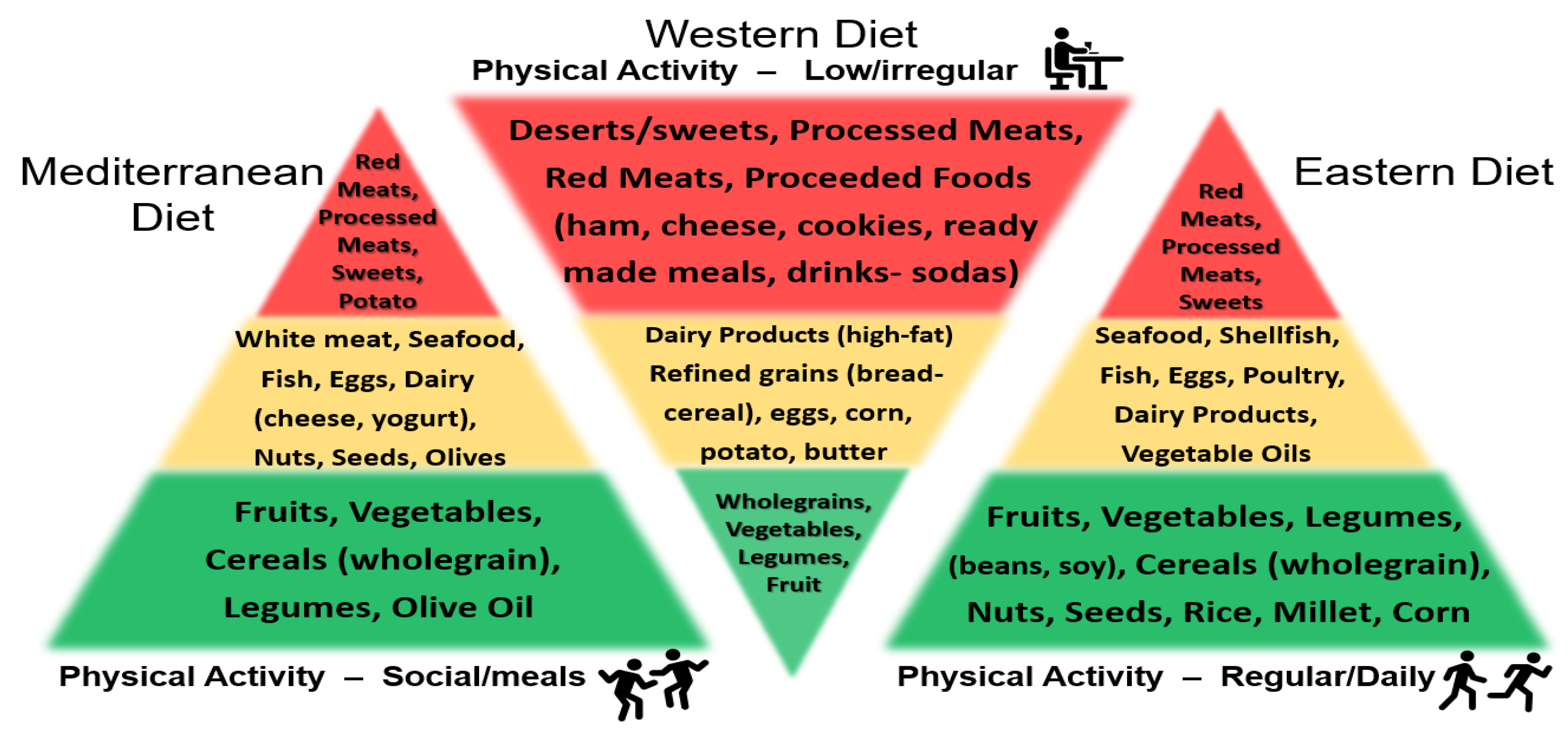
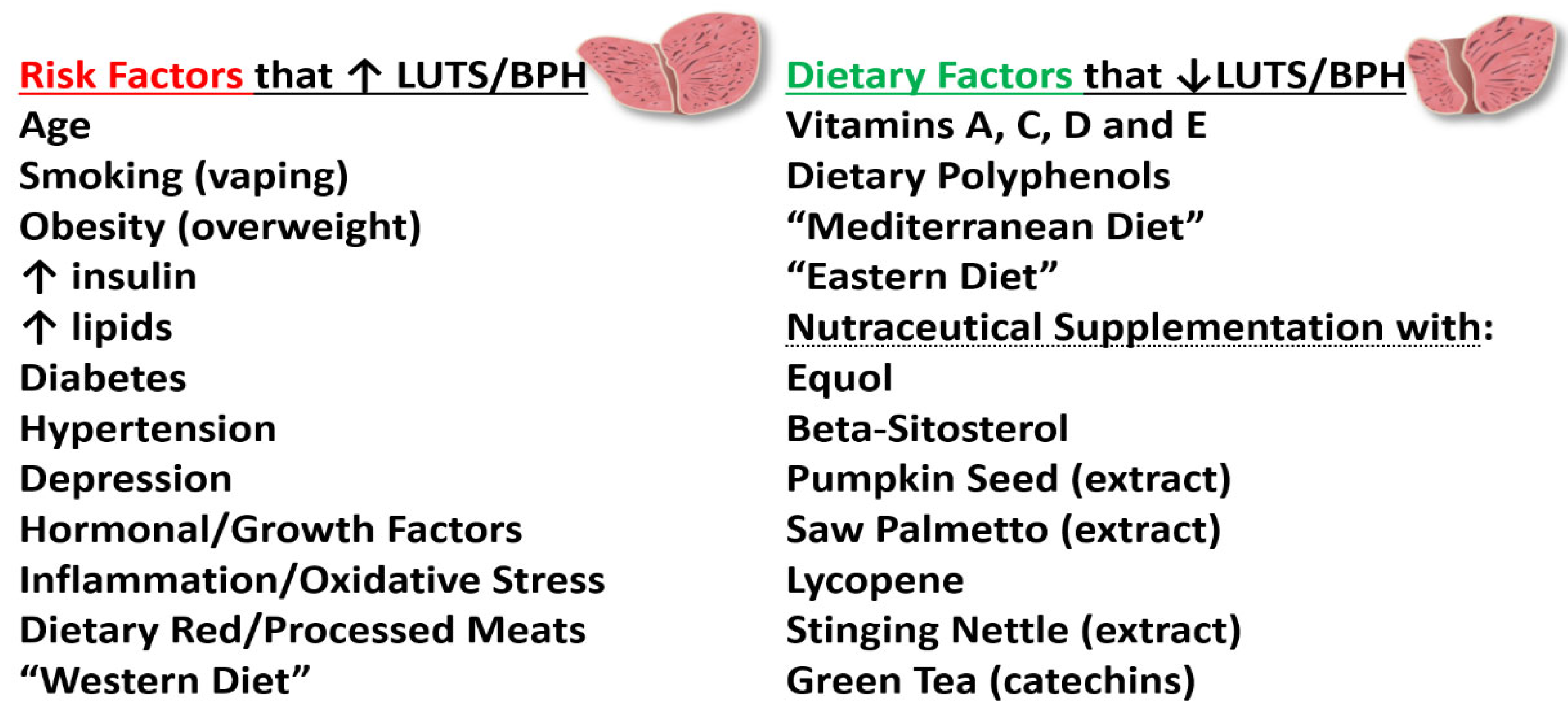
4.1. 4′,7-Isoflavandiol (Equol) and BPH
4′,7-Isoflavandiol (Equol) is classified as a polyphenol and isoflavonoid compound, which is relatively new to nutraceutical applications since it was discovered in the early 1908s in humans [
107,
108,
109] (see “equol hypothesis” below). Isoflavonoids are well-known plant secondary metabolites that have gained research attention due to their multiple nutraceutical and pharmaceutical applications [
103,
105,
107]. In plants, isoflavonoids play an important role in the plant’s defense that can provide a competitive advantage to survive and flourish under environmental challenges such as heat and drought [
108]. In the late 1990s, the ‘equol hypothesis’ was proposed, which implied health benefits in humans with adequate equol levels (for protection against breast and prostate cancer); thereafter, increased research attention on this isoflavonoid compound has flourished [
103,
105,
107,
110,
111].
Equol has a chiral center at carbon 3, and thus, can exist in two mirror image forms or enantiomers (S-equol and R-equol), which (1) are both biologically active and can specifically bind free 5α-DHT, an important aspect for BPH treatment [
13,
107,
109], (2) can be found in plants, food, and animal products [
13,
110], (3) are classified as phytoestrogens that act like selective estrogen receptor modulators (SERMS) that bind ERβ receptors in the prostate to protect against BPH [
13,
110,
111,
112,
113], (4) provide an inhibitory action on the aromatase enzyme that is responsible for estrogen biosynthesis and cellular proliferation [
114,
115] and, (5) can be biosynthesized to high purity for commercial use in human topical and oral applications [
116]. Comparisons of the chemical structures of 17β-Estradiol and Equol are shown in
Figure 6.
At this point, it is important to note that the terms soy, isoflavones and phytoestrogens can be used collectively, since soybeans are a high source of isoflavonoids, and in turn, flavonoids are phytoestrogens. Thus, these terms can be used interchangeably [
116]. Conversely, from a scientific perspective isoflavones should not be equated with estrogen, and soy foods should not be equated with isoflavones [
116,
117], which have been shown to be safe [
117]. Finally, the controversies and misinformation about phytoestrogens have been addressed in a major recent review in detail [
116]. Since everyone is exposed to and consumes phytoestrogen molecules everyday regardless of age, gender, or geographic location around the world, how we understand their effect(s) is a matter of perspective [
116].
Earlier studies showed that persons, who could produce equol in response to consuming soy isoflavones, were classified as “equol producers”, which implied health benefits [
13,
109,
112]. It should be noted that this terminology represents individuals that display equol levels of around 10 to 20 ng/mL or more, which is an arbitrary biomarker [
13], whereas in comparison to most other mammals (except pigs) the levels of equol range from 800 to over 2500 ng/mL [
13]. Miyake et al., 2022, reviewed prior studies that showed that supplementing healthy men (
n = 28) with dietary soy isoflavones resulted in no changes in estrogen or testosterone levels, but an 18 percent decline in serum 5α-DHT levels was observed versus before supplementation [
26], which suggested that in equol producers this may account for lower BPH levels in Asian men [
13,
110,
118,
119].
Additionally, the presence of estrogen-related-receptor gamma (ERR-γ) appears to play an important role in prostate health, where it has been shown to slow proliferation/growth in androgen-sensitive prostate cancer cells [
13]. In fact, equol has affinity for ERR-γ and is known to increase the transcriptional activity of ERR-γ along with binding to ERβ to increase positive estrogen-like influences in the prostate [
13,
120].
In other clinical randomized, double-blind studies (60 and 41 subjects, respectively), examined the safety and pharmacokinetics of equol evaluating dosing from 10 to 320 mg per day in healthy adult volunteers [
121]; however, the results of these studies have not been published in the public domain. Nevertheless, previous clinical investigations on equol’s pharmacokinetics indicated that (1) gastrointestinal (GI) absorption is approximately 80% in humans and (2) the half-life (T ½ of either S- or R-equol) after oral ingestion was 6 to 8 h [
13,
109] where in non-equol producers baseline equol levels ranged between 2 and 4 ng/mL, but increased from 121 to 137 ng/mL after approximately 45 min with a single 6 mg equol dose (
n =18) [
118] (see
Figure 7A,B).
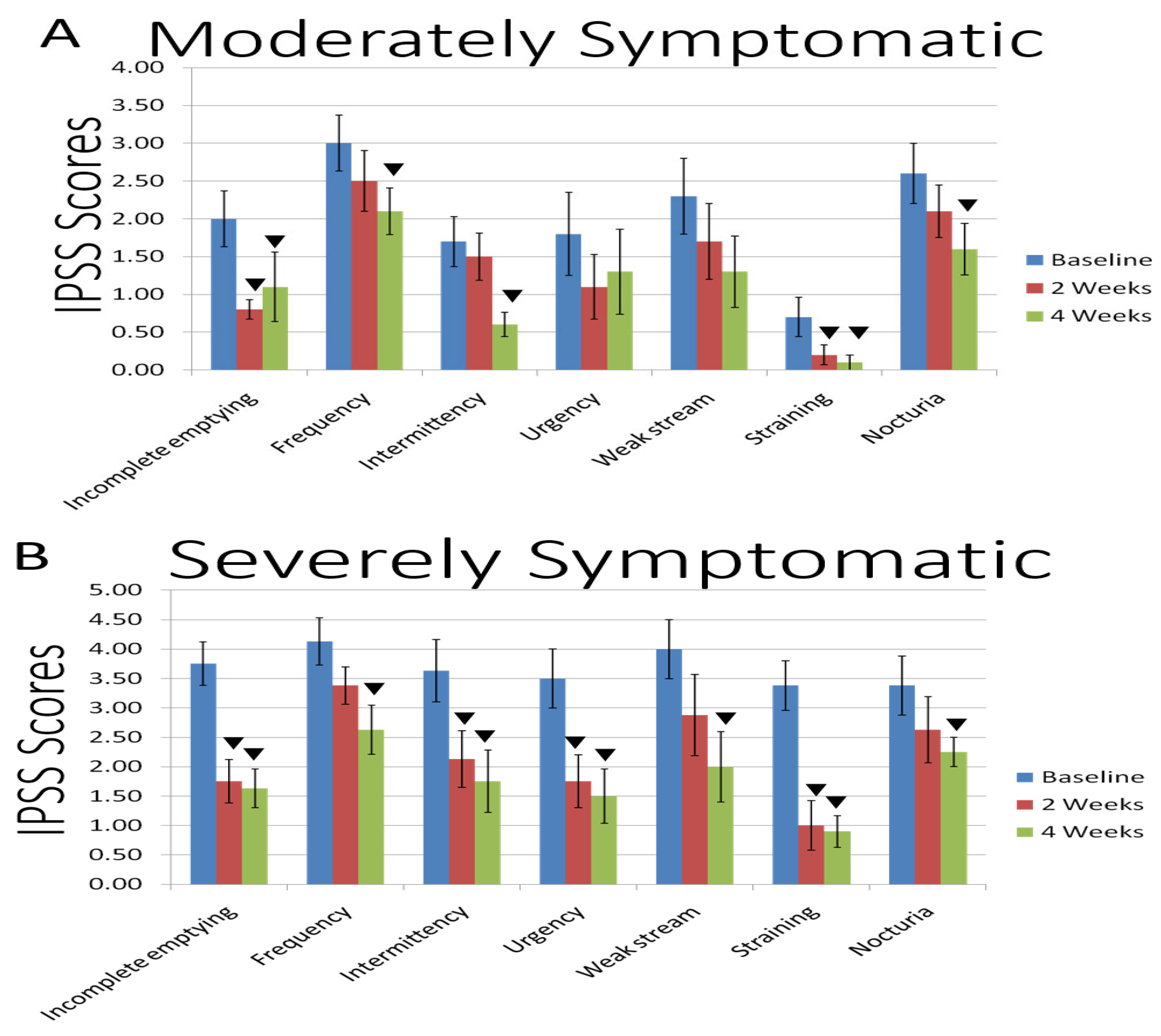
The overall IPSS values from a pilot clinical study that evaluated equol as a potential treatment for BPH symptoms are shown in
Figure 7A,B and
Table 2 (below). Equol at 6 mg was taken by oral capsule supplement twice per day (breakfast and dinner) in men with moderate or severe BPH symptoms [
13]. The mean age was 55.8 years and the body mass index was 27.9 in the moderately symptomatic group (
n = 10) and the mean age was 58.0 years old with a body mass index of 27.2 in the severely symptomatic group (
n = 8) [
13].
Equol as a nutraceutical supplement is currently available as a therapy to address BPH (and women’s menstrual) symptoms as complementary and alternative medicines (CAM), where some companies using a US Federal Drug Administration (FDA) dossier(s) evaluated equol as generally recognized as safe (GRAS) by self-affirmation based on data from oral or topical applications for over 5 years without side effects in more than 20,000 to over 50,000 human subjects, respectively (by application) [
13,
81,
116,
122,
123].
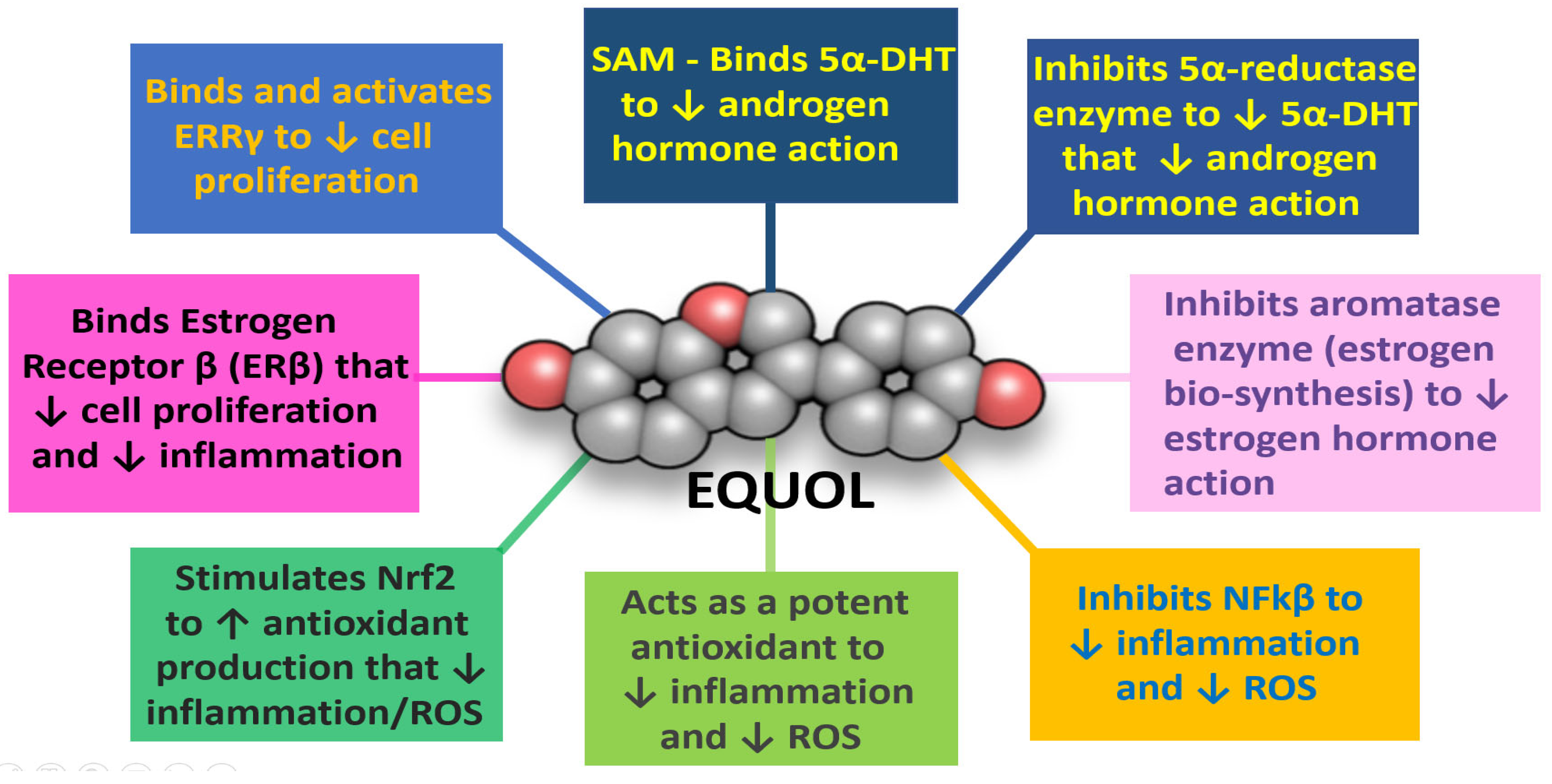
Equol’s mechanisms of action: (1) directly by binding free serum 5α-DHT [
13,
119], (2) as a 5α-reductase enzyme inhibitor [
119], (3) as a moderate aromatase enzyme inhibitor [
114,
115], (4) by inhibiting NFkβ and stimulating nuclear factor-erythroid factor 2-related factor 2 (Nrf2) expression to decrease inflammation and enhance antioxidant/detoxification enzyme expression, respectively, (5) as a SERM to protect the prostate by binding ERβ [
13,
113], (6) by binding estrogen-related-receptor-gamma (ERR-γ) that appears to play an important role in prostate health [
13,
118], and (7) for approximately 6 to 8 h (T ½ half-life) after taken orally [
109] without side effects; then equol may provide beneficial efficacy to treat BPH symptoms that are not present in current pharmaceutical treatments (see
Figure 8).
Finally, the gut microbiota-assisted synthesis, cellular interactions and synergistic aspects of equol as a potent anticancer isoflavone has been reported [
124]. In summary, one recent review covered “equol producers”, which are a subset of certain populations have the ability to convert daidzein into the more potent equol compound under the action of the gut microflora that exerts strong and multifaceted anticancer actions. These include apoptotic, cell cycle arrest mechanisms, antiestrogen and anticancer actions via ER subtypes, antioxidant and anti-inflammatory, and synergism with current anticancer agents such as tamoxifen [
124].
4.2. Beta-Sitosterol (BST) and BPH
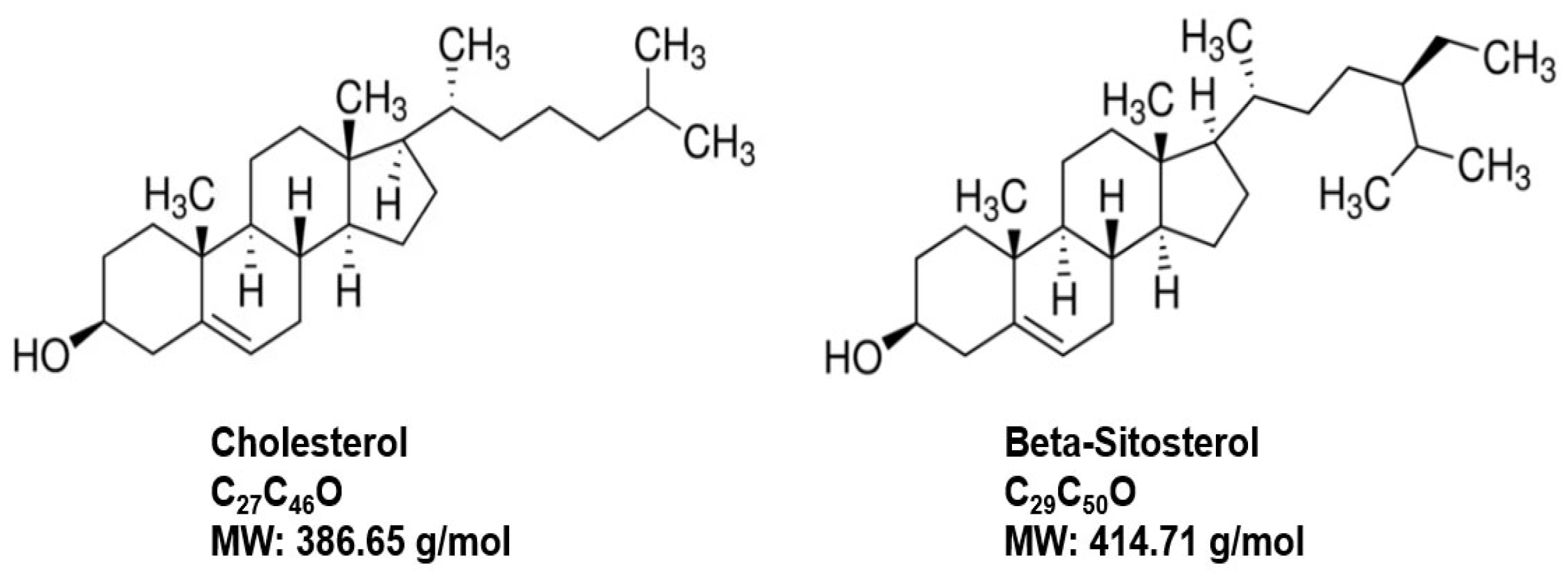
Phytosterol are biologically active compounds found naturally in plant cell membranes, with a chemical composition similar to animal cell membranes that are composed of cholesterol among the lipid-bilayer components [
125,
126,
127] (
Figure 9). Beta-sitosterol (BST), a white powdery organic substance, is one of the most abundant naturally occurring phytosterols in plants and is found in many food products such as cereals, fruits, nuts, vegetables, and vegetable oils, etc. [
125,
126,
127]. BST has GRAS status in the US with applications in medicine, agriculture, and chemical industries [
125,
127]. A long history of research on BST from 1922 until today includes antioxidant, anxiolytic, lipid-lowering, antidiabetic, anti-inflammatory, anticancer and immunomodulatory, respiratory, wound healing and anti-viral protective effects among many other applications, in addition to its use for BPH, which have been reviewed elsewhere [
125,
126,
127,
128].
Early pharmacokinetic studies on BST showed low GI absorption at approximately 5–10%, a half-life (T ½) of around 3 h, and effective oral dosing ranging from 60 to 195 mg per day [
129]. Additionally, in the late 1990s a multicentric, placebo-controlled, double-blind clinical trial (
n = 177 men) reported by Klippel et al. examined BST (at 130 mg per day) for the treatment of BPH over a six-month interval [
130]. IPSS values, peak urinary flow rates (Qmax), and post-void residual urinary volumes (PVR) were recorded pre-, during and post-treatment. The obtained results indicated significant improvements over placebo control levels, increased Qmax (4.5 mL/s) and decreased PVR values (33.5 mL) in the BST-treated men, which suggested that BST was an effective option for the treatment of BPH [
130].
A more recent clinical study by Cosentino et al., 2018, examined the efficacy and safety of a combination dietary supplement containing curcumin, beta-sitosterol, and proanthocyanidins, in 100 men with LUTS caused by BPH [
131]. Dosing was one tablet per day for 3 months, and the measured parameters included IPSS values and urinary flow levels. The study results suggested that the combination dietary supplement therapy was effective for almost all the IPSS parameters qualified with significantly increased urinary flow rates by a majority of the enrolled subjects without adverse side effects [
131].
Sudeep et al., 2019, compared the efficacy of BST enriched saw palmetto (VISPO) versus conventional saw palmetto (SPO) at 200 or 400 mg/Kg body weight administrated orally daily for 28 days in alleviating BPH parameters using a testosterone-induced BPH rat model [
132]. The measurement parameters included serum testosterone levels and prostate tissue analysis (histology plus inflammatory and apoptotic biomarkers via Western blot assays). In general, the obtained data showed experimental evidence that BST-enriched saw palmetto (VISPO) was more efficacious compared to the saw palmetto treatment (SPO) only (versus control animal values), which decreased prostate hyperplasia, testosterone levels, and for alterations in the expression of inflammatory and apoptotic biomarkers [
132].
In a follow-up clinical double-blind, placebo-controlled randomized comparative investigation, the same authors, Sudeep et al., 2020, examined the efficacy and safety of a BST-enriched saw palmetto (VISPO) (3% BST) versus conventional saw palmetto (SPO) (0.2% BST) in adult men (ages 40–65 years of age,
n = 33 per treatment group) with symptomatic BPH for 12 weeks [
133]. Dosing was twice per day of capsules (containing 200 mg of extract—active ingredient), while placebo capsules contained maltodextrin for 12 weeks. The measured parameters included IPSS values, quantified urine flow (Qmax), 5α-reductase enzyme activity, prostate-specific antigen (PSA) analysis, and the Aging Male Symptoms (AMS) scale and Androgen Deficiency in Aging Male (ADAM) questionnaires. The overall results demonstrated that BST-enriched saw palmetto (VISPO) was significantly superior to conventional saw palmetto (SPO) for a majority of the quantified parameters that were evaluated, except that 5α-reductase enzyme activity was not significantly reduced [
133]. This study claims to provide the first clinical evidence of improved efficacy of saw palmetto due to the enriched content of BST to improve BPH symptoms in men via the synergistic effects of the functional active ingredients in this dietary supplement [
133].
Lastly, the molecular mechanisms of BST in preventing cell proliferation via pro-apoptotic, anti-metastatic, anti-invasive, and chemo-sensitizing actions that include factors such as p53 protein, B cell lymphoma-2 (Bcl-2), ROS, cell cycle blockade, interleukins, vascular endothelial growth factor (VEGF), mitogen activated protein kinase (MAPK), and PCNA pathways with applications to prostate disorders, but tumor progression, in general, have been recently reviewed [
134].
4.3. Pumpkin Seed Extract and BPH
Pumpkin (
Cucuribita pepo) is an herbaceous vine, originally native to America, now grown worldwide and has high nutritional and protein value (where lipids comprise 50% and protein 30% of the seeds) [
135,
136]. Pumpkin seeds contain, in addition to protein and fatty acids, vitamins [folates, niacin (B3), vitamins A, C, and especially E], minerals (zinc, phosphorus, manganese, potassium, magnesium, copper and iron) plus phyto-nutrients such as β-carotene, β-crypto-xanthin, lutein, and zeaxanthin) [
135,
136,
137] (
Table 3). Notably, the high zinc levels in pumpkin seeds are thought to ameliorate BPH symptoms by providing optimal levels of this important mineral along with its 5α-reductase enzyme inhibiting actions via the phyto-nutrients such as the phytosterols [
137,
138,
139]. For example, a daily intake of zinc is required, because the body has no way to store it long term [
140]. Recall, zinc is an essential trace mineral involved in many beneficial physiological functions, but pertaining to BPH it protects against oxidative stress and assists with DNA repair [
140].
Pumpkin seeds have several of the same attributes as β-sitosterol (antioxidant, anti-inflammatory, cholesterol/blood pressure/diabetic control, etc.) [
135,
136,
137]. Notably, while pumpkin seeds have been used traditionally for a variety of therapeutic applications one of the most frequent uses, both traditional and modern, continues to be for BPH [
135,
136,
137]. The nutritional supplement doses for pumpkin seeds range from 100 to 1000 mg per day. This section summarizes the most recent findings involving pumpkin seed (extracts in most cases) for addressing BPH symptoms.
In 2021, Kang et al. reported using a rat model that pumpkin seed oil ameliorated BPH parameters by lowering prostate 5α-reductase enzyme expression, which confirmed previous results demonstrating this benefit [
139].
In 2019, Leibbrand et al. used a single-arm, monocenter pilot study of 60 men with symptoms of BPH (IPSS of 14.8), that investigated the effects of a proprietary oil-free hydroethanolic pumpkin seed extract [
141]. Subjects ingested oil-free hydroethanolic pumpkin seed extract (500 mg) once daily (before going to bed) for 3 months. After 12 weeks of supplementation, a significant symptom reduction from baseline (average, 30.1%) was observed based on total IPSS. Symptom alleviation also had a high impact on QOL and was significant after 8 and 12 weeks of intervention. Nocturia significantly decreased over time, as confirmed by IPSS questionnaire and bladder diary. Postvoid residual urine volume was significantly reduced at the end of intervention (from 83.67 mL at baseline to 63.11 mL after 12 weeks). In general, the results indicate that the oil-free hydroethanolic pumpkin seed extract was well tolerated (without adverse effects) and provided significant health benefits for BPH-related symptoms [
141].
In 2021, Zerafatjou et al. investigated the effects of a pumpkin seed (720 mg) oil versus tamsulosin (0.4 mg) for BPH symptom relief in a single-blind randomized clinical trial for 3 months [
142] with a total of 73 men (mean age 63.6 years old; weight, height and BMI were similar), 34 in the tamsulosin and 39 in the pumpkin seed group. There was a significant decrease in IPSS and significant increase in QOL values for both treatment groups, but the changes from baseline to 1 and at 3 months was significantly greater in the tamsulosin versus the pumpkin seed group. None of the pumpkin seed-treated subjects experienced any adverse effects, whereas dizziness (6%), headache (3%) and retrograde ejaculation (3%), and erythema with pruritus were observed in the tamsulosin group. The overall outcome of this study was that pumpkin seed treatment decreased BPH symptoms without adverse effects while tamsulosin appeared to be more effective (than the pumpkin seed treatment), but with side effects [
142], suggesting that men may select natural therapeutic nutritional supplementation over pharmaceutical drug treatments based upon recent surveys [
21,
22].
In 2022, Theil et al. in a 24-month clinical study of 83 men (mean age 65.2 ± 8.7 years) with moderate LUTS/BPH symptoms, showed that pumpkin seed extract (500 mg capsules) taken twice per day significantly improved IPSS levels after 12 and 24 months in over 50% of the subjects [
143]. The proportion of patients with QOL scores at baseline (mostly satisfied) was 11 percent that rose to 62 percent and 73 percent at 12 and 24 months, respectively. No adverse events were reported, and the AMS and International Index of Erectile Function (HEF-5) scores indicated no negative impact on function during the treatment interval [
143]. The authors concluded that pumpkin seed extract provided significant relief in men with moderate LUTS/BPH symptoms without side effects.
Vahlensieck et al., in 2022, in a meta-analysis of two randomized placebo-controlled trials over 12-month evaluated the beneficial effects of pumpkin seed extract on LUTS and quality of life in men with BPH [
144]. Specifically, the safety and efficacy of a well-tolerated proprietary pumpkin seed soft extract (PSE) was investigated in the Bach [
145] and GRANU studies [
146]. Both trials examined LUTS/BPH patients with IPSS > 13 at baseline. The original Bach study demonstrated positive effects of PSE compared to placebo, but no difference between treatments was observed in the GRANU study. In the combined studies, 687 subjects were in the PSE group, while 702 patients were in the placebo-control group [
144,
145,
146]. For the variables study, baseline IPSS, and center size had a relevant influence on treatment response. Vahlensieck et al. concluded that although the Bach and the GRANU study showed contradictory results, the analysis in a pooled analysis pointed towards an advantage of PSE; namely, more patients in the PSE group showed an IPSS improvement of at least 5 points after 12 months [
144,
146]. Therefore, the results of this meta-analysis suggest that patients with moderate LUTS/BPH may benefit from PSE treatment in terms of symptomatic relief.
Finally, in 2020, Nemer et al. examined a combination treatment of pumpkin seed, isoflavonoids, and cranberry mix in LUTS/BPH subjects in a multicenter open-label one-arm pilot study [
147]. Male patients were recruited, who still had LUTS/BPH symptoms despite current use of alpha-1 blockers, which included 128 men (61.8 + 9.9 years of age). The treatment mix tablet contained 550 mg of pumpkin seed extract, 50 mg of soy isoflavones and 50 mg of cranberry taken twice per day for 90 days, and IPSS, QOL, and HEF-5 were scored at baseline, 30, and 90 days. IPSS values significantly decreased from baseline from a value of fifteen compared to 30 days of a score of eleven to 90 days where the IPSS score was nine. QOL values significantly increased from baseline, while HEF-5 levels also improved [
147]. The results suggested that the combination treatment of pumpkin seed extract, soy isoflavones and cranberry relieved LUTS/BPH symptoms in men and had a mild beneficial effect on erectile function. Remarkably, the obtained results were in men who were on alpha-1 blockers that did not previously alleviate their LUTS/BPH symptoms.
4.4. Saw Palmetto and BPH
Saw palmetto has a long historical record starting in 1575 [
148]. Native Americans (in the Florida region) were known to use the fruit of saw palmetto (
Serenoa repens) from the American dwarf palm tree (3 to 9 feet tall) to treat urinary and reproductive disorders [
148]. In April 1877, Read and Abraham A. Solomons, manufacturing pharmacists in Savannah, introduced saw palmetto to the medical profession at the second annual meeting of the Georgia Pharmaceutical Association held in Atlanta, Georgia [
148]. Interest in saw palmetto products increased in the early 1990s, as more scientific evidence of their safety and efficacy was published, particularly in Germany, where federal regulations at the time required a high level of scientific evidence for phytomedicinal products, which already had been on the market for decades. Since 2000, consumer interest in saw palmetto products has continued to grow in the United States, and saw palmetto was the 13th top-selling herbal dietary supplement ingredient in natural retail outlets in 2020 [
148,
149]. Since then, saw palmetto extract (SPE) has been commonly used to treat BPH. It has been used in Europe for decades, while increased use in the US over the past 30 years came about because of side effects from pharmaceuticals that treat BPH and the numerous scientific reports which provided evidence for saw palmetto’s safety and efficacy in treating LUTS and BPH-related symptoms [
148,
149,
150,
151,
152].
There are three main types of saw palmetto extracts: hexanic, supercritical CO
2 and ethanolic, with each having different phytochemical compositions [
151,
152]. However, the one most tested for efficacy and safety has been the hexanic
S. repens extract (HSE) [
149,
150,
151]. Saw palmetto in US supplement products (liquid) contain more than 90 percent fatty acids (laurate, myristic, palmitate, oleate, and linoleate acids), and low levels of phytosterols (campesterol, stigmasterol and β-sitosterol [
149,
150,
151,
152,
153] (
Table 4 and
Table 5).
Notably, the HSE was first approved in Europe for BPH in 1981 [
154]. The recommended dosage at 320 mg once daily or 160 mg twice daily, administered orally [
149,
150,
151,
152], where long-term use has been reported as well tolerated. However, in some long-term studies the low frequency adverse events reported included headache and abdominal pain, but without the adverse sexual effects observed with pharmaceutical alpha-1 blockers or 5RIs [
154,
155].
The mechanisms of action of HSE include (1) anti-androgen by non-selective inhibition of both type I and type II isoenzymes of 5α-reductase versus finasteride, which selectively inhibits only the type II 5α-reductase enzyme [
149], (2) pro-apoptotic by inhibition of the growth of the prostate via blockade of insulin-like growth factor-1, plus the increased B-cell lymphoma-associated X (Bax)/Bcl2 ratio observed in prostate tissue sample from subjects (via oral dosing of HSE at 320 mg daily for 3 months compared to untreated control values) [
149,
156], and (3) anti-inflammatory actions by significantly reduced expression of inflammation-regulated genes including IL-6, CCL-5, CCL-2, COX-2, and iNos plus the downregulation of other pro-inflammatory factors [
149,
150,
151,
152]. Finally, it has been suggested that HSE may relax lower urinary tract smooth muscle by the inhibition of alpha-1 adrenergic and muscarinic receptors [
149].
Active ingredients in HSE include the following: (1) fatty acids (lauric and myristic acid) have been shown to inhibit both 5α-reductase type I and type II enzymes, while olieic acid and linolenic acid inhibit the type I enzyme, plus several fatty acids showed inhibition of alpha-1 adrenergic receptors that may relax smooth muscle contraction in the prostate [
149,
152,
156] and (2) phytosterols, which are relatively low in HSE but may contribute to reduced prostatic inflammation [
149,
150,
151,
152].
Over the past 40 years, there have been many reports of the chemistry, pharmacology, and clinical studies on saw palmetto, HSE and other aspects of this phytochemical product which have been reported in several reviews and meta-analyses [
103,
148,
151,
153,
154]. However, in brief, the reports from the past 5 years are summarized herein.
Vela-Navarrete et al. in 2018 examined the efficacy and safety of HSE (Permixon
®, Castres, France) for the treatment of LUTS/BPH by conducting a systematic review and meta-analysis of randomized controlled trials and observational investigations that covered 27 studies with a total of 447 patients [
150]. The authors concluded that HSE (Permixon
®) (at 320 mg per day or 160 mg taken twice per day over weeks to months) significantly reduced nocturia and improved Qmax compared to placebo control values and had similar efficacy to tamsulosin and (short-term use of) 5α-reductase inhibitors in relieving LUTS/BPH. The HSE (Permixon
®) appeared to be an efficacious and well-tolerated therapeutic option for the long-term medical treatment of LUTS/BPH [
150].
Kwon in 2019, and Csikos et al. in 2021, reviewed the use of HSE for BPH, and reported the benefits of the fatty acid and phytosterol components including the mechanisms of actions such as the anti-androgenic, pro-apoptotic and anti-inflammatory effects from several studies [
103,
150]. All the authors cited randomized clinical trials about the efficacy of HSE. However, Kwon pointed out that the variability of therapeutic efficacy of the HSE (formulas) may be due, in part, to the lack of standardization of HSE (formulations) [
149]. On this point, the work of Penugonda and Lindshield (2013) showed variability of saw palmetto (active ingredients) in seven different nutraceutical supplements sold in the U.S. [
153].
In 2022, Blair reviewed HSE (Permixon
®) for the symptomatic relief of BPH symptoms by examining 21 different studies (representing 361 patients) that, in some cases, specifically compared Permixon
® to alpha-1 blockers or 5α-reductase inhibitors [
152]. The rationale for this review was to address men wishing to avoid the side effects of alpha-1 blockers and/or 5α-reductase inhibitors, where HSE Permixon
® treatment avoided these adverse events especially those related to sexual function. The analysis indicated that the efficacy of HSE was similar to that of an alpha-1 blocker in terms of improving voiding and storage symptoms, increasing urinary flow, and reducing prostate inflammation and volume [
152]. HSE Permixon
® was also as effective as 5α-reductase inhibitors and/or alpha-1 blockers at improving LUTS/BPH parameters and QOL. In general, HSE was well tolerated and offered a therapeutic option for the treatment of BPH [
152].
In 2022, De Nunzio et al. reported on the combination therapy of alpha-1 blockers with HSE versus monotherapy in the treatment of LUTS/BPH from five different clinical studies (mean age ranged from 55.3 to 68.3, representing 1292 subjects with HSE dosing at 320 mg/day and alpha-1 blocker(s) dosing 0.2 to 0.4 mg/day from 6 to 13.5 months) [
157]. The authors concluded that HSE in combination with alpha-1 blockers provided greater symptom relief and fewer adverse events (sexual dysfunction) in patients with LUTS/BPH than with alpha-1 blockers alone. The implications were that combination therapy of HSE and alpha-1 blockers for moderate LUTS/BPH subjects might be an effective strategy for preserving their sexual function, especially having higher BMI and/or metabolic syndrome disorders [
157].
Finally, Nickel et al. in 2022, in a major review/evaluation of the available scientific evidence (safety and efficacy) of HSE for LUTS/BPH treatment using national and international urological guidelines by an international panel of urology experts generated consensus statements, which supported HSE use [
158]. Specifically, the general overall conclusion was that HSE should be considered as a treatment option for men with mild-to-moderate LUTS/BPH symptoms as an alternative to watchful waiting [
158].
4.5. Lycopene and BPH
A natural antioxidant, lycopene contained in plants has gained importance in preventing oxidation of fats/oils and foods, also as a food additive, along with its many human health benefits [
159,
160]. Lycopene is a lipophilic carotenoid hydrocarbon pigment found in red, pink, and orange fruit and vegetables such as tomatoes, apricots, melons, papayas, grapes, peaches, watermelons, and cranberries [
159,
160]. However, tomatoes are the most studied due to lycopene’s abundance in this fruit [
159,
160]. In 1876, Millardet, first discovered this compound (in tomatoes), and it was later named lycopene by Schunck in 1903 [
160]. Lycopene is the most abundant carotenoid found in human serum and has been recognized as the most effective antioxidant among all the carotenoids [
160]. Lycopene has 11 conjugated double bonds in its structure (
Figure 10). The extended conjugated double bond system of these compounds is an important feature in the carotenoids responsible for their attractive colors because it forms a light absorbing chromophore [
159,
160].
Lycopene’s mechanism(s) of actions include (1) antioxidant characteristics by serving as a singlet oxygen and peroxyl radical scavenger, plus the combinations of lycopene with other antioxidants such as vitamin C and E express high scavenging activity levels to combat free radicals, and hence, oxidative stress, and also protect against DNA damage in both normal or cancerous human cells and lymphocytes [
159,
160,
161]; (2) anti-inflammatory properties that result from its lipophilic nature, which has a close association with the cell membrane and enables it to regulate the inflammatory mediator signaling pathways and activate the expression of antioxidant genes (such as preventing the production of different types of cytokines (such as IL1, IL6, IL8, and TNF-α), chemokines, nitric oxide (NO), and cyclooxygenase that modulate the immune system response; it also inhibits the Nfkβ signaling pathway [
159,
160,
161], and (3) anti-proliferation properties, where lycopene alters IFG-1 to decrease cellular proliferation. However, its stability is a critical factor for its functional aspects. Physical and chemical factors such as elevated temperature, exposure to oxygen and light, metallic ions (e.g., Cu
2+ and Fe
3+), extremes in pH, and active surfaces affect its stability [
160].
From recent clinical studies on lycopene, Li et al. in 2019 examined the efficacy and safety of lycopene in 127 patients with moderate to severe LUTS/BPH by IPSS classifications [
162]. Men were treated with oral lycopene (500 mg twice per day) for 16 weeks. At 8 and 16 weeks, they compared IPSS and QOL scores, prostate volume, PSA levels, Qmax, PVR, and incidence of adverse effects before and after treatment. The results showed that all the quantified parameters were significantly altered for the improvement of LUTS/BPH symptoms, except there was no significant difference in prostate volume before versus after treatment, and no adverse effects were reported by any of the patients [
162].
Cicero et al. in 2019, and Kutwin et al. in 2022 published comprehensive reviews on lycopene as a dietary supplement for the treatment of LUTS/BPH [
119,
163]. In brief, oral lycopene at doses ranging from 10 mg to 500 mg, usually taken twice per day from 2 weeks to up to 6 months, in general, significantly improved LUTS/BPH symptoms (covering over 1000 subjects within an age range of 45–80 years) without adverse effects. The patients were classified as having moderate or severe LUTS/BPH symptoms according to IPSS values before lycopene treatment was initiated [
119,
163]. Furthermore, other comprehensive reviews on the health benefits of lycopene have been reported elsewhere [
159,
160,
161].
In 2022, Carrasco et al. conducted a pilot study of 20 men (10 healthy versus 10 with moderate BPH symptoms via IPSS), mean age was 50 years old, both groups consumed lycopene (at 8 mg twice per day) for 30 days [
164]. The measured outcomes included sleep quality, PSA, C reactive protein (serum), and total antioxidant levels (in urine). The obtained results showed improvement in IPSS values, PSA, and total antioxidant levels (in urine) in both the treatment groups, but especially in men with moderate BPH symptoms sleep quality significantly increased, and nocturia significantly decreased [
164].
Combination phytochemical therapy was investigated by Morgia et al. in 2018 in a phase IV clinical study that compared HSE (320 mg) + selenium (50 mcg) + lycopene (5 mg) versus the phosphodiesterase-5 inhibitor (tadalafil at 5 mg) taken orally once per day for 6 months for the treatment of LUTS in 404 men (50 to 80 years of age) in a multi-center randomized controlled study [
165]. The parameter outcomes included IPSS, Qmax and PVR, where both treatments significantly improved IPSS values (including the QOL scores), but were not significantly different from each other. Qmax values were significantly improved in the combination therapy and the phosphodiesterase-5 inhibitor treatment. For adverse events, four out of 101 patients (or 1 percent) in the combination therapy and 10 out of 120 subjects (or 8%) in the tadalafil treatment reported side effects. The authors concluded that the combination therapy of HSE + selenium + lycopene was equivalent to tadalafil treatment for improvements in the IPSS and Qmax parameters [
165].
In 2021, Cormio et al. conducted a phase II prospective, randomized, double-blinded, placebo-controlled study aimed at determining the efficacy and safety of the novel whole tomato-based food supplement in reducing LUTS of patients with histologically validated BPH (via PBX) [
166,
167]. The food supplement combination therapy was studied in 40 men (mean age 65 years old) that contained carotenoids (500 mg), lycopene (190 mg) and flavonoids (200 mg) as the main active ingredients in a 5 g sachet taken once per day for 2 months. Compared to placebo control values, there was a trend in the combination therapy treatment men for reduction in PSA levels, while there were significant improvements in IPSS and QOL scores at the end of the study [
166]. The authors concluded that the tomato-based food supplement represented an efficacious option for the treatment of symptomatic BPH compared to pharmaceutical treatments, since this food supplement was free of side effects and highly accepted among the patients tested [
166].
Finally, the anti-inflammatory and healing properties of oral lycopene along with dutasteride treatment on reducing transurethral resection of the prostate (TURP) bleeding were examined by Nugroho et al. in 2019 [
168]. Twenty-two patients diagnosed with BPH were randomly assigned to two groups (
n = 11 each). Thirty days prior to TURP, men in the control group were given daily oral dutasteride 0.5 mg and a placebo pill and subjects in the treatment group were given dutasteride 0.5 mg and lycopene 30 mg daily. For this study, the parameters measured were pre- and post-TURP plasma erythrocyte count and micro-vessel density (MVD) of resected prostate tissue stained for histology evaluation. The mean MVD in the control group was significantly higher versus the intervention group (28.2 ± 12.3 vs. 18.3 ± 7.6 vessel/mm
2) and reduction of post-TURP plasma erythrocytes was significantly higher in the control group compared to the intervention group (−0.34 ± 0.18 vs. −0.17 ± 0.12). The results suggested that daily consumption of dutasteride and lycopene for at least 30 days reduced the formation of blood vessels in the prostate and reduced blood loss in post-TURP examinations [
168].
4.6. Stinging Nettle and BPH
Nettle (
Urtica dioica) is a perennial herb native to Europe, Asia, North Africa, and North America that grows to a height of 3 to 7 feet [
169,
170]. It is often referred to as “Stinging Nettle” because it has “trichomes”, or hollow hairs, on its leaves and stem, which act like needles that inject histamine, formic acid, and other chemicals that produce a stinging sensation [
169,
170]. Nettle, unlike many other plants, tends to produce only male or female flowers throughout each plant, thus giving it the name dioica, meaning “two houses” [
169,
170,
171]. Nettle use has been recorded as far back as the Bronze Age (3000 BCE—1200 BCE), and there are records (59–45 BCE) where Julius Caesar’s troops rubbed it on their limbs to help them stay awake and alert during the night in battle [
169,
170,
171]. In addition to its use in herbal therapies, nettle also has been used as a textile (similar to flax) in the 1500–1600s in Scotland and during WWI and WWII as a substitute for cotton. There are many human health applications of stinging nettle (food, cosmetic, etc., which have been reviewed elsewhere [
169,
170,
171,
172,
173,
174], including its use in enhancing farmed fish immunity to be more resistant against bacterial infections [
173,
174]. However, the most recognized human health supplement application of stinging nettle (root) is for the treatment of BPH [
119,
175,
176,
177,
178,
179].
Stinging nettle can be extracted from fresh herbal plants by industrial aqueous, alcoholic-organic and/or hydroalcoholic methods, and distillation processes, where good yields can be 0.74 L of hydrolate from 1 Kg of starting material [
172,
179]. The nutritional composition and various compounds in stinging nettle have been identified (
Table 6, below), including protein, dietary fiber, flavonoids, phenolic acids, carotenoids, organic acids, and fatty acids [
173,
175].
Stinging Nettle’s mechanism(s) of actions include (1) antioxidant characteristics by serving as a superoxide scavenging agent, and/or stimulating the increase in catalase (CAT), superoxide dismutase (SOD), and glutathione along with protecting against lipid peroxidation, and DNA damage [
172,
173,
174], (2) anti-inflammatory properties by inhibiting COX-1 and COX-2, NFkβ signaling pathway, IL-1 and IL-2, interferon (INF), and TNF-α2 [
172,
173,
174], (3) anti-proliferation properties, where stinging nettle inhibits not only the binding of androgens to their transporter proteins [i.e., sex hormone binding globulin (SHBG)], but also their binding to prostate membrane receptors that contribute to the proliferative effects on prostate tissues along with inhibiting the aromatase enzyme (for estrogen biosynthesis), thus altering prostate growth [
172,
173,
174] and, (4) anti-bacterial/viral activities by inhibiting Gram-negative and Gram-positive bacterium and viruses (respiratory syncytial and cytomegalovirus) by its component compounds of alkaloids, flavonoids, phenols, and saponins [
173,
174,
180,
181,
182].
One of the largest randomized, double-blind, placebo-controlled clinical studies to examine the effects of stinging nettle was reported in 2005, where 558 patients (55 to 72 years of age) were administrated 120 mg of
U. dioica root extract for 6 months [
183]. The obtained results showed that 81 percent of the men displayed significant improvement in LUTS/BPH symptoms, Qmax, and QOL scores [
183]. Additionally, PVR levels were significantly decreased with a modest decrease in prostate size, but no alterations in PSA or testosterone levels were observed with stinging nettle oral supplementation [
183]. Since 2005, other clinical studies have been performed that are summarized below (including combination therapies), and general reports/reviews are available elsewhere [
119,
169,
170,
171,
172,
173,
174,
175,
176,
177,
182].
In 2020, Karami et al. reported a randomized controlled trial investigating the efficacy of stinging nettle root extract in 30 LUTS/BPH patients (administered 450 mg tablet extract per day for 12 weeks) and in 30 men that served as controls (placebo group) [
184]. The measured parameters were IPSS, C-reactive protein levels, malondialdehyde (MDA), and SOD activities. The results revealed immediate effects (within days) in improving IPSS values, a small effect on C-reactive protein, intermediate effects on MDA and SOD activities, without any adverse effects after 3 months of stinging nettle on the oral supplementation treatment [
184].
Ibishev et al. in 2019 reported a comparative study investigating HSE alone (at 320 mg taken once per day) versus HSE (at 160 mg) in combination with stinging nettle (at 120 mg) taken twice per day for 3 months in 51 men per treatment group (age ranged from 48 to 64 years) to determine of efficacy of each treatment [
185]. The results showed a significant improvement in LUTS/BPH parameters via IPSS values, significant increase in Qmax with a significant decrease in PVR in both treatment groups. However, when group comparisons were made the combination treatment displayed better results compared to HSE therapy alone for addressing LUTS/BPH symptoms in men [
185].
Kirscher-Hermans et al., in 2019, reviewed four randomized, placebo-controlled clinical trials that examined the efficacy of a promising herbal preparation (called WS PRO 160/120 available in Germany) for the treatment of patients with LUTS/BPH related symptoms [
186]. This herbal supplement contains 160 mg of HSE plus 120 mg of stinging nettle (root) extracts, and the four reviewed studies represented 980 patients (mean age ranged from 65 to 68 years) that were administered one capsule twice per day for 24 to 60 weeks. In all the trials, significant symptomatic improvement was recorded via IPSS, QOL and Qmax values while PVR was significantly reduced compared to placebo parameters and comparable with the 5α-reductase inhibitor (finasteride) or the alpha-1 blocker, tamsulosin. The adverse events were few with the herbal combination treatment compared to the tolerability and safety profiles of the reference drugs (5RI or alpha-1 blocker), which were included in two out of the four studies. The authors concluded that the combination HSE and stinging nettle extract treatment was a valid alternative in the treatment of patients with moderate BPH, especially with the view to sexual function and good quality of life especially for long-term use [
186].
Finally, an in vitro study by Saponaro et al. in 2020 evaluated the antioxidant and anti-inflammatory activity of a combined formulation of HSE and stinging nettle extract using a human model of BPH (i.e., BPH-1 cells) [
187]. The results confirmed both the antioxidant and anti-inflammatory effects of the combination treatment by a reduction in ROS, IL-6 and IL-8 production and a reduction in NFkβ translocation inside the nucleus using the human androgen-independent prostate model, PC3 cells. The authors concluded that the combination treatment of HSE and stinging nettle extract supported the hypothesis that ROS reduction is directly implicated in the decrease of the NFkβ pathway to cause reduced expression of inflammatory cytokines. Consequently, the reduced inflammation contributed to the mitigation of prostate enlargement and BPH symptoms [
187].
4.7. Green Tea
Green tea has an interesting history. It originated in China around 3000 BCE, where a later written account described the discovery that occurred accidently when the Chinese Emperor Shennong mistakenly drank water with a dead tea leaf boiled inside [
188,
189]. He found the flavor refreshing; thus, a new beverage was born. It was not available to the Chinese general public until the 14th century for liquid refreshment and medicinal purposes. Then, it spread to many different neighboring regions, and in the 19th century it traveled West with European explorers, where today its Great Britain’s national beverage (along with black tea) [
188,
189]. Today, green tea is one of the most popular drinks in the world and the least oxidized compared to other teas [
190]. In the last 30 years, the popularity of green tea has greatly increased and has been extensively investigated for its many health benefit including its protective role against various types of human cancers [
10,
12,
119,
190,
191,
192,
193]; however, this narrative overview is focused on BPH.
Green tea comes from the
Camellia sinenis plant that is an evergreen shrub or small tree native to East Asia and Southeast Asia, where fresh leaves contain caffeine (around 4 percent), are a rich source of polyphenols and catechins along with theanine, vitamins (B2, C, E, and folic acid), saponins, GABA and minerals (potassium, calcium, phosphorus, etc.) [
190,
194]. The polyphenols in green tea are credited with beneficial properties against several diseases/disorders via biological and molecular mechanisms [
190,
195].
The distinctive polyphenolic compounds present in green tea are called catechins, which make up 80–90% of the flavonoids and about 40% of the water- soluble solids [
190]. The four major catechins are (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECG), and (-)-epigallocatechin gallate (EGCG) [
190,
195]. The distribution of the catechins varies; however, EGCG accounts for around 60%, followed by EGC at 20%, ECG at 14% and EC at 6% [
190,
195] (
Figure 11). EGCG is the major catechin in green tea that accounts for most of the research conducted to date on various disorders and human cancers [
192,
193,
195]. For example, one cup of green tea contains up to 200 mg of EGCG, although some reports suggest three to five cups of green tea per day provides a minimum of catechins per day [
196]. The bioavailability of EGCG has been determined in adult health volunteers (men
n = 5, women
n = 5; mean age 30 years old) after a single dose of green tea extract supplement (approximately 200 mg) by pharmacokinetic studies where the Cmax (maximum concentration) was approximately 6 mg/mL/Kg in men and women, while the T ½ (half-life) was highest in men at 154 min vs. 117 min in women [
196]. This recent report on the bioavailability of green tea catechins confirms previous pharmacokinetic studies in humans [
195,
197].
Notably, green tea has been extensively studied; however, the controversies regarding its benefits and risks still exist, but the numerous health-promoting benefits outweigh its few reported risk effects [
197,
198]. Nevertheless, this is a matter of perspective, since herbal dietary supplements may present potential hepatotoxicity, if consumption exceeds the recommended dosage [
198]. Conversely, all clinical studies have reported that green tea in liquid or dietary supplements (at various doses) is well tolerated without side effects [
10,
119,
190,
192,
193,
194,
195,
196,
197].
There are several mechanisms of action for the health benefits of green tea, since extensive investigations from in vitro, animal, and human results are available [
10,
11,
12,
103,
119], but preclinical and clinical evidence of green tea has focused on the catechin, EGCG for almost all reported research reports [
195,
196,
197,
198,
199]. Thus, EGCG will be the major coverage for the mechanisms of action involving green tea [
103,
105,
106,
119,
190,
191,
195,
196,
197,
198,
199].
First, EGCG has been shown to induce apoptosis, arrest the cell cycle and inhibit cell proliferation (DNA replication) [
103,
105,
119,
199]. Flow cytometric analysis found a linear dose relationship between EGCG and the promotion of apoptosis via DNA fragmentation in LNCap and DU245 cell lines [
105,
111,
199]. Furthermore, ECGC altered the transcription factors p53 and NFβ that lead to a change in the ration of Bax/Bcl-2 in a manner that favored apoptosis [
10,
111,
199]. Additional studies showed that EGCG induced G0/G1 phase cell cycle arrest in both androgen-sensitive and androgen-insensitive cell lines in a dose-dependent manner [
12,
111,
199]. EGCG also inhibits the expression of the highly conserved mini-chromosome maintenance (MCM) gene and protein (specifically MCM7) that are essential for genome replication, which is associated with the progression of cell proliferation and growth [
195,
199].
Additionally, EGCG is involved in regulating MAP kinases (P38/JNK/ERK), PI3K-Akt, and PKC to reduce cellular proliferation and growth [
10,
12,
111,
199]. For example, EGCG reduced the expression of ERK1 and 2 (MAP kinases) by 50–60% in vitro observed in DU-145 cells as well as other MAP kinases (p38, JNK) [
10,
12,
111,
199]. Additionally, green tea polyphenols decreased expression of PI3K (by 70%) and Akt (by 65%) in LNCap and DU-145 cell cultures [
190,
199]. Taken together, the results indicated that the green tea component compound, EGCG, induced apoptosis and arrested the cell cycle to decrease cell proliferation in prostate cells [
111,
195,
199] (
Figure 12).
Second, EGCG acts as an anti-inflammatory agent that targets the NFkβ and COX-2 inflammatory pathways, where EGCG was shown to decrease the DNA binding activity of NFkβ and reduced the expression of the p65 subunit of NFkβ in LNCap cells [
190,
191,
195]. EGCG inhibited COX-2 as well as iNOS expression in vitro, which suggested an anti-inflammatory action in addition to inhibiting the NFkβ pathway [
10,
12,
111]. Using a rat model, Zhou et al. in 2018 reported the ameliorative effects of EGCG against testosterone-induced benign prostatic hyperplasia and fibrosis [
200]. The authors reported that the EGCG treatment improved prostatic hyperplasia and collagen deposition by attenuated prostatic oxidative stress and inflammation via reduced expression of AR, ERα, HIFα, TGF-β1, TGF-βR1 and p-Smad3, while enhancing the expression of ERβ in prostate tissue [
200] (
Figure 12).
Third, EGCG inhibited insulin-like growth factor and insulin-like growth factor binding proteins (IGFBPs) levels by 70 to 80% in prostate tissues of in vivo animal studies (TRAMP mice) [
105,
190,
199]. A combination of green tea polyphenols (at 0.1% or 0.6%) in the drinking water of the mice displayed significant reductions in the insulin-like growth factor parameters compared to control values [
199] (
Figure 12).
Fourth, green tea and/or EGCG reduced the expression of androgen receptor (AR), prostate-specific antigen (PSA) levels and inhibited the 5α-reductase enzyme (responsible for 5α-DHT biosynthesis) in in vitro (cell culture) studies and in vivo using athymic nude mice [
10,
12,
105,
199]. In brief, in LNCap cells ECGC and/or green tea extract in concentrations of 10–20 µM significantly decreased AR gene and protein expression; in observations from in vivo data with nude mice, the administration of 0.1% green tea extract in the drinking water resulted in reduced prostate volume, and serum PSA levels [
199]. However, in a systematic review and meta-analysis of randomized controlled studies, Sharifi-Zahabi et al., in 2021, reported that green tea had no significant effect on PSA levels [
201]. However, the authors noted that this lack of support may be due to the heterogeneity among the studies analyzed, where larger sample sizes are required to make a definitive determination on the relationship between green tea’s effect on PSA levels [
201]. In a cell-free assay, ECG and EGCG inhibited the 5α-reductase type I enzyme with an IC50 of 12 µM and 15 µM, respectively, which suggested decreased production of the potent androgen, 5α-DHT, in ameliorating BPH symptoms [
199]. Lastly, Mitsunari et al. in 2021 reviewed in detail the changes in hormone-related molecules (androgens, estrogens, 5α-reductase) and eight different growth factors by green tea polyphenols [
105] (
Figure 12).
Fifth, EGCG acts as an antioxidant, stimulates Nrf2 (to increase antioxidant and detoxifying enzymes), decreases oxidative stress and enhances fertility [
105,
202,
203,
204,
205]. Prior studies by Na and Surh, in 2008, showed that EGCG stimulated the master antioxidant and detoxifying signal Nrf2 that in turn induced expression of glutathione S-transferase, glutathione peroxidase, glutamate cysteine ligase, hemeoxygenase-1 (HO1), etc., to decrease oxidative stress [
202]. Yao et al., in 2019, examined the effects of dietary EGCG on oxidative stress and the metabolism/toxicity of acetaminophen in the liver, where rats were fed diets with (0.5%) of EGCG or without EGCG supplementation for four weeks and were then injected intraperitoneally with acetaminophen (1 g/kg) [
203]. The results showed that EGCG lowered hepatic oxidative stress and cytochrome P450 (CYP) 1A2, 2E1, and 3A, and UDP-glucurosyltransferase activities prior to acetaminophen injection. After acetaminophen challenge, the elevations in plasma alanine aminotransferase activity and histological changes in the liver were ameliorated by EGCG treatment. Thus, EGCG reduced cytochrome P450 (CYP)-mediated acetaminophen bioactivation, oxidative stress, and apoptosis, as well as increased autophagy and lower accumulation of toxic products in the liver [
203]. Notably, in 2021, Mitsunari et al. reviewed in detail the changes in antioxidants, oxidative stress, and inflammatory-related molecules by green tea polyphenols [
105]. Zhang et al., 2020, reported in a review the physiological activities of EGCG in improving fertility in humans and mammals due to its detoxifying and antioxidant effects, especially to reduce ROS [
204]. In 2022, Beyaz et al. reviewed the antioxidant properties of EGCG that acts as a scavenger of free radicals, has redox characteristics, prevents the formation of reactive oxygen species, and has been shown to reduce cellular damage caused by oxidative stress [
206] (
Figure 12).
Sixth, the anti-infective and immune properties of EGCG have been studied for several years [
207,
208,
209]. For example, Steinmann et al., in 2012, reviewed the actions of EGCG that bind to lipid membranes, which altered folic acid metabolism of bacteria and fungi by inhibiting the cytoplasmic enzyme dihydrofolate reductase [
207]. In 2018, Reygaert reviewed the antimicrobial properties of the catechins that have been shown to be effective against several viruses, parasites, fungi, and even prions, but also noted that the anti-inflammatory and antioxidant effects of catechins may support/contribute to this mechanism of action [
208]. Finally, Sun et al. in 2022 reported a comprehensive review on the effects of green tea and its components on the immune function, where the authors indicated that in immune-related diseases tea polyphenols are the most significant compounds that modify immune functions (of macrophages, natural killer cells, mast cells, basophils, and eosinophils, neutrophils, dendritic, T and B cells) for potential therapeutic applications in humans [
209] (
Figure 12).
Since green tea and its catechins have been extensively studied, almost all recent clinical investigations have examined prostate cancer rather than BPH [
192,
210,
211]. However, presented herein are a few available clinical studies on green tea (catechins) covering BPH that have been published or are currently being conducted.
In 2014, Katz et al. examined the safety and efficacy of a green and black tea blend in 40 men (mean age 56 years old) with moderate to severe LUTS/BPH in a randomized, double-blind, placebo-controlled clinical trial [
212]. In three treatment groups, men took capsules once per day for a total of (a) 500 mg tea blend (
n = 15), (b) 1000 mg tea blend (
n = 13) or (c) placebo-control (
n = 12) for 12 weeks and the parameter outcomes included American Urologic Association symptom scores (AUAss), uroflowmetry (Qmean), PVR, C-reactive protein levels, a health survey (HS), and International Index of Erectile Function (IIEF) at 6 and 12 weeks of treatment. The results showed that oral administration of the green/black tea blend significantly improved LUTS/BPH symptoms (via the AUAss, Qmean urine flow and PVR values in the treatment groups versus placebo-controls) and the quality of life and physical function/sexual desire improved by the HS and IIEF scores in as little as 6 weeks, where the tea blend treatment was well tolerated without adverse effects [
212].
In 2021, Mitsunari et al. reviewed the potential clinical usefulness of polyphenols (including green tea), where the authors concluded that no well-designed clinical trials with large populations have been conducted to determine the clinical use and safety in humans [
105]. However, in 2020 a randomized double-blinded Phase II clinical trial began to evaluate the bioavailability, safety, effectiveness and validate the mechanism by which a standardized formulation of whole Green Tea Catechin, (Sunphenon
® 90D, Minneapolis, MN, USA) containing 405 mgs vs. placebo, administered for 24 months in a cohort of 135 men to manage prostate health progression (via active surveillance), which will conclude in 2024 [
213].
While green tea and its catechins molecules have received much research attention over the past 20 years, further clinical studies are warranted to determine its safety and efficacy in treating LUTS/BPH in men.
Finally, other important aspects of green (and black) tea are the benefits of polyphenols on mediating gut microbiota where there is a two-way relationship that involves the metabolism and mechanism(s) of action to improve human health [
214,
215]. For example, ellagic acid is a polyphenolic compound present in green tea and other natural sources including pomegranate, strawberries, blackberries, raspberries, and walnuts, etc. [
216]. Furthermore, upon ingestion of green tea or EGCG, microbial gut enzymes convert EGCG into gallic acid and epigallocatechin (EGC) [
216,
217]. Gallic acid can be subsequently degraded into urolithins and pyrogallol by decarboxylation (from gallic acid and ellagitannins) that were discovered almost 20 years ago in animal models and in humans [
216,
217]. In fact, urolithins (A and B) have been shown to accumulate in human breast, colon, and prostate tissue [
216]. Previous studies have shown that urolithins disrupt the regulatory mechanisms in the G1 phase of the cell cycle upregulating apoptosis, control proliferation of epithelial cells, and decrease serum concentrations of prostate-specific antigen (PSA) as well as androgen receptor (AR) levels within prostate cells [
216,
217]. Moreover, the gut microbial metabolite pyrogallol is a more potent inducer of Nrf2-associated gene expression compared to its parent green tea compound EGCG [
218], which suggests strong antioxidant and detoxifying effects. Recently, secondary polyphenol metabolites such as urolithins have been reported as anticancer compounds that can mediate cell cycle arrest, inhibit estrogen biosynthesis via the aromatase enzyme, induce apoptosis, promote autophagy, reduce oxidative stress, enhance antioxidant activities, and regulate growth factors [
219].