Antimicrobial Resistance and Genomic Characterization of Salmonella Infantis from Different Sources
Abstract
:1. Introduction
2. Results
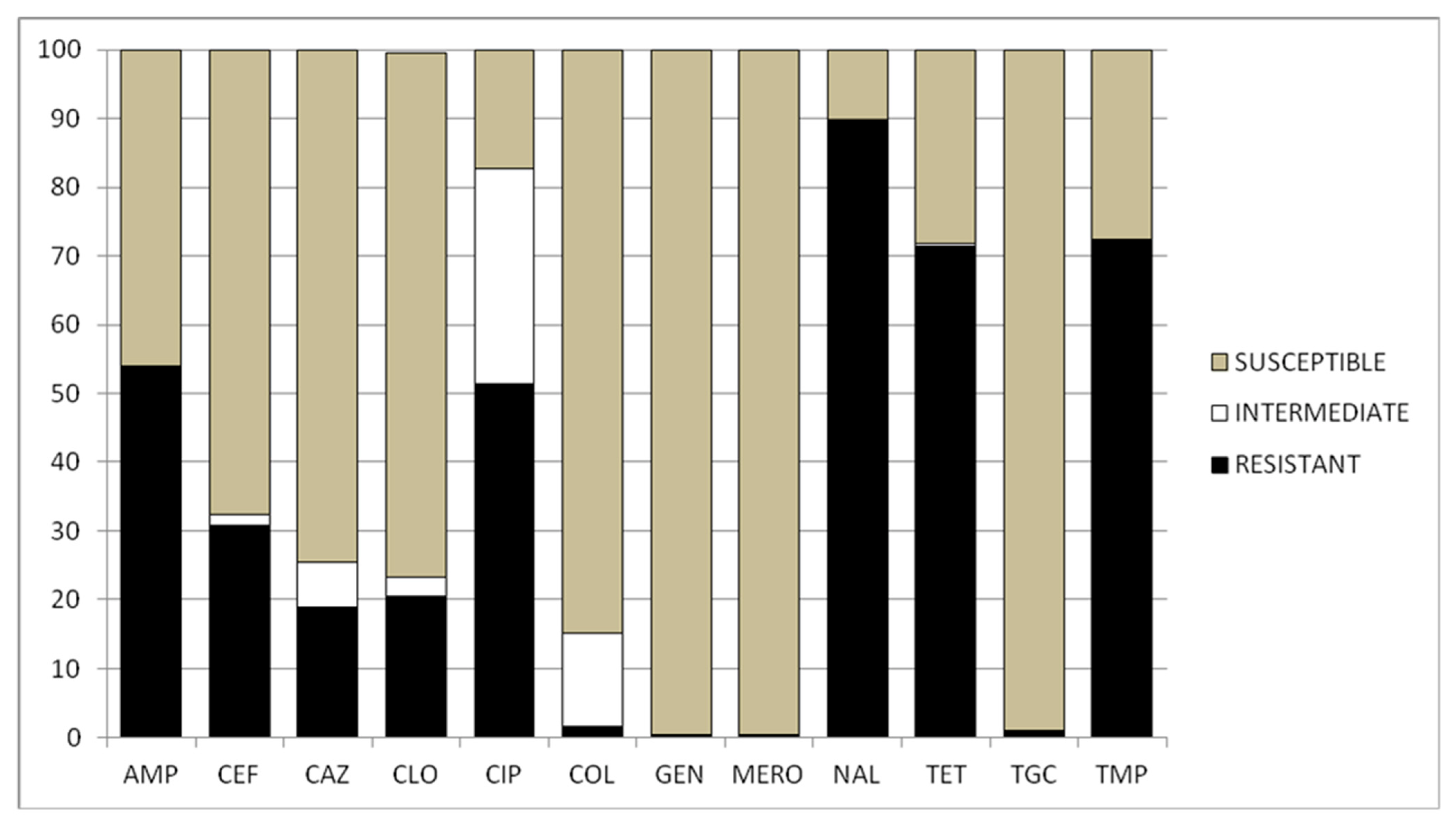
2.1. Minimal Inhibitory Concentration
2.2. MLVA
3. Discussion
4. Materials and Methods
4.1. Detection and Serotyping
4.2. Minimal Inibitory Concentration
4.3. Molecular Assays
4.3.1. DNA Extraction
4.3.2. MLVA Assay
4.4. Clustering Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar] [CrossRef]
- Leati, M.; Busani, L.; Zaccherini, A.; Ruocco, L.; Amato, S.D.; Villa, L.; Barco, L.; Ricci, A.; Cibin, V. The Challenging Task to Select Salmonella Target Serovars in Poultry: The Italian Point of View. Epidemiol. Infect. 2021, 149, e160. [Google Scholar] [CrossRef] [PubMed]
- Rakitin, A.L.; Yushina, Y.K.; Zaiko, E.V.; Bataeva, D.S.; Kuznetsova, O.A.; Semenova, A.A.; Ermolaeva, S.A.; Beletskiy, A.V.; Kolganova, T.V.; Mardanov, A.V.; et al. Evaluation of Antibiotic Resistance of Salmonella Serotypes and Whole-Genome Sequencing of Multiresistant Strains Isolated from Food Products in Russia. Antibiotics 2022, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Juniora, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-Analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [Green Version]
- Drauch, V.; Kornschober, C.; Palmieri, N.; Hess, M.; Hess, C. Infection Dynamics of Salmonella Infantis Strains Displaying Different Genetic Backgrounds–with or without PESI-like Plasmid–Vary Considerably. Emerg. Microbes Infect. 2021, 10, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Perilli, M.; Scattolini, S.; Telera, G.C.; Cornacchia, A.; Tucci, P.; Sacchini, F.; Sericola, M.; Romantini, R.; Marotta, F.; Di Provvido, A.; et al. Distribution of Salmonella spp. Serotypes Isolated from Poultry in Abruzzo and Molise Regions (Italy) during a 6-Year Period. Microorganisms 2022, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Peruzy, M.F.; Proroga, Y.T.R.; Capuano, F.; Mancusi, A.; Montone, A.M.I.; Cristiano, D.; Balestrieri, A.; Murru, N. Occurrence and Distribution of Salmonella Serovars in Carcasses and Foods in Southern Italy: Eleven-Year Monitoring (2011–2021). Front. Microbiol. 2022, 13, 1005035. [Google Scholar] [CrossRef] [PubMed]
- Bogomazova, A.N.; Gordeeva, V.D.; Krylova, E.V.; Soltynskaya, I.V.; Davydova, E.E.; Ivanova, O.E.; Komarov, A.A. Mega-Plasmid Found Worldwide Confers Multiple Antimicrobial Resistance in Salmonella Infantis of Broiler Origin in Russia. Int. J. Food Microbiol. 2020, 319, 108497. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.L.; Heuzenroeder, M.W. A Comparison of Three Molecular Typing Methods for the Discrimination of Salmonella Enterica Serovar Infantis. FEMS Immunol. Med. Microbiol. 2008, 53, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA (European Food Safety Authority). Scientific report on the European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef] [Green Version]
- Sodagari, H.R.; Wang, P.; Robertson, I.; Habib, I.; Sahibzada, S. Non-Typhoidal Salmonella at the Human-Food-of-Animal-Origin Interface in Australia. Animals 2020, 10, 1192. [Google Scholar] [CrossRef]
- Peruzy, M.F.; Capuano, F.; Proroga, Y.T.R.; Cristiano, D.; Carullo, M.R.; Murru, N. Antimicrobial Susceptibility Testing for Salmonella Serovars Isolated from Food Samples: Five-Year Monitoring (2015–2019). Antibiotics 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, X.; Fernández, J.; Rodríguez-Lozano, J.; Calvo, J.; Rodicio, R.; Rodicio, M.R. Genomic Analysis of Two MDR Isolates of Salmonella Enterica Serovar Infantis from a Spanish Hospital Bearing the BlaCTX-M-65 Gene with or without FosA3 in PESI-like Plasmids. Antibiotics 2022, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Di Marcantonio, L.; Romantini, R.; Marotta, F.; Chiaverini, A.; Zilli, K.; Abass, A.; Di Giannatale, E.; Garofolo, G.; Janowicz, A. The Current Landscape of Antibiotic Resistance of Salmonella Infantis in Italy: The Expansion of Extended-Spectrum Beta-Lactamase Producers on a Local Scale. Front. Microbiol. 2022, 13, 812481. [Google Scholar] [CrossRef] [PubMed]
- EU’s JIACRA Reports 2016–18. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/analysis-antimicrobial-consumption-resistance-jiacra-reports (accessed on 23 November 2022).
- Neoh, H.M.; Tan, X.E.; Sapri, H.F.; Tan, T.L. Pulsed-Field Gel Electrophoresis (PFGE): A Review of the “Gold Standard” for Bacteria Typing and Current Alternatives. Infect. Genet. Evol. 2019, 74, 103935. [Google Scholar] [CrossRef]
- Savas, S.; Çetinkaya, S. Molecular Typing of Different Salmonella Serotypes by Multiple-Locus Analysis of Tandem Repeats. J. Oleo Sci. 2020, 69, 1585–1589. [Google Scholar] [CrossRef]
- Nagy, T.; Szmolka, A.; Wilk, T.; Kiss, J.; Szabó, M.; Pászti, J.; Nagy, B.; Olasz, F. Comparative Genome Analysis of Hungarian and Global Strains of Salmonella Infantis. Front. Microbiol. 2020, 11, 539. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Prevalence and Characterisation of Salmonella in Slaughtered Cattle and Beef in Poland. Bull. Vet. Inst. Pulawy 2013, 57, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Cetin, E.; Temelli, S.; Eyigor, A. Salmonella Prevalence and Serovar Distribution in Healthy Slaughter Sheep and Cattle Determined by ISO 6579 and VIDAS UP Salmonella Methods. J. Food Sci. Technol. 2019, 56, 5317–5325. [Google Scholar] [CrossRef]
- Hussain, M.A.; Wang, W.; Sun, C.; Gu, L.; Liu, Z.; Yu, T.; Ahmad, Y.; Jiang, Z.; Hou, J. Molecular Characterization of Pathogenic Salmonella spp. from Raw Beef in Karachi, Pakistan. Antibiotics 2020, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Mudadu, A.G.; Spanu, C.; Pantoja, J.C.F.; Dos Santos, M.C.; De Oliveira, C.D.; Salza, S.; Piras, G.; Uda, M.T.; Virgilio, S.; Giagnoni, L.; et al. Association between Escherichia coli and Salmonella Spp. Food Safety Criteria in Live Bivalve Molluscs from Wholesale and Retail Markets. Food Control. 2022, 137, 108942. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); ECDC (European Centre for Disease Prevention and Control). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, 7209. [Google Scholar] [CrossRef]
- WHO Report. 2019. Available online: www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 12 December 2022).
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The Application of Antibiotics in Broiler Production and the Resulting Antibiotic Resistance in Escherichia coli: A Global Overview. Poult. Sci. 2019, 98, 1791–1804. [Google Scholar] [CrossRef]
- Crabb, H.K.; Allen, J.L.; Devlin, J.M.; Firestone, S.M.; Wilks, C.R.; Gilkerson, J.R. Salmonella spp. Transmission in a Vertically Integrated Poultry Operation: Clustering and Diversity Analysis Using Phenotyping (Serotyping, Phage Typing) and Genotyping (MLVA). PLoS ONE 2018, 13, e0201031. [Google Scholar] [CrossRef] [Green Version]
- Guibourdenche, M.; Roggentin, P.; Mikoleit, M.; Fields, P.I.; Bockemühl, J.; Grimont, P.A.D.; Weill, F.X. Supplement 2003–2007 (No. 47) to the White-Kauffmann-Le Minor Scheme. Res. Microbiol. 2010, 161, 26–29. [Google Scholar] [CrossRef]
- Proroga, Y.T.R.; Mancusi, A.; Peruzy, M.F.; Carullo, M.R.; Montone, A.M.I.; Fulgione, A.; Capuano, F. Characterization of Salmonella Typhimurium and Its Monophasic Variant 1,4, [5],12:I:- Isolated from Different Sources. Folia Microbiol. 2019, 64, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Kjeldsen, M.K.; Torpdahl, M.; Pedersen, K.; Nielsen, E.M. J of Applied Microbiology-2015-Kjeldsen-Development and Comparison of a Generic Multiple-locus Variable-number Tandem. J. Appl. Microbiol. 2015, 119, 1707–1717. [Google Scholar] [CrossRef]
- Lindstedt, B.A.; Vardund, T.; Aas, L.; Kapperud, G. Multiple-Locus Variable-Number Tandem-Repeats Analysis of Salmonella Enterica Subsp. Enterica Serovar Typhimurium Using PCR Multiplexing and Multicolor Capillary Electrophoresis. J. Microbiol. Methods 2004, 59, 163–172. [Google Scholar] [CrossRef]
- Bergamini, F.; Iori, A.; Massi, P.; Pongolini, S. Multilocus Variable-Number of Tandem-Repeats Analysis of Salmonella Enterica Serotype Gallinarum and Comparison with Pulsed-Field Gel Electrophoresis Genotyping. Vet. Microbiol. 2011, 149, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Tien, Y.Y.; Wang, Y.W.; Tung, S.K.; Liang, S.Y.; Chiou, C.S. Comparison of Multilocus Variable-Number Tandem Repeat Analysis and Pulsed-Field Gel Electrophoresis in Molecular Subtyping of Salmonella enterica Serovars Paratyphi A. Diagn. Microbiol. Infect. Dis. 2011, 69, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P.R. Reproducibility and Indices of Discriminatory Power of Microbial Typing Methods. J. Clin. Microbiol. 1990, 28, 1903–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaluga, J.; Stragier, P.; Van Vaerenbergh, J.; Maes, M.; De Vos, P. Multilocus Variable-Number-Tandem-Repeats Analysis (MLVA) Distinguishes a Clonal Complex of Clavibacter Michiganensis Subsp. Michiganensis Strains Isolated from Recent Outbreaks of Bacterial Wilt and Canker in Belgium. BMC Microbiol. 2013, 13, 126. [Google Scholar] [CrossRef] [Green Version]


| Origin | N° | Source | N° | Sampling Site | |
|---|---|---|---|---|---|
| Mammals | cow/calves | 2 | meat and meat products | 2 | retail market |
| water buffalo | 2 | fecal swabs | 2 | farms | |
| pig | 3 | sponge | 3 | slaughterhouse | |
| wild boars | 2 | tissue/sponge | 2 | hygiene controls after hunting | |
| human | 7 | fecal swabs | 5 | hospital | |
| urine | 1 | ||||
| blood | 1 | ||||
| poultry | 162 | poultry meat | 26 | retail market (butcher’s shop) | |
| surface swabs and sponges | 14 | farms (broiler) | |||
| surface swabs and sponges | 8 | farms (laying hens) | |||
| poultry products | 78 | retail market (large-scale distribution) | |||
| eggs | 19 | retail market (large-scale distribution) | |||
| neck skin samples | 17 | slaughterhouse | |||
| mussels | 7 | mussels | 5 | farms | |
| mussels | 2 | retail market | |||
| Total | 185 | 185 | |||
| AMP | CEF | CAZ | CLO | CIP | COL | GEN | MERO | NAL | TET | TGC | TMP | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | N. | % | |
| Poultry | R | 95 | 58.64 | 54 | 33.33 | 34 | 20.99 | 74 | 45.68 | 91 | 56.17 | 3 | 1.85 | 1 | 0.62 | 1 | 0.62 | 157 | 96.91 | 124 | 76.54 | 2 | 1.23 | 126 | 77.78 |
| I | 0 | 0 | 3 | 1.85 | 118 | 72.84 | 5 | 3.09 | 53 | 32.72 | 23 | 14.20 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.62 | 0 | 0 | 0 | 0 | |
| S | 67 | 41.36 | 105 | 64.81 | 10 | 6.17 | 83 | 51.23 | 18 | 11.11 | 136 | 83.95 | 161 | 99.38 | 161 | 99.38 | 5 | 3.09 | 37 | 22.84 | 160 | 98.77 | 36 | 22.22 | |
| Mussels | R | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 29 |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S | 7 | 100 | 7 | 100 | 7 | 100 | 6 | 86 | 7 | 100 | 5 | 71 | 7 | 100 | 7 | 100 | 7 | 100 | 7 | 100 | 7 | 100 | 5 | 71 | |
| Humans | R | 5 | 71.43 | 3 | 42.86 | 1 | 14.29 | 0 | 0 | 4 | 57.14 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 71.43 | 5 | 71.43 | 0 | 0.00 | 4 | 57.14 |
| I | 0 | 0 | 0 | 0 | 2 | 28.57 | 0 | 0 | 1 | 14.29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S | 2 | 28.57 | 4 | 57.14 | 4 | 57.14 | 7 | 100.00 | 2 | 28.57 | 7 | 100.00 | 7 | 100.00 | 7 | 100.00 | 2 | 28.57 | 2 | 28.57 | 7 | 100.00 | 3 | 42.86 | |
| Other mammals | R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 44.44 | 3 | 33.33 | 0 | 0 | 2 | 22.22 |
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 44.44 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S | 9 | 100 | 9 | 100 | 9 | 100 | 9 | 100 | 5 | 55.56 | 9 | 100 | 9 | 100 | 9 | 100 | 5 | 55.56 | 6 | 66.67 | 9 | 100 | 7 | 77.78 | |
| VNTR | ADP | Shannon Diversity Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Locus (n = 3) | Sty19 | |||||||||
| Locus Size (bp) | 56 | 66 | 69 | 0.24 | 0.48 | |||||
| Number of strains | 160 | 9 | 16 | |||||||
| Locus ID | ST1 | ST2 | ST3 | |||||||
| Locus (n = 6) | SG2 | |||||||||
| Locus Size (bp) | 154 | 286 | 299 | 310 | 66 | 77 | 0.55 | 1.16 | ||
| Number of strains | 120 | 12 | 26 | 6 | 14 | 7 | ||||
| Locus ID | CG1 | CG2 | CG3 | CG4 | CG5 | CG6 | ||||
| Locus (n = 5) | STTR3 | |||||||||
| Locus Size (bp) | 232 | 264 | 331 | 490 | 495 | 0.25 | 0.59 | |||
| Number of strains | 8 | 7 | 4 | 7 | 159 | |||||
| Locus ID | SRA1 | SRA2 | SRA3 | SRA4 | SRA5 | |||||
| Locus (n = 4) | STTR5 | |||||||||
| Locus Size (bp) | 286 | 297 | 303 | 309 | 0.53 | 1.01 | ||||
| Number of strains | 32 | 120 | 20 | 13 | ||||||
| Locus ID | SRB1 | SRB2 | SRB3 | SRB4 | ||||||
| Locus (n = 1) | STTR9 | |||||||||
| Locus Size (bp) | 154 | 0.0 | 0.0 | |||||||
| Number of strains | ||||||||||
| Locus ID | SRC1 | |||||||||
| Cluster | Province | Source | AMR Profile | MLVA | Date | Sampling Site |
|---|---|---|---|---|---|---|
| 1 | AV | wild boar | susceptible | 56;154;286;66;495 | 19 February 2020 | hunting house |
| AV | wild boar | susceptible | 56;154;286;66;495 | 7 April 2020 | hunting house | |
| 2 | SA | cow | TRIM;TET;NAL;CIP | 56;154;297;66;495 | 6 June 2018 | supermarket |
| SA | cow | TRIM;TET;NAL;CIP | 56;154;297;66;495 | 6 June 2018 | supermarket | |
| BN | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 24 November 2020 | supermarket | |
| NA | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 15 January 2020 | supermarket | |
| BN | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 8 June 2021 | supermarket | |
| BN | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 8 June 2021 | supermarket | |
| BN | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 8 June 2021 | supermarket | |
| NA | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 14 July 2020 | slaughterhouse | |
| NA | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 15 July 2020 | supermarket | |
| NA | poultry | TRIM;NAL;CIP;CLO;CAZ;FOT;AMP | 56;154;297;66;495 | 11 October 2021 | supermarket | |
| 4 | SA | w buffalo | susceptible | 69;154;297;153;232 | 25 March 2021 | farm |
| SA | w buffalo | susceptible | 56;154;309;310;495 | 15 March 2019 | farm | |
| 6 | NA | poultry | TRIM;TET;NAL;CIP;AMP | 56;154;292;66;493 | 14 January 2019 | supermarket |
| AV | human | TRIM;TET;NAL;CIP;AMP | 66;154;297;153;495 | 4 November 2019 | hospital | |
| SA | poultry | TET;NAL;CIP | 69;154;309;66;495 | 29 July 2020 | supermarket | |
| AV | human | TET;NAL;CIP | 66;154;297;153;495 | 20 September 2020 | hospital |
| Antibiotics | MIC Breakpoints (µg/mL) | ||
|---|---|---|---|
| Susceptible (S) | Intermediate (I) | Resistant (R) | |
| Ampicillin (AMP) | <8 | 8 | >8 |
| Cefotaxime (CEF) | <0.5 | 0.5 | >0.5 |
| Ceftazidime (CAZ) | <2 | 2 | >2 |
| Chloramphenicol (CLO) | <16 | 16 | >16 |
| Ciprofloxacin (CIP) | <0.06 | 0.06 | >0.06 |
| Colistin (COL) | <2 | 2 | >2 |
| Gentamicin (GEN) | <2 | 2 | >2 |
| Meropenem (MERO) | <0.12 | 0.12 | >0.12 |
| Nalidixic Acid (NAL) | <16 | 16 | >16 |
| Tetracycline (TET) | <8 | 8 | >8 |
| Tigecycline (TGC) | <1 | 1 | >1 |
| Trimethoprim (TMP) | <2 | 2 | >2 |
| VNTR | Size (bp) | Primers | References |
|---|---|---|---|
| STTR3 | 27/33 | F- PET-CCCCCTAAGCCCGATAATGG | [32] |
| R- TGACGCCGTTGCTGAAGGTAAT | |||
| STTR5 | 6 | F- VIC-ATGGCGAGGCGAGCAGCAG | [32] |
| R- GGTCAGGCCGAATAGCAGGA | |||
| STTR9 | 9 | F- 6FAM-AGAGGCGCTGCGATTGACGA | [32] |
| R- CATTTTCCACAGCGGCAGTTTTT | |||
| SG2 | 8 | F- NED-GTGATGATCATGGCGGACT | [33] |
| R- CAGGTGGAACAGGAACTTC | |||
| Sty19 | 9 | F- 6FAM-CATCGTATTGTCAGGGTGGA | [34] |
| R- TTCCCTGCGAGGAAAAGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montone, A.M.I.; Cutarelli, A.; Peruzy, M.F.; La Tela, I.; Brunetti, R.; Pirofalo, M.G.; Folliero, V.; Balestrieri, A.; Murru, N.; Capuano, F. Antimicrobial Resistance and Genomic Characterization of Salmonella Infantis from Different Sources. Int. J. Mol. Sci. 2023, 24, 5492. https://doi.org/10.3390/ijms24065492
Montone AMI, Cutarelli A, Peruzy MF, La Tela I, Brunetti R, Pirofalo MG, Folliero V, Balestrieri A, Murru N, Capuano F. Antimicrobial Resistance and Genomic Characterization of Salmonella Infantis from Different Sources. International Journal of Molecular Sciences. 2023; 24(6):5492. https://doi.org/10.3390/ijms24065492
Chicago/Turabian StyleMontone, Angela Michela Immacolata, Anna Cutarelli, Maria Francesca Peruzy, Immacolata La Tela, Roberta Brunetti, Maria Gerarda Pirofalo, Veronica Folliero, Anna Balestrieri, Nicoletta Murru, and Federico Capuano. 2023. "Antimicrobial Resistance and Genomic Characterization of Salmonella Infantis from Different Sources" International Journal of Molecular Sciences 24, no. 6: 5492. https://doi.org/10.3390/ijms24065492
APA StyleMontone, A. M. I., Cutarelli, A., Peruzy, M. F., La Tela, I., Brunetti, R., Pirofalo, M. G., Folliero, V., Balestrieri, A., Murru, N., & Capuano, F. (2023). Antimicrobial Resistance and Genomic Characterization of Salmonella Infantis from Different Sources. International Journal of Molecular Sciences, 24(6), 5492. https://doi.org/10.3390/ijms24065492








