Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Food Supplements
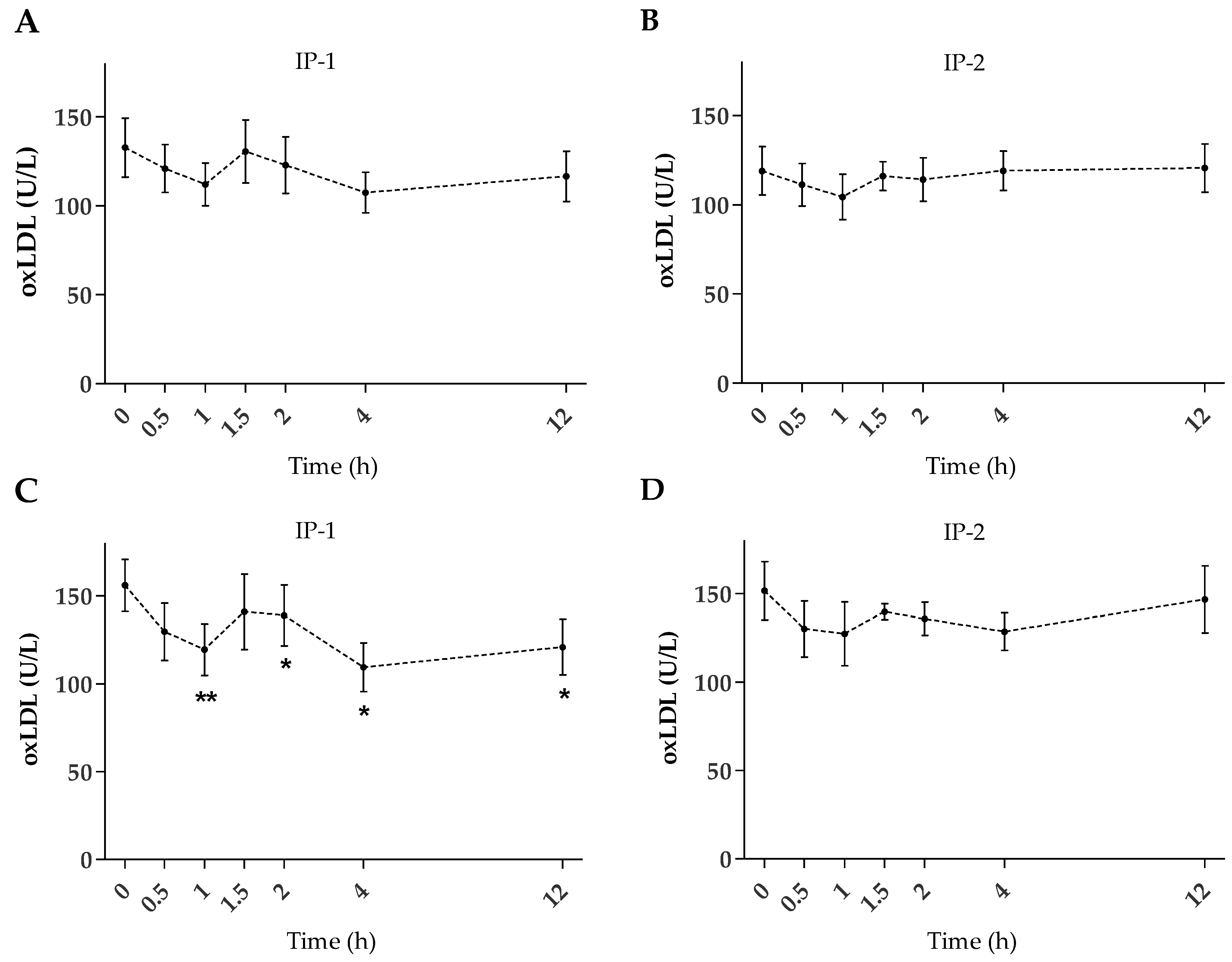
2.2. Plasma oxLDL
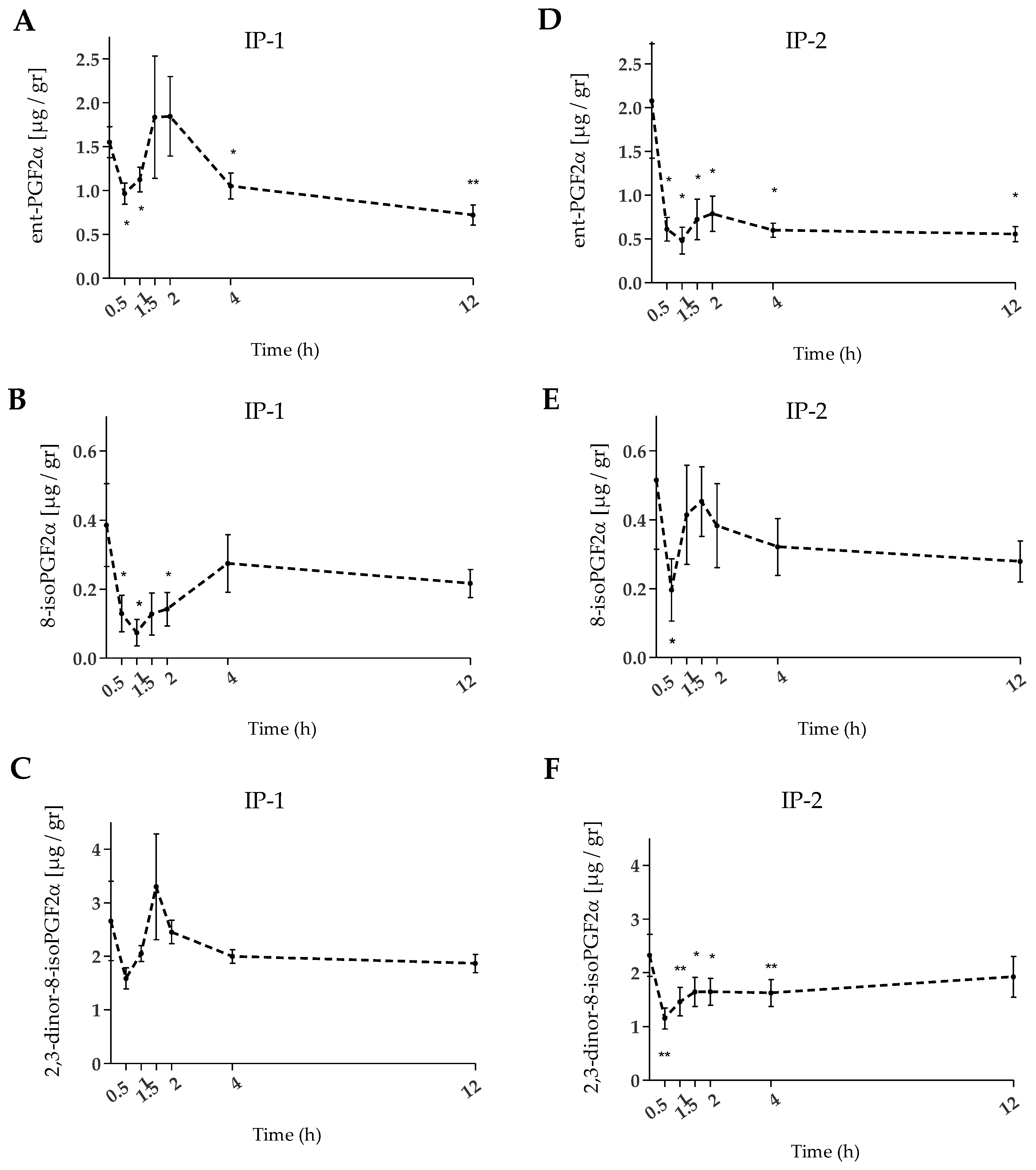
2.3. Urinary F2-IsoPs
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. Investigational Products (IPs)
4.3. Study Design
4.4. Participants
4.5. Sampling
4.6. Analysis of Plasma oxLDL
4.7. Analysis of Urinary F2-IsoPs
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosen, R.C.; Weiner, D.N. Cardiovascular disease and sleep-related erections. J. Psychosom. Res. 1997, 42, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Deshmukh, A.; Sachdeva, R.; Lu, J.; Mehta, J.L. Oxidized Low-Density Lipoprotein and Atherosclerosis Implications in Antioxidant Therapy. Am. J. Med. Sci. 2011, 342, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and Atherosclerosis. Mediat. Inflamm. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2019, 38, 333–342. [Google Scholar] [CrossRef]
- Carr, A.C.; McCall, M.R.; Frei, B. Oxidation of LDL by Myeloperoxidase and Reactive Nitrogen Species: Reaction Pathways and Antioxidant Protection. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1716–1723. [Google Scholar] [CrossRef] [Green Version]
- Arai, H. Oxidative Modification of Lipoproteins. Subcell. Biochem. 2013, 77, 103–114. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N.D. Vegetarian Dietary Patterns and Cardiovascular Disease. Prog. Cardiovasc. Dis. 2018, 61, 54–61. [Google Scholar] [CrossRef]
- Willett, W. Diet and health: What should we eat? Science 1994, 264, 532–537. [Google Scholar] [CrossRef] [Green Version]
- Zock, P.L.; Katan, M.B. Diet, LDL oxidation, and coronary artery disease. Am. J. Clin. Nutr. 1998, 68, 759–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-García, F.; Peragón, J. HPLC analysis of oleuropein, hydroxytyrosol, and tyrosol in stems and roots of Olea europaea L. cv. Picual during ripening. J. Sci. Food Agric. 2010, 90, 2295–2300. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alagna, F.; Mariotti, R.; Panara, F.; Caporali, S.; Urbani, S.; Veneziani, G.; Esposto, S.; Taticchi, A.; Rosati, A.; Rao, R.; et al. Olive phenolic compounds: Metabolic and transcriptional profiling during fruit development. BMC Plant Biol. 2012, 12, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez-Tortosa, M.C.; Urbano, G.; Lopez-Jurado, M.; Nestares, T.; Gomez, M.C.; Mir, A.; Ros, E.; Mataix, J.; Gil, A. Extra-virgin olive oil increases the resistance of LDL to oxidation more than refined olive oil in free-living men with peripheral vascular disease. J. Nutr. 1999, 129, 2177–2183. [Google Scholar] [CrossRef] [Green Version]
- Marcelino, G.; Hiane, P.A.; Freitas, K.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Regulation EC No. 432/2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Off. J. Eur. Union 2012, L136, 1. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32012R0432 (accessed on 3 February 2022).
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-Robles, A.; Rivas, A.; Lorenzo-Tovar, M.L.; Monteagudo, C.; Mariscal-Arcas, M.; Olea-Serrano, F. Estimation of the intake of phenol compounds from virgin olive oil of a population from southern Spain. Food Addit. Contam. Part A 2014, 31, 1460–1469. [Google Scholar] [CrossRef]
- Bender, C.; Straßmann, S.; Heidrich, P. Cellular Antioxidant Effects and Bioavailability of Food Supplements Rich in Hydroxytyrosol. Appl. Sci. 2021, 11, 4763. [Google Scholar] [CrossRef]
- Bender, C.; Strassmann, S.; Golz, C. Oral bioavailability and metabolism of hydroxytyrosol from food supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef] [PubMed]
- Piłacik, B.; Nofer, T.W.; Wasowicz, W. F2-isoprostanes biomarkers of lipid peroxidation: Their utility in evaluation of oxidative stress induced by toxic agents. Int. J. Occup. Med. Environ. Health 2002, 15, 19–27. [Google Scholar] [PubMed]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-Isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10 (Suppl. S1), S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; Roberts, L.J., II. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011, 50, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxidized LDL ELISA Kit: Directions for Use 10-1143-01 v 23.0. Available online: https://www.mercodia.com/product/oxidized-ldl-elisa (accessed on 25 May 2021).
- Gimeno, E.; Fitó, M.; Lamuela-Raventós, R.; Castellote, A.; Covas, M.; Farré, M.; de la Torre-Boronat, M.C.; López-Sabater, M. Effect of ingestion of virgin olive oil on human low-density lipoprotein composition. Eur. J. Clin. Nutr. 2002, 56, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Weinbrenner, T.; Fitó, M.; Torre, R.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; Albaladejo, M.F.; Abanades, S.; Schroder, H.; et al. Olive Oils High in Phenolic Compounds Modulate Oxidative/Antioxidative Status in Men. J. Nutr. 2004, 134, 2314–2321. [Google Scholar] [CrossRef] [Green Version]
- Marrugat, J.; Covas, M.-I.; Fitó, M.; Schröder, H.; Miró-Casas, E.; Gimeno, E.; Carmen López-Sabater, M.; de la Torre, R.; Farré, M. Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation. Eur. J. Nutr. 2004, 43, 140–147. [Google Scholar] [CrossRef]
- Covas, M.I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef]
- Covas, M.I.; Nyyssönen, K.; Poulsen, H.E.; Kaikkonen, J.; Zunft, H.J.F.; Kiesewetter, H.; Gaddi, A.; de la Torre, R.; Mursu, J.; Bäumler, H.; et al. The effect of polyphenols in olive oil on heart disease risk factors. Ann. Intern. Med. 2006, 145, 333–341. [Google Scholar] [CrossRef]
- De la Torre-Carbot, K.; Chávez-Servín, J.L.; Jaúregui, O.; Castellote, A.I.; Lamuela-Raventós, R.M.; Nurmi, T.; Poulsen, H.E.; Gaddi, A.V.; Kaikkonen, J.; Zunft, H.-F.; et al. Elevated Circulating LDL Phenol Levels in Men Who Consumed Virgin Rather Than Refined Olive Oil Are Associated with Less Oxidation of Plasma LDL. J. Nutr. 2010, 140, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Castañer, O.; Covas, M.-I.; Khymenets, O.; Nyyssonen, K.; Konstantinidou, V.; Zunft, H.-F.; de la Torre, R.; Muñoz-Aguayo, D.; Vila, J.; Fitó, M. Protection of LDL from oxidation by olive oil polyphenols is associated with a downregulation of CD40-ligand expression and its downstream products in vivo in humans. Am. J. Clin. Nutr. 2012, 95, 1238–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, M.A.; Gualtieri, P.; Gratteri, S.; Ali, W.; Sergi, D.; Muscoli, S.; Cammarano, A.; Bernardini, S.; Di Renzo, L.; Romeo, F. Effects of postprandial hydroxytyrosol and derivates on oxidation of LDL, cardiometabolic state and gene expression. J. Cardiovasc. Med. 2019, 20, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sarapis, K.; George, E.S.; Marx, W.; Mayr, H.L.; Willcox, J.; Esmaili, T.; Powell, K.L.; Folasire, O.S.; Lohning, A.E.; Garg, M.; et al. Extra virgin olive oil high in polyphenols improves antioxidant status in adults: A double-blind, randomized, controlled, cross-over study (OLIVAUS). Eur. J. Nutr. 2022, 61, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Valls, R.-M.; Farràs, M.; Suárez, M.; Fernández-Castillejo, S.; Fitó, M.; Konstantinidou, V.; Fuentes, F.; Lopez-Miranda, J.; Giralt, M.; Covas, M.-I.; et al. Effects of functional olive oil enriched with its own phenolic compounds on endothelial function in hypertensive patients. A randomised controlled trial. Food Chem. 2015, 167, 30–35. [Google Scholar] [CrossRef]
- Guertin, K.A.; Grant, R.K.; Arnold, K.B.; Burwell, L.; Hartline, J.; Goodman, P.J.; Minasian, L.M.; Lippman, S.M.; Klein, E.; Cassano, P.A. Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radic. Biol. Med. 2016, 95, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Hermans, N.; Van der Auwera, A.; Breynaert, A.; Verlaet, A.; De Bruyne, T.; Van Gaal, L.; Pieters, L.; Verhoeven, V. A red yeast rice-olive extract supplement reduces biomarkers of oxidative stress, OxLDL and Lp-PLA2, in subjects with metabolic syndrome: A randomised, double-blind, placebo-controlled trial. Trials 2017, 18, 302. [Google Scholar] [CrossRef] [Green Version]
- Lafay, S.; Jan, C.; Nardon, K.; Lemaire, B.; Ibarra, A.; Roller, M.; Houvenaeghel, M.; Juhel, C.; Cara, L. Grape extract improves antioxidant status and physical performance in elite male athletes. J. Sports Sci. Med. 2009, 8, 468. [Google Scholar]
- Visioli, F.; Caruso, D.; Galli, C.; Viappiani, S.; Galli, G.; Sala, A. Olive oils rich in natural catecholicpheols decrease isoprostane excretion in humans. Biochem. Biophys. Res. Commun. 2000, 278, 797–799. [Google Scholar] [CrossRef]
- Ruano, J.; Lopez-Miranda, J.; Fuentes, F.; Moreno, J.A.; Bellido, C.; Perez-Martinez, P.; Lozano, A.; Gómez, P.; Jiménez, Y.; Pérez Jiménez, F. Phenolic content of virgin olive oil improves ischemic reactive hyperemia in hypercholesterolemic patients. J. Am. Coll. Cardiol. 2005, 46, 1864–1868. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.; Nascetti, S.; Lopez-Sabater, M.C.; Elosua, R.; Salonen, J.T.; Nyyssonen, K.; Poulsen, H.E.; Zunft, H.J.; Kiesewetter, H.; de la Torre, K.; et al. Changes in LDL fatty acid composition as a response to olive oil treatment are inversely related to lipid oxidative damage: The EUROLIVE study. J. Am. Coll. Nutr. 2008, 27, 314–320. [Google Scholar] [CrossRef]
- Kendall, M.; Batterham, M.; Obied, H.; Prenzler, P.D.; Ryan, D.; Robards, K. Zero effect of multiple dosage of olive leaf supplements on urinary biomarkers of oxidative stress in healthy humans. Nutrition 2009, 25, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, T.; Serafini, M. Antioxidant Modulation of F2-Isoprostanes in Humans: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1202–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawson, J.A.; Rokach, J.; FitzGerald, G.A. Isoprostanes: Formation, analysis and use as indices of lipid peroxidation in vivo. J. Biol. Chem. 1999, 274, 24441–24444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, L.J.; Morrow, J.D. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic. Biol. Med. 2000, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.; Delanty, N.; Lawson, J.A.; FitzGerald, G.A. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 1996, 94, 19–25. [Google Scholar] [CrossRef]
- Budinsky, A.; Wolfram, R.; Oguogho, A.; Efthimiou, Y.; Stamatopoulos, Y.; Sinzinger, H. Regular ingestion of opuntia robusta lowers oxidation injury. Prostaglandins Leukot. Essent. Fat. Acids 2001, 65, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, M.; Block, G.; Hudes, M.; Morrow, J.D.; Norkus, E.P.; Traber, M.G.; Cross, C.E.; Packer, L. Antioxidant supplementation decreases lipid peroxidation biomarker F(2)-isoprostanes in plasma of smokers. Cancer Epidemiol. Biomark. Prev. 2002, 11, 7–13. [Google Scholar]
- Dietrich, M.; Block, G.; Benowitz, N.L.; Morrow, J.D.; Hudes, M.; Jacob, P.; Norkus, E.P.; Packer, L. Vitamin C supplementation decreases oxidative stress biomarker f2-isoprostanes in plasma of nonsmokers exposed to environmental tobacco smoke. Nutr. Cancer 2003, 45, 176–184. [Google Scholar] [CrossRef]
- Dhawan, V.; Jain, S. Effect of garlic supplementation on oxidized low density lipoproteins and lipid peroxidation in patients of essential hypertension. Mol. Cell Biochem. 2004, 266, 109–115. [Google Scholar] [CrossRef]
- Santus, P.; Sola, A.; Carlucci, P.; Fumagalli, F.; Di Gennaro, A.; Mondoni, M.; Carnini, C.; Centanni, S.; Sala, A. Lipid peroxidation and 5-lipoxygenase activity in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 838–843. [Google Scholar] [CrossRef] [Green Version]
- Block, G.; Jensen, C.D.; Morrow, J.D.; Holland, N.; Norkus, E.P.; Milne, G.L.; Hudes, M.; Dalvi, T.B.; Crawford, P.B.; Fung, E.B.; et al. The effect of vitamins C and E on biomarkers of oxidative stress depends on baseline level. Free Radic. Biol. Med. 2008, 45, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nutritional Value * [g/25 mL] | IP-1 | IP-2 |
|---|---|---|
| Energy (kJ) | 18.75 | 138.75 |
| Fat | 0.00 | 0.01 |
| of which saturates | 0.00 | 0.00 |
| Carbohydrate | 1.04 | 8.01 |
| of which sugars | 0.26 | 6.88 |
| Protein | 0.05 | 0.09 |
| Salt | 0.01 | 0.005 |
| Potassium | 0.201 | 0.161 |
| HT and Derivatives (in the free forms) [mg/25mL] | ||
| HT | 30.6 | 61.5 |
| Ole | 0.04 | 0.07 |
| HVA | n.d | n.d |
| DOPAC | n.d | n.d |
| Total polyphenols * | 274.5 | 252.5 |
| Time (h) | IP-1 (n = 9) | IP-2 (n = 7) | ||
|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |
| 0 | 4.593 | 0.88 | 4.797 | 1.11 |
| 0.5 | 2.680 * | 0.18 | 1.955 * | 0.26 |
| 1 | 3.246 | 0.19 | 2.353 * | 0.27 |
| 1.5 | 5.259 | 1.22 | 2.815 | 0.39 |
| 2 | 4.438 | 0.65 | 2.813 * | 0.39 |
| 4 | 3.322 | 0.21 | 2.542 * | 0.30 |
| 12 | 2.801 * | 0.24 | 2.757 * | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, C.; Candi, I.; Rogel, E. Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans. Int. J. Mol. Sci. 2023, 24, 5521. https://doi.org/10.3390/ijms24065521
Bender C, Candi I, Rogel E. Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans. International Journal of Molecular Sciences. 2023; 24(6):5521. https://doi.org/10.3390/ijms24065521
Chicago/Turabian StyleBender, Cecilia, Ilaria Candi, and Eva Rogel. 2023. "Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans" International Journal of Molecular Sciences 24, no. 6: 5521. https://doi.org/10.3390/ijms24065521
APA StyleBender, C., Candi, I., & Rogel, E. (2023). Efficacy of Hydroxytyrosol-Rich Food Supplements on Reducing Lipid Oxidation in Humans. International Journal of Molecular Sciences, 24(6), 5521. https://doi.org/10.3390/ijms24065521






