67, 3–12. [Google Scholar]
- Kojima, Y.; Urano, T.; Kojima, K.; Serizawa, K.; Takada, Y.; Takada, A. The significant enhancement of fibrinolysis by calcium ion in a cell free system: The shortening of euglobulin clot lysis time by calcium ion. Thromb. Haemost. 1994, 72, 113–118. [Google Scholar] [CrossRef]
- Ap Gwynn, I.; Evans, P.M.; Jones, B.M.; Chandler, J.A. Effects of divalent cations on calcium levels, aggregation and morphology of human blood platelets in vitro. Cytobios 1980, 27, 97–106. [Google Scholar] [PubMed]
- Venter, C.; Oberholzer, H.M.; Bester, J.; van Rooy, M.J.; Bester, M.J. Ultrastructural, Confocal and Viscoelastic Characteristics of Whole Blood and Plasma After Exposure to Cadmium and Chromium Alone and in Combination: An Ex Vivo Study. Cell Physiol. Biochem. 2017, 43, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.; Kaji, T.; Sakamoto, M.; Kozuka, H. Effects of cadmium on the release of tissue plasminogen activator and plasminogen activator inhibitor type 1 from cultured human vascular smooth muscle cells and fibroblasts. Toxicology 1996, 106, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Sakuma, M.; Fujie, T.; Kaji, T.; Yamamoto, C. Cadmium induces plasminogen activator inhibitor-1 via Smad2/3 signaling pathway in human endothelial EA.hy926 cells. J. Toxicol. Sci. 2021, 46, 249–253. [Google Scholar] [CrossRef]
- Smith, A.G.; Smith, A.N. Effect of cobaltous chloride on aggregation of platelets from normal and afibrinogenaemic human blood. Toxicol. Lett. 1984, 23, 349–352. [Google Scholar] [CrossRef]
- Shumilla, J.A.; Barchowsky, A. Inhibition of protein synthesis by chromium(VI) differentially affects expression of urokinase and its receptor in human type II pneumocytes. Toxicol. Appl. Pharmacol. 1999, 158, 288–295. [Google Scholar] [CrossRef]
- Greil, W.; Patscheke, H.; Brossmer, R. Effect of lithium and other monovalent cations on the ADP induced platelet aggregation in human platelet rich plasma. FEBS Lett. 1972, 26, 271–273. [Google Scholar] [CrossRef][Green Version]
- McGee, M.P.; Teuschler, H.; Liang, J. Electrostatic interactions during activation of coagulation factor IX via the tissue factor pathway: Effect of univalent salts. Biochim. Biophys. Acta 1999, 1453, 239–253. [Google Scholar] [CrossRef][Green Version]
- Whiss, P.A.; Andersson, R.G. Divalent cations and the protein surface co-ordinate the intensity of human platelet adhesion and P-selectin surface expression. Blood Coagul. Fibrinolysis 2002, 13, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Ward, T.D.; Ford, P.M. Effects of cupric chloride on coagulation in human plasma: Role of fibrinogen. J. Thromb. Thrombolysis 2018, 46, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bogert, A.B. The effect of copper ions on clot lysis time in the fibrinolytic assay of streptokinase. Thromb. Diath. Haemorrh. 1965, 13, 477–483. [Google Scholar] [CrossRef]
- Bucci, J.C.; Trelle, M.B.; McClintock, C.S.; Qureshi, T.; Jørgensen, T.J.; Peterson, C.B. Copper(II) Ions Increase Plasminogen Activator Inhibitor Type 1 Dynamics in Key Structural Regions That Govern Stability. Biochemistry 2016, 55, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Pretorius, E.; Oberholzer, H.M.; van der Spuy, W.J. Interaction of fibrin with red blood cells: The role of iron. Ultrastruct. Pathol. 2012, 36, 79–84. [Google Scholar] [CrossRef]
- Pretorius, E.; Lipinski, B. Differences in morphology of fibrin clots induced with thrombin and ferric ions and its pathophysiological consequences. Heart Lung Circ. 2013, 22, 447–449. [Google Scholar] [CrossRef]
- Lipinski, B.; Pretorius, E. Hydroxyl radical-modified fibrinogen as a marker of thrombosis: The role of iron. Hematology 2012, 17, 241–247. [Google Scholar] [CrossRef]
- Pretorius, E.; Bester, J.; Vermeulen, N.; Lipinski, B. Oxidation inhibits iron-induced blood coagulation. Curr. Drug Targets 2013, 14, 13–19. [Google Scholar] [CrossRef][Green Version]
- Pretorius, E.; Vermeulen, N.; Bester, J.; Lipinski, B.; Kell, D.B. A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: The use of scanning electron microscopy. Toxicol. Mech. Methods 2013, 23, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Jankun, J.; Landeta, P.; Pretorius, E.; Skrzypczak-Jankun, E.; Lipinski, B. Unusual clotting dynamics of plasma supplemented with iron(III). Int. J. Mol. Med. 2014, 33, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Pretorius, E. Iron and carbon monoxide enhance coagulation and attenuate fibrinolysis by different mechanisms. Blood Coagul. Fibrinolysis 2014, 25, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Pretorius, E. Iron-enhanced coagulation is attenuated by chelation: Thrombelastographic and ultrastructural analysis. Blood Coagul. Fibrinolysis 2014, 25, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Henderson, J. Sonoclot®-based method to detect iron enhanced coagulation. J. Thromb. Thrombolysis 2016, 42, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Jacobsen, W.K. Iron modulates the alpha chain of fibrinogen. Biometals 2016, 29, 235–238. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Z.; Li, Y.V. Metal ion chelation enhances tissue plasminogen activator (tPA)-induced thrombolysis: An in vitro and in vivo study. J. Thromb. Thrombolysis 2022, 53, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Bauters, A.; Holt, D.J.; Zerbib, P.; Rogosnitzky, M. Gallium nitrate induces fibrinogen flocculation: An explanation for its hemostatic effect? Biometals 2013, 26, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Mirowski, M.; Wierzbicki, R. Coagulation and fibrinolysis in rats poisoned with mercuric chloride. Pol. J. Pharmacol. Pharm. 1988, 40, 365–371. [Google Scholar]
- Nielsen, V.G. Lethal concentrations of mercury or lead do not affect coagulation kinetics in human plasma. J. Thromb. Thrombolysis 2019, 48, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Maseko, P.B.; van Rooy, M.; Taute, H.; Venter, C.; Serem, J.C.; Oberholzer, H.M. Whole blood ultrastructural alterations by mercury, nickel and manganese alone and in combination: An ex vivo investigation. Toxicol. Ind. Health 2021, 37, 98–111. [Google Scholar] [CrossRef]
- Nanninga, L.B.; Guest, M.M. Blocking of inhibition of fibrinolysin by mercurials and oxidizing agents. Arch. Biochem. Biophys. 1965, 109, 607–614. [Google Scholar] [CrossRef]
- La Gioia, A.; Petrini, M.; Carulli, G.; Lofaro, A. Lithium and rubidium activities on platelet aggregation. Drugs Exp. Clin. Res. 1991, 17, 313–316. [Google Scholar] [PubMed]
- Hargreaves, L.N.; Hayes, P.C. The influence of lithium and calcium ions on the aggregation of human blood platelets. Thromb. Res. 1978, 13, 79–83. [Google Scholar] [CrossRef]
- Imandt, L.; Tijhuis, D.; Wessels, H.; Haanen, C.; van den Berg, R.; Thomas, C. Lithium stimulates thromboxane B2 formation in human platelets. Prostaglandins Med. 1981, 7, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Dehpour, A.R.; Kiani, K.; Ghafourifar, P.; Mousavizadeh, K. Conditions that lithium inhibits or potentiates vasopressin V1-receptor-mediated platelet aggregation and [Ca++]i mobilization. Gen. Pharmacol. 1995, 26, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Riches, P.L.; Gow, I.F. The effect of lithium on bovine blood platelet function. Biochem. Soc. Trans. 1998, 26, S391. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.C.; Hargreaves, L.N. Lithium inhibition of ristocetin-induced fibrinogen precipitation. Thromb. Res. 1978, 12, 567–568. [Google Scholar] [CrossRef]
- Ravn, H.B.; Kristensen, S.D.; Vissinger, H.; Husted, S.E. Magnesium inhibits human platelets. Blood Coagul. Fibrinolysis 1996, 7, 241–244. [Google Scholar] [CrossRef]
- Ravn, H.B.; Lassen, J.F.; Bergenhem, N.; Kristensen, A.T. Intravenous magnesium does not influence the activity of the coagulation cascade. Blood Coagul. Fibrinolysis 2001, 12, 223–228. [Google Scholar] [CrossRef]
- Van den Besselaar, A.M. Magnesium and manganese ions accelerate tissue factor-induced coagulation independently of factor IX. Blood Coagul. Fibrinolysis 2002, 13, 19–23. [Google Scholar] [CrossRef]
- Stewart, D.; Marder, V.J.; Starkman, S.; Saver, J.L. Magnesium sulfate neither potentiates nor inhibits tissue plasminogen activator-induced thrombolysis. J. Thromb. Haemost. 2006, 4, 1575–1579. [Google Scholar] [CrossRef] [PubMed]
- Riondino, S.; Pulcinelli, F.M.; Pignatelli, P.; Gazzaniga, P.P. Involvement of the glycoproteic Ib-V-IX complex in nickel-induced platelet activation. Environ. Health Perspect. 2001, 109, 225–228. [Google Scholar] [CrossRef][Green Version]
- Andrew, A.; Barchowsky, A. Nickel-induced plasminogen activator inhibitor-1 expression inhibits the fibrinolytic activity of human airway epithelial cells. Toxicol. Appl. Pharmacol. 2000, 168, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Klei, L.R.; Barchowsky, A. Nickel requires hypoxia-inducible factor-1 alpha, not redox signaling, to induce plasminogen activator inhibitor-1. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L607–L615. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Klei, L.R.; Barchowsky, A. AP-1-dependent induction of plasminogen activator inhibitor-1 by nickel does not require reactive oxygen. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L616–L623. [Google Scholar] [CrossRef]
- Davis, R.B.; Holtz, G.C. Effects of lead acetate on platelet aggregation, ultrastructure and serotonin release. Thromb. Diath. Haemorrh. 1971, 26, 455–466. [Google Scholar] [CrossRef]
- Sharp, D.S.; Beswick, A.; Renaud, S.; Toothill, C.; Elwood, P.C. Blood lead and platelet aggregation—Evidence for a causal association. Thromb. Haemost. 1991, 66, 604–608. [Google Scholar] [CrossRef]
- Nielsen, V.G. Platinoid effects on human plasmatic coagulation kinetics: A viscoelastic analysis. J. Thromb. Thrombolysis 2021, 51, 577–583. [Google Scholar] [CrossRef]
- Solymoss, B.; Selye, H.; Gabbiani, G. Predisposition to thrombosis not reflected by the blood coagulogram. J. Clin. Pathol. 1966, 19, 331–333. [Google Scholar] [CrossRef]
- Muñoz-Hernández, M.A.; Muñoz-Tejedor, D.; Alda, J.O.; Escanero, J.F. Influence of stable strontium on platelets factor 4 release in sheep blood. Arch. Farmacol. Toxicol. 1986, 12, 135–138. [Google Scholar]
- Togna, G.; Gallozzi, S.; Caprino, L. Influence of strontium chloride on blood platelet function. Arch. Toxicol. Suppl. 1989, 13, 366–369. [Google Scholar] [PubMed]
- González-Villalva, A.; Piñón-Zárate, G.; De la Peña Díaz, A.; Flores-García, M.; Bizarro-Nevares, P.; Rendón-Huerta, E.P.; Colín-Barenque, L.; Fortoul, T.I. The effect of vanadium on platelet function. Environ. Toxicol. Pharmacol. 2011, 32, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.S.; Lopes-Pires, M.; Pugh, N. Zinc: An endogenous and exogenous regulator of platelet function during hemostasis and thrombosis. Platelets 2021, 32, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, I. Contact activation in human plasma is triggered by zinc ion modulation of factor XII (Hageman factor). Blood Coagul. Fibrinolysis 1993, 4, 671–678. [Google Scholar] [CrossRef]
- Fatah, K.; Hessel, B. Effect of zinc ions on fibrin network structure. Blood Coagul. Fibrinolysis 1998, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Fredenburgh, J.C.; Weitz, J.I. Zinc: An important cofactor in haemostasis and thrombosis. Thromb. Haemost. 2013, 109, 421–430. [Google Scholar] [CrossRef]
- Henderson, S.J.; Xia, J.; Wu, H.; Stafford, A.R.; Leslie, B.A.; Fredenburgh, J.C.; Weitz, D.A.; Weitz, J.I. Zinc promotes clot stability by accelerating clot formation and modifying fibrin structure. Thromb. Haemost. 2016, 115, 533–542. [Google Scholar] [CrossRef]
- Husain, S.S. Fibrin affinity of urokinase-type plasminogen activator. Evidence that Zn2+ mediates strong and specific interaction of single-chain urokinase with fibrin. J. Biol. Chem. 1993, 268, 8574–8579. [Google Scholar] [CrossRef] [PubMed]
- Marx, P.F.; Bouma, B.N.; Meijers, J.C. Role of zinc ions in activation and inactivation of thrombin-activatable fibrinolysis inhibitor. Biochemistry 2002, 41, 1211–1216. [Google Scholar] [CrossRef]
- Henderson, S.J.; Stafford, A.R.; Leslie, B.A.; Kim, P.Y.; Vaezzadeh, N.; Ni, R.; Fredenburgh, J.C.; Weitz, J.I. Zinc delays clot lysis by attenuating plasminogen activation and plasmin-mediated fibrin degradation. Thromb. Haemost. 2015, 113, 1278–1288. [Google Scholar] [CrossRef]
- Elias, T.P. An in vitro study of the effect of arsenic (As2O3) on blood clotting. Acta Haematol. 1969, 41, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, R.; Pino, M.; Hurtado, B.; García de Frutos, P.; Caballo, C.; Escolar, G.; Gomis, R.; Diaz-Ricart, M. Role of sodium tungstate as a potential antiplatelet agent. Drug Des. Dev. Ther. 2015, 26, 2777–2786. [Google Scholar] [CrossRef]
- Thorisdottir, H.; Ratnoff, O.D.; Maniglia, A.J. Activation of Hageman factor (factor XII) by bismuth subgallate, a hemostatic agent. J. Lab. Clin. Med. 1988, 112, 481–486. [Google Scholar] [PubMed]
- Callanan, V.; Curran, A.J.; Smyth, D.A.; Gormley, P.K. The influence of bismuth subgallate and adrenaline paste upon operating time and operative blood loss in tonsillectomy. J. Laryngol. Otol. 1995, 109, 206–208. [Google Scholar] [CrossRef]
- Kriklava, J. Thromboelastographic examination (TEG) in uranium miners. Strahlentherapie 1971, 141, 185–192. [Google Scholar]
- Huang, H.H.; Chen, Z.H.; Nguyen, D.T.; Tseng, C.M.; Chen, C.S.; Chang, J.H. Blood Coagulation on Titanium Dioxide Films with Various Crystal Structures on Titanium Implant Surfaces. Cells 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Amiri, R.J.; Pirzadeh, M.; Moghadamnia. A.A. Aluminum Poisoning with Emphasis on Its Mechanism and Treatment of Intoxication. Emerg. Med. Int. 2022, 2022, 1480553. [Google Scholar] [CrossRef]
- Merchant, B. Gold, the noble metal and the paradoxes of its toxicology. Biologicals 1998, 26, 49–59. [Google Scholar] [CrossRef]
- Moroz, L.A. Effect of gold sodium thiomalate on fibrinolysis. J. Rheumatol. Suppl. 1979, 5, 149–153. [Google Scholar]
- Bhoelan, B.S.; Stevering, C.H.; van der Boog, A.T.; van der Heyden, M.A. Barium toxicity and the role of the potassium inward rectifier current. Clin. Toxicol. 2014, 52, 584–593. [Google Scholar] [CrossRef]
- Seidler, A.; Euler, U.; Müller-Quernheim, J.; Gaede, K.I.; Latza, U.; Groneberg, D.; Letzel, S. Systematic review: Progression of beryllium sensitization to chronic beryllium disease. Occup. Med. 2012, 62, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Schleef, R.R. Calcium-dependent stabilization of type I plasminogen activator inhibitor within platelet alpha-granules. J. Biol. Chem. 1996, 271, 2754–2761. [Google Scholar] [CrossRef]
- Plug, T.; Meijers, J.C. Stimulation of thrombin- and plasmin-mediated activation of thrombin-activatable fibrinolysis inhibitor by anionic molecules. Thromb. Res. 2016, 146, 7–14. [Google Scholar] [CrossRef]
- Sakata, Y.; Tateno, K.; Tamaki, T.; Aoki, N. Calcium-dependent binding of alpha 2-plasmin inhibitor to fibrin. Thromb. Res. 1979, 16, 279–282. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.; Song, W.; Hong, D.; Huang, L.; Li, Y. A review on Cadmium Exposure in the Population and Intervention Strategies Against Cadmium Toxicity. Bull. Environ. Contam. Toxicol. 2021, 106, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Brocato, J.; Costa, M. Oral Chromium Exposure and Toxicity. Curr. Environ. Health Rep. 2015, 2, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.; Zanoni, L.Z. Clinical effects of cesium intake. Biol. Trace Elem. Res. 2010, 135, 1–9. [Google Scholar] [CrossRef]
- Brewer, G.J. The risks of copper toxicity contributing to cognitive decline in the aging population and to Alzheimer’s disease. J Am. Coll. Nutr. 2009, 28, 238–242. [Google Scholar] [CrossRef]
- Scheiber, I.; Dringen, R.; Mercer, J.F. Copper: Effects of deficiency and overload. Met. Ions Life Sci. 2013, 13, 359–387. [Google Scholar]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef]
- Rosenmund, A.; Haeberli, A.; Straub, P.W. Blood coagulation and acute iron toxicity. Reversible iron-induced inactivation of serine proteases in vitro. J. Lab. Clin. Med. 1984, 103, 524–533. [Google Scholar]
- Thompson, J.L., 3rd; Nielsen, V.G.; Castro, A.R.; Chen, A. Heme oxygenase derived carbon monoxide and iron mediated plasmatic hypercoagulability in a patient with calcific mitral valve disease. J. Thromb. Thrombolysis 2015, 39, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G.; Pretorius, E.; Bester, J.; Jacobsen, W.K.; Boyle, P.K.; Reinhard, J.P. Carbon monoxide and iron modulate plasmatic coagulation in Alzheimer’s disease. Curr. Neurovasc. Res. 2015, 12, 31–39. [Google Scholar] [CrossRef][Green Version]
- Nielsen, V.G.; Sobieski, M.A., 2nd; Slaughter, M.S. Left Ventricular Assist Device-Associated Carbon Monoxide and Iron-Enhanced Hypercoagulation: Impact of Concurrent Disease. ASAIO J. 2015, 61, 417–423. [Google Scholar] [CrossRef]
- Shah, N.; Welsby, I.J.; Fielder, M.A.; Jacobsen, W.K.; Nielsen, V.G. Sickle cell disease is associated with iron mediated hypercoagulability. J. Thromb. Thrombolysis 2015, 40, 182–185. [Google Scholar] [CrossRef]
- Nielsen, V.G.; Kulin, W.; LaWall, J.S.; MacFarland, F.N.; Chen, A.; Hadley, H.A.; DaDeppo, A.J.; Steinbrenner, E.B.; Matika, R.W. Chronic Migraineurs Form Carboxyhemefibrinogen and Iron-Bound Fibrinogen. CNS Neurol. Disord. Drug Targets 2015, 14, 1079–1085. [Google Scholar] [CrossRef]
- Chitambar, C.R. Medical applications and toxicities of gallium compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef]
- Magos, L.; Clarkson, T.W. Overview of the clinical toxicity of mercury. Ann. Clin. Biochem. 2006, 43, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Dennison, U.; Clarkson, M.; O’Mullane, J.; Cassidy, E.M. The incidence and clinical correlates of lithium toxicity: A retrospective review. Ir. J. Med. Sci. 2011, 180, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Mordes, J.P.; Wacker, W.E. Excess magnesium. Pharmacol. Rev. 1977, 29, 273–300. [Google Scholar]
- Michalke, B.; Fernsebner, K. New insights into manganese toxicity and speciation. J. Trace Elem. Med. Biol. 2014, 28, 106–116. [Google Scholar] [CrossRef]
- Takagi, J.; Petre, B.M.; Walz, T.; Springer, T.A. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 2002, 110, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Schaumlöffel, D. Nickel species: Analysis and toxic effects. J. Trace Elem. Med. Biol. 2012, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P. International perspectives of lead exposure and lead toxicity. Neurotoxicology 1993, 14, 9–14. [Google Scholar]
- Ibels, L.S.; Pollock, C.A. Lead intoxication. Med. Toxicol. 1986, 1, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Boulikas, T.; Vougiouka, M. Cisplatin and platinum drugs at the molecular level. (Review). Oncol. Rep. 2003, 10, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Fieve, R.R.; Meltzer, H.L. Proceedings: Rubidium salts--toxic effects in humans and clinical effects as an antidepressant drug. Psychopharmacol. Bull. 1974, 10, 38–50. [Google Scholar]
- Düzenli, S.; Bakuridze, K.; Gepdiremen, A. The effects of ruthenium red, dantrolene and nimodipine, alone or in combination, in NMDA induced neurotoxicity of cerebellar granular cell culture of rats. Toxicol. Vitr. 2005, 19, 589–594. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef]
- Tanida, E.; Usuda, K.; Kono, K.; Kawano, A.; Tsuji, H.; Imanishi, M.; Suzuki, S.; Ohnishi, K.; Yamamoto, K. Urinary scandium as predictor of exposure: Effects of scandium chloride hexahydrate on renal function in rats. Biol. Trace Elem. Res. 2009, 130, 273–282. [Google Scholar] [CrossRef]
- Sánchez-González, C.; López-Chaves, C.; Rivas-García, L.; Galindo, P.; Gómez-Aracena, J.; Aranda, P.; Llopis, J. Accumulation of scandium in plasma in patients with chronic renal failure. Sci. World J. 2013, 2013, 782745. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, M. Strontium overload and toxicity: Impact on renal osteodystrophy. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S2), 30–34. [Google Scholar] [CrossRef] [PubMed]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef]
- Gruzewska, K.; Michno, A.; Pawelczyk, T.; Bielarczyk, H. Essentiality and toxicity of vanadium supplements in health and pathology. J. Physiol. Pharmacol. 2014, 65, 603–611. [Google Scholar] [PubMed]
- Fatola, O.I.; Olaolorun, F.A.; Olopade, F.E.; Olopade, J.O. Trends in vanadium neurotoxicity. Brain Res. Bull. 2019, 145, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–257. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, V.G.; Goff, T.; Hunsaker, B.D.; Neves, C.D. The Gilded Clot: Review of Metal-Modulated Platelet Activation, Coagulation, and Fibrinolysis. Int. J. Mol. Sci. 2023, 24, 3302. https://doi.org/10.3390/ijms24043302
Nielsen VG, Goff T, Hunsaker BD, Neves CD. The Gilded Clot: Review of Metal-Modulated Platelet Activation, Coagulation, and Fibrinolysis. International Journal of Molecular Sciences. 2023; 24(4):3302. https://doi.org/10.3390/ijms24043302
Chicago/Turabian StyleNielsen, Vance G., Tanner Goff, Brent D. Hunsaker, and Coulter D. Neves. 2023. "The Gilded Clot: Review of Metal-Modulated Platelet Activation, Coagulation, and Fibrinolysis" International Journal of Molecular Sciences 24, no. 4: 3302. https://doi.org/10.3390/ijms24043302
APA StyleNielsen, V. G., Goff, T., Hunsaker, B. D., & Neves, C. D. (2023). The Gilded Clot: Review of Metal-Modulated Platelet Activation, Coagulation, and Fibrinolysis. International Journal of Molecular Sciences, 24(4), 3302. https://doi.org/10.3390/ijms24043302





