Phototoxic Potential of Different DNA Intercalators for Skin Cancer Therapy: In Vitro Screening
Abstract
:1. Introduction
2. Results and Discussions
2.1. Cytotoxicity in HaCaT and MET1 Cells
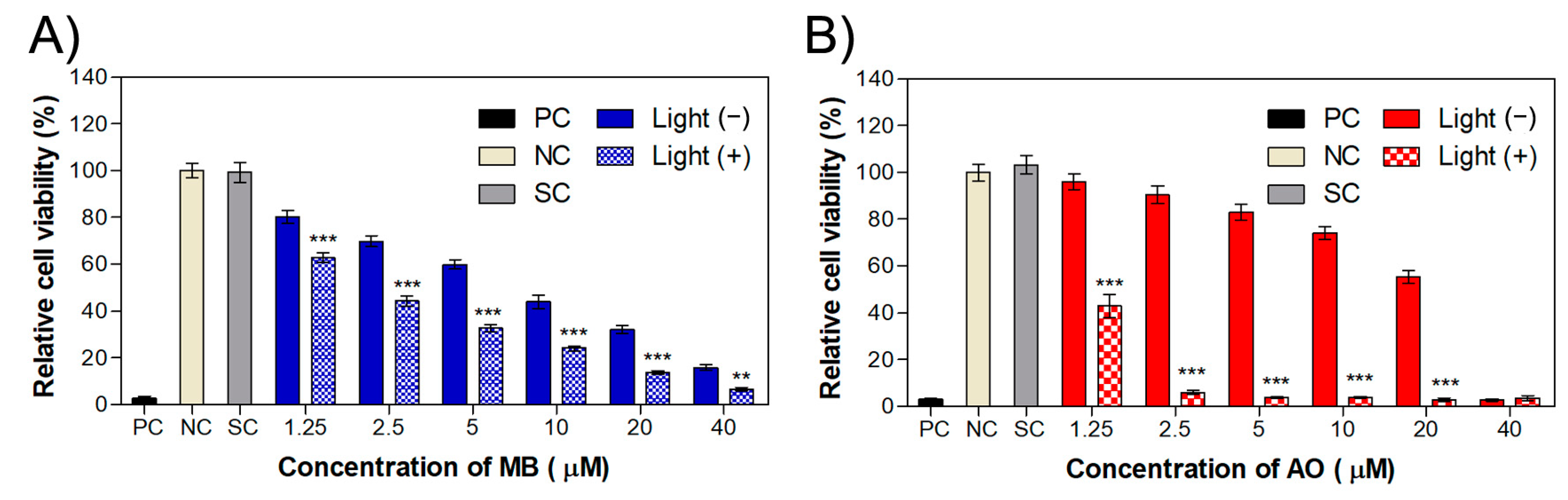
2.2. Phototoxicity in MET1 Cells
2.3. Intracellular ROS Production in MET1 Cells
2.4. Cytotoxicity and Phototoxicity in WM983b Cells
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Cytotoxicity
3.4. Phototoxicity of MET1 Cells
3.5. Intracellular ROS Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, X.; Li, Y.; Hamblin, M.R. Photodynamic Therapy in Dermatology beyond Non-Melanoma Cancer: An Update. Photodiagnosis Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jung, H.; Park, H. Topical PDT in the Treatment of Benign Skin Diseases: Principles and New Applications. Int. J. Mol. Sci. 2015, 16, 23259–23278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tampa, M.; Sarbu, M.I.; Matei, C.; Mitran, C.I.; Mitran, M.I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.R. Photodynamic Therapy: A Hot Topic in Dermato-Oncology (Review). Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhao, H.; Fu, L.; Cui, J.; Yang, Y. Global Trends and Research Progress of Photodynamic Therapy in Skin Cancer: A Bibliometric Analysis and Literature Review. Clin. Cosmet. Investig. Dermatol. 2023, 16, 479–498. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The Potential of Photodynamic Therapy (PDT)—Experimental Investigations and Clinical Use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef]
- Crous, A.; Chizenga, E.; Hodgkinson, N.; Abrahamse, H. Targeted Photodynamic Therapy: A Novel Approach to Abolition of Human Cancer Stem Cells. Int. J. Opt. 2018, 2018, 7317063. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; He, Y.-Y. Recent Advances in the Prevention and Treatment of Skin Cancer Using Photodynamic Therapy. Expert. Rev. Anticancer. Ther. 2010, 10, 1797–1809. [Google Scholar] [CrossRef] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An Updated Overview on the Development of New Photosensitizers for Anticancer Photodynamic Therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Mall, C.; Solanki, P.P. Evaluation of Mixed Dye Combination by Spectral Study for the Application as Photosensitizer in Photogalvanic Cells for Solar Energy Conversion and Storage. Surf. Interfaces 2022, 29, 101688. [Google Scholar] [CrossRef]
- Raab, O. Über Die Wirkung Fluoreszierender Stoffe Auf Infusoren. Zeitschr Biol. 1900, 39, 524–546. [Google Scholar]
- Lamch, Ł.; Kulbacka, J.; Dubińska-Magiera, M.; Saczko, J.; Wilk, K.A. Folate-Directed Zinc (II) Phthalocyanine Loaded Polymeric Micelles Engineered to Generate Reactive Oxygen Species for Efficacious Photodynamic Therapy of Cancer. Photodiagnosis Photodyn. Ther. 2019, 25, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fadeel, D.; Al-Toukhy, G.M.; Elsharif, A.M.; Al-Jameel, S.S.; Mohamed, H.H.; Youssef, T.E. Improved Photodynamic Efficacy of Thiophenyl Sulfonated Zinc Phthalocyanine Loaded in Lipid Nano-Carriers for Hepatocellular Carcinoma Cancer Cells. Photodiagnosis Photodyn. Ther. 2018, 23, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mehraban, N.; Musich, P.; Freeman, H. Synthesis and Encapsulation of a New Zinc Phthalocyanine Photosensitizer into Polymeric Nanoparticles to Enhance Cell Uptake and Phototoxicity. Appl. Sci. 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.M.; Oliveira, T.C.; de Gomes, V.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene Blue Photodynamic Therapy Induces Selective and Massive Cell Death in Human Breast Cancer Cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [Green Version]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-Activated Green Drugs: How We Can Use Them in Photodynamic Therapy and Mass-Produce Them with Biotechnological Tools. Phytomedicine Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Siewert, B.; Stuppner, H. The Photoactivity of Natural Products—An Overlooked Potential of Phytomedicines? Phytomedicine 2019, 60, 152985. [Google Scholar] [CrossRef]
- Machado, F.C.; Adum de Matos, R.P.; Primo, F.L.; Tedesco, A.C.; Rahal, P.; Calmon, M.F. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Breast Adenocarcinoma Cell Line. Bioorg. Med. Chem. 2019, 27, 1882–1890. [Google Scholar] [CrossRef]
- Duse, L.; Pinnapireddy, S.R.; Strehlow, B.; Jedelská, J.; Bakowsky, U. Low Level LED Photodynamic Therapy Using Curcumin Loaded Tetraether Liposomes. Eur. J. Pharm. Biopharm. 2018, 126, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of Targeted Curcumin (CUR) Loaded PLGA Nanoparticles for in Vitro Photodynamic Therapy on Human Glioblastoma Cell Line. Photodiagnosis Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- de Matos, R.P.A.; Calmon, M.F.; Amantino, C.F.; Villa, L.L.; Primo, F.L.; Tedesco, A.C.; Rahal, P. Effect of Curcumin-Nanoemulsion Associated with Photodynamic Therapy in Cervical Carcinoma Cell Lines. Biomed Res. Int. 2018, 2018, 4057959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paula Rodrigues, R.; Tini, I.R.P.; Soares, C.P.; da Silva, N.S. Effect of Photodynamic Therapy Supplemented with Quercetin in HEp-2 Cells. Cell Biol. Int. 2014, 38, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Mandal, N.; Patel, G.; Kirar, S.; Reddy, Y.N.; Kushwah, V.; Jain, S.; Kalia, Y.N.; Bhaumik, J.; Banerjee, U.C. Co-Administration of Zinc Phthalocyanine and Quercetin via Hybrid Nanoparticles for Augmented Photodynamic Therapy. Nanomedicine 2021, 33, 102368. [Google Scholar] [CrossRef]
- Yang, J.; Xu, Y.; Jiang, M.; Zou, D.; Yang, G.; Shen, L.; Zou, J. Photochemical Property of Two Ru(II) Compounds Based on 5-(2-Pyrazinyl)Tetrazole for Cancer Phototherapy by Changing Auxiliary Ligand. J. Inorg. Biochem. 2019, 193, 124–129. [Google Scholar] [CrossRef]
- Vardevanyan, P.O.; Antonyan, A.P.; Parsadanyan, M.A.; Shahinyan, M.A.; Hambardzumyan, L.A. Mechanisms for Binding between Methylene Blue and DNA. J. Appl. Spectrosc. 2013, 80, 595–599. [Google Scholar] [CrossRef]
- Pereira, E.; do Quental, L.; Palma, E.; Oliveira, M.C.; Mendes, F.; Raposinho, P.; Correia, I.; Lavrado, J.; di Maria, S.; Belchior, A.; et al. Evaluation of Acridine Orange Derivatives as DNA-Targeted Radiopharmaceuticals for Auger Therapy: Influence of the Radionuclide and Distance to DNA. Sci. Rep. 2017, 7, 42544. [Google Scholar] [CrossRef] [Green Version]
- Andrews, W.J.; Ray, S.; Panova, T.; Engel, C.; Panov, K.I. DNA Intercalators Inhibit Eukaryotic Ribosomal RNA Synthesis by Impairing the Initiation of Transcription. Genes 2021, 12, 1412. [Google Scholar] [CrossRef]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bora, U. Interactions of Curcumin and Its Derivatives with Nucleic Acids and Their Implications. Mini-Rev. Med. Chem. 2013, 13, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gu, J.; Liu, Y.; Xu, K.; Song, J.; Wang, X.; Yu, D.; Wu, H. Epigallocatechin Gallate-Loaded Tetrahedral DNA Nanostructures as a Novel Inner Ear Drug Delivery System. Nanoscale 2022, 14, 8000–8011. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, Á.; Gil, A. Elucidating the Intercalation of Methylated 1,10-Phenanthroline with DNA: The Important Weight of the CH/H Interactions and the Selectivity of CH/π and CH/n Interactions. RSC Adv. 2021, 11, 1553–1563. [Google Scholar] [CrossRef]
- Cusumano, M.; di Pietro, M.L.; Giannetto, A.; Vainiglia, P.A. The Intercalation to DNA of Bipyridyl Complexes of Platinum(II) with Thioureas. J. Inorg. Biochem. 2005, 99, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Kucková, L.; Jomová, K.; Švorcová, A.; Valko, M.; Segľa, P.; Moncoľ, J.; Kožíšek, J. Synthesis, Crystal Structure, Spectroscopic Properties and Potential Biological Activities of Salicylate—Neocuproine Ternary Copper(II) Complexes. Molecules 2015, 20, 2115–2137. [Google Scholar] [CrossRef] [Green Version]
- González-Larraza, P.G.; López-Goerne, T.M.; Padilla-Godínez, F.J.; González-López, M.A.; Hamdan-Partida, A.; Gómez, E. IC 50 Evaluation of Platinum Nanocatalysts for Cancer Treatment in Fibroblast, HeLa, and DU-145 Cell Lines. ACS Omega 2020, 5, 25381–25389. [Google Scholar] [CrossRef]
- Badisa, R.B.; Ayuk-Takem, L.T.; Ikediobi, C.O.; Walker, E.H. Selective Anticancer Activity of Pure Licamichauxiioic-B Acid in Cultured Cell Lines. Pharm. Biol. 2006, 44, 141–145. [Google Scholar] [CrossRef]
- Aung, T.; Qu, Z.; Kortschak, R.; Adelson, D. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef] [Green Version]
- Skvortsov, D.A.; Kalinina, M.A.; Zhirkina, I.V.; Vasilyeva, L.A.; Ivanenkov, Y.A.; Sergiev, P.V.; Dontsova, O.A. From Toxicity to Selectivity: Coculture of the Fluorescent Tumor and Non-Tumor Lung Cells and High-Throughput Screening of Anticancer Compounds. Front. Pharmacol. 2021, 12, 713103. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Baldi, A.; De Luca, A.; Maiorano, P.; D’Angelo, C.; Giordano, A. Curcumin as an Anticancer Agent in Malignant Mesothelioma: A Review. Int. J. Mol. Sci. 2020, 21, 1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-Inducing Effects of Quercetin in Vitro and in Vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Ohishi, T.; Miyoshi, N.; Oishi, Y.; Nakamura, Y.; Isemura, M. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules 2020, 25, 4553. [Google Scholar] [CrossRef]
- Melgoza, D.; Hernández-Ramírez, A.; Peralta-Hernández, J.M. Comparative Efficiencies of the Decolourisation of Methylene Blue Using Fenton’s and Photo-Fenton’s Reactions. Photochem. Photobiol. Sci. 2009, 8, 596–599. [Google Scholar] [CrossRef]
- Kofler, B.; Romani, A.; Pritz, C.; Steinbichler, T.; Schartinger, V.; Riechelmann, H.; Dudas, J. Photodynamic Effect of Methylene Blue and Low Level Laser Radiation in Head and Neck Squamous Cell Carcinoma Cell Lines. Int. J. Mol. Sci. 2018, 19, 1107. [Google Scholar] [CrossRef] [Green Version]
- Romio, K.B.; dos Santos, K.F.; da Silva, R.J.; Pedro, M.F.C.; Kalck, A.S.; da Silva Sousa, M.; Possamai, L.M.; Souto, P.C.S.; Silva, J.R.; de Souza, N.C. Incorporation of Triclosan and Acridine Orange into Liposomes for Evaluating the Susceptibility of Candida Albicans. J. Photochem. Photobiol. B Biol. 2017, 173, 514–521. [Google Scholar] [CrossRef]
- Shenderovich, I. The Partner Does Matter: The Structure of Heteroaggregates of Acridine Orange in Water. Molecules 2019, 24, 2816. [Google Scholar] [CrossRef] [Green Version]
- Osman, H.; Elsahy, D.; Saadatzadeh, M.R.; Pollok, K.E.; Yocom, S.; Hattab, E.M.; Georges, J.; Cohen-Gadol, A.A. Acridine Orange as a Novel Photosensitizer for Photodynamic Therapy in Glioblastoma. World Neurosurg. 2018, 114, e1310–e1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Lin, J.F.; Tsai, T.F.; Chen, H.E.; Chou, K.Y.; Yang, S.C.; Tang, Y.M.; Hwang, T.I.S. Acridine Orange Exhibits Photodamage in Human Bladder Cancer Cells under Blue Light Exposure. Sci. Rep. 2017, 7, 14103. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Zhu, K.; Liu, Z.; Huang, J. Prooxidant Effects of Epigallocatechin-3-Gallate in Health Benefits and Potential Adverse Effect. Oxidative Med. Cell. Longev. 2020, 2020, 9723686. [Google Scholar] [CrossRef]
- Pires, F.; Santos, J.F.; Bitoque, D.; Silva, G.A.; Marletta, A.; Nunes, V.A.; Ribeiro, P.A.; Silva, J.C.; Raposo, M. Polycaprolactone/Gelatin Nanofiber Membranes Containing EGCG-Loaded Liposomes and Their Potential Use for Skin Regeneration. ACS Appl. Bio Mater. 2019, 2, 4790–4800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Elias, R.J. Antioxidant and Pro-Oxidant Activity of (−)-Epigallocatechin-3-Gallate in Food Emulsions: Influence of PH and Phenolic Concentration. Food Chem. 2013, 138, 1503–1509. [Google Scholar] [CrossRef]
- Mun, S.T.; Bae, D.H.; Ahn, W.S. Epigallocatechin Gallate with Photodynamic Therapy Enhances Anti-Tumor Effects in Vivo and in Vitro. Photodiagnosis Photodyn. Ther. 2014, 11, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Luna, M.; Rucker, N.; Wong, S.; Gomer, C.J. Pro-Apoptotic and Anti-Inflammatory Properties of the Green Tea Constituent Epigallocatechin Gallate Increase Photodynamic Therapy Responsiveness. Lasers Surg. Med. 2011, 43, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of Curcumin in Different Solvent and Solution Media: UV–Visible and Steady-State Fluorescence Spectral Study. J. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef]
- Abdel Fadeel, D.A.; Kamel, R.; Fadel, M. PEGylated Lipid Nanocarrier for Enhancing Photodynamic Therapy of Skin Carcinoma Using Curcumin: In-Vitro/in-Vivo Studies and Histopathological Examination. Sci. Rep. 2020, 10, 10435. [Google Scholar] [CrossRef]
- Al Hageh, C.; Al Assaad, M.; El Masri, Z.; Samaan, N.; El-Sibai, M.; Khalil, C.; Khnayzer, R.S. A Long-Lived Cuprous Bis-Phenanthroline Complex for the Photodynamic Therapy of Cancer. Dalton Trans. 2018, 47, 4959–4967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mari, C.; Pierroz, V.; Rubbiani, R.; Patra, M.; Hess, J.; Spingler, B.; Oehninger, L.; Schur, J.; Ott, I.; Salassa, L.; et al. DNA Intercalating Ru II Polypyridyl Complexes as Effective Photosensitizers in Photodynamic Therapy. Chem.—A Eur. J. 2014, 20, 14421–14436. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Blacque, O.; Goldner, P.; Chao, H.; Gasser, G. Towards Long Wavelength Absorbing Photodynamic Therapy Photosensitizers via the Extension of a [Ru(Bipy)3]2+ Core. Eur. J. Inorg. Chem. 2019, 2019, 3704–3712. [Google Scholar] [CrossRef]
- Griendling, K.K.; Touyz, R.M.; Zweier, J.L.; Dikalov, S.; Chilian, W.; Chen, Y.-R.; Harrison, D.G.; Bhatnagar, A. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System. Circ. Res. 2016, 119, e39–e75. [Google Scholar] [CrossRef] [Green Version]
- Klimaszewska-Wisniewska, A.; Halas-Wisniewska, M.; Tadrowski, T.; Gagat, M.; Grzanka, D.; Grzanka, A. Paclitaxel and the Dietary Flavonoid Fisetin: A Synergistic Combination That Induces Mitotic Catastrophe and Autophagic Cell Death in A549 Non-Small Cell Lung Cancer Cells. Cancer Cell Int. 2016, 16, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many Ways to Resistance: How Melanoma Cells Evade Targeted Therapies. Biochim. Biophys. Acta—Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
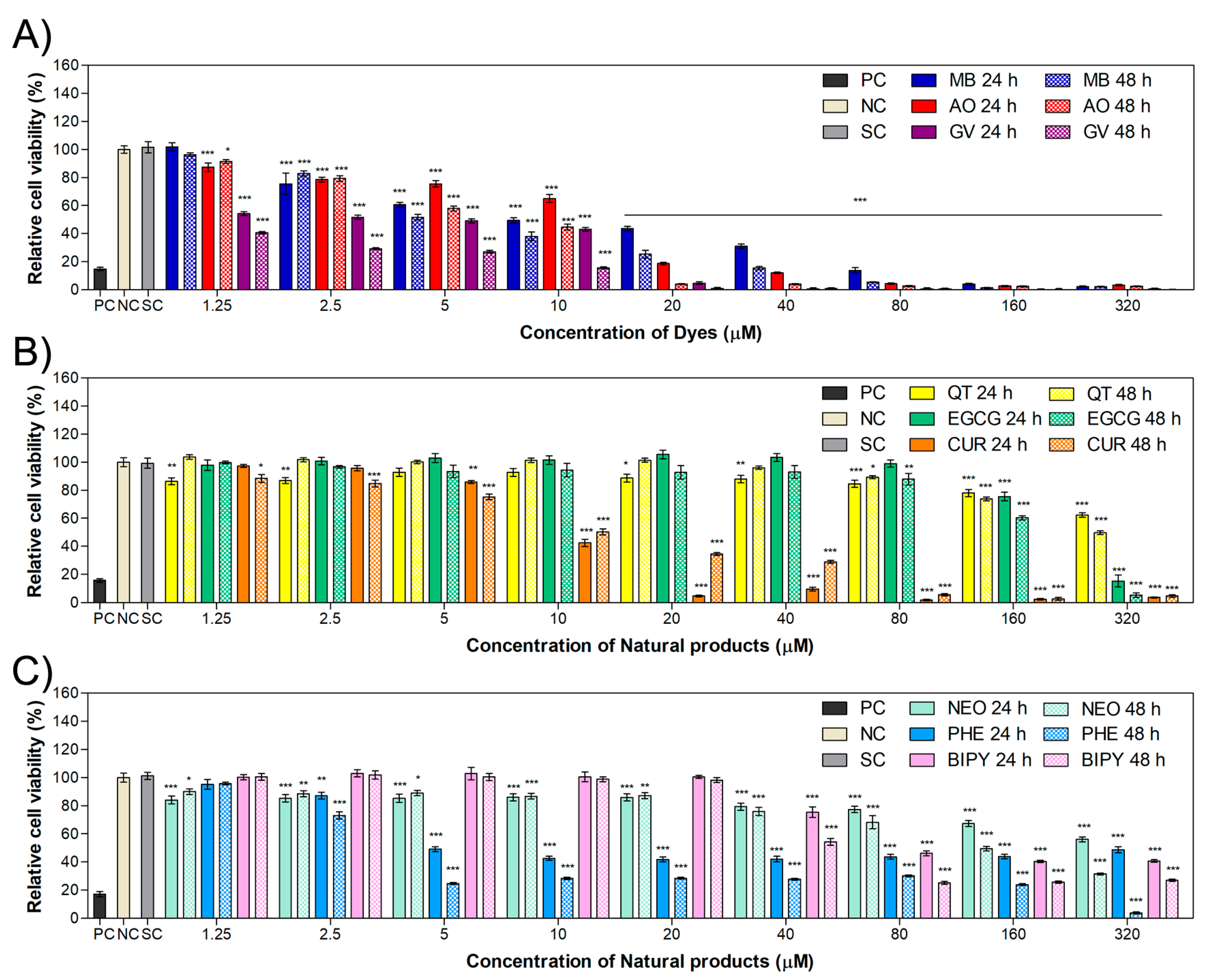



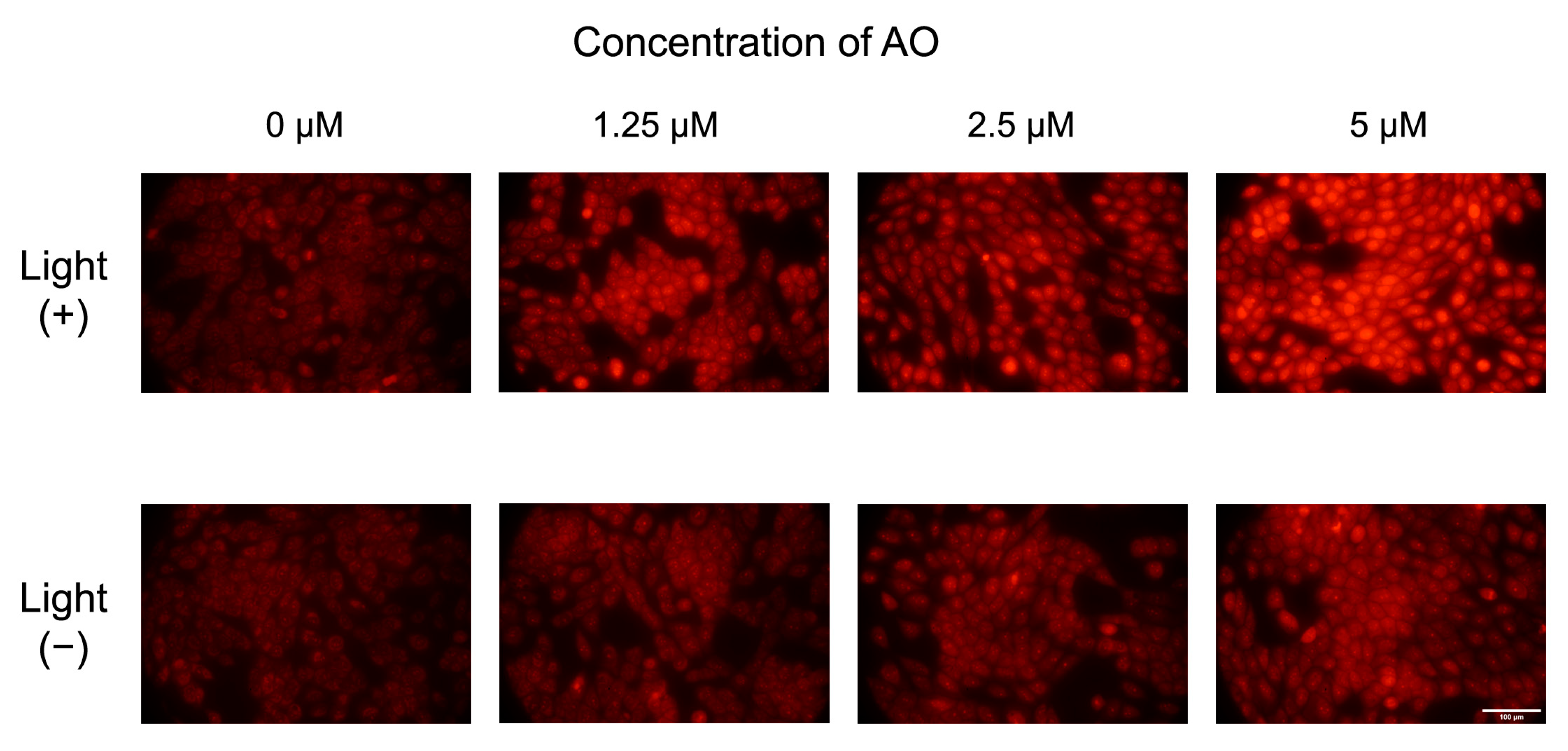
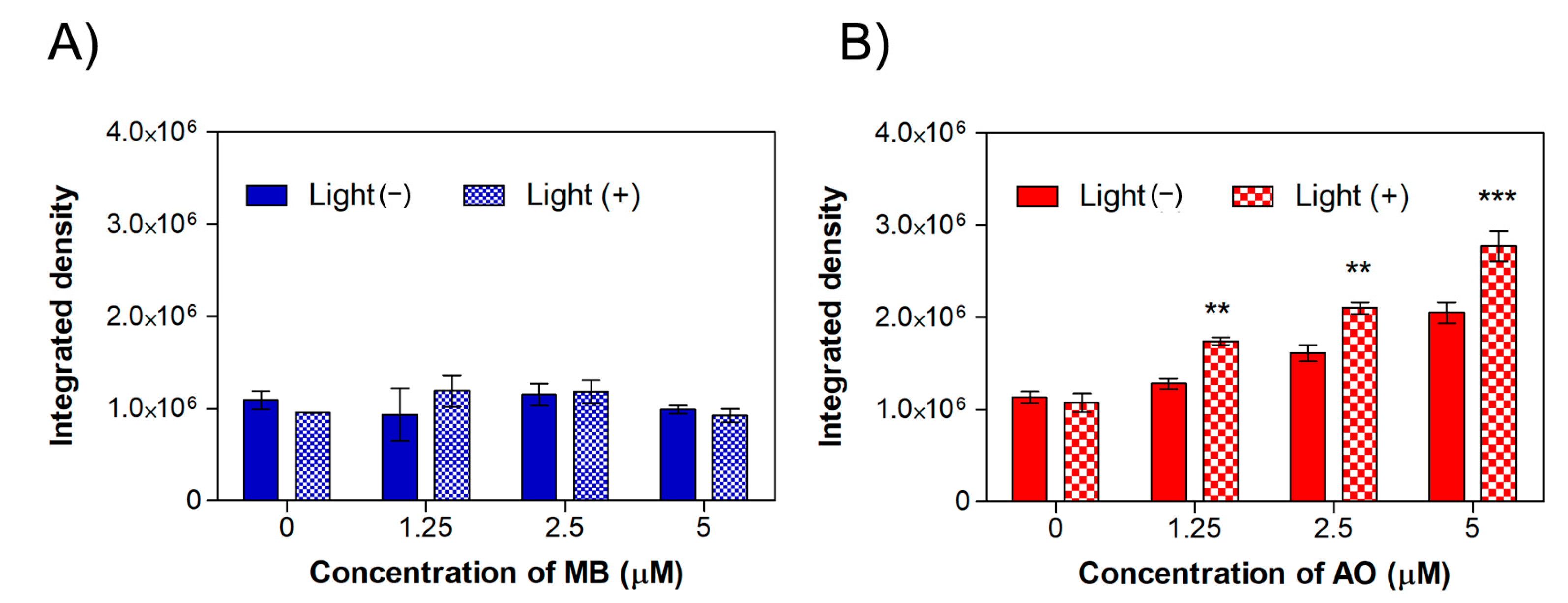
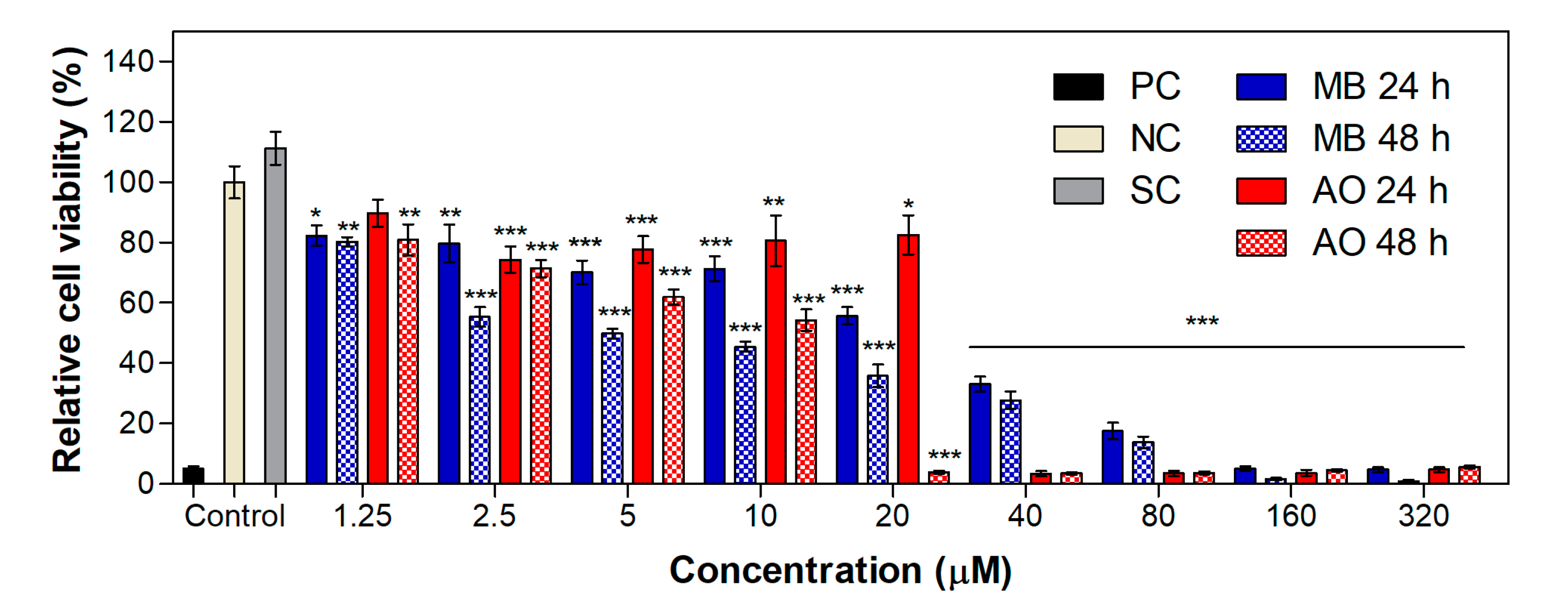
| IC50 (µM) | ||||
|---|---|---|---|---|
| MET1 Cells | HaCaT Cells | |||
| Molecule | 24 h | 48 h | 24 h | 48 h |
| Methylene blue | 14.2 | 4.2 | 11.6 | 7.1 |
| Acridine orange | 15.1 | 10.3 | 10.5 | 6.5 |
| Gentian violet | 3.9 | 1.2 | 2.8 | 0.9 |
| Quercetin | >320 | 110 | >320 | 315 |
| Curcumin | 26.0 | 7.2 | 9.0 | 11.7 |
| Epigallocatechin gallate | 174 | 136 | 211 | 174 |
| Neocuproine | >320 | >320 | >320 | 159 |
| 1,10-phenanthroline | 124 | 7.7 | 35.2 | 5.9 |
| 2,2′-bipyridyl | 275 | 97.2 | 125 | 57.8 |
| IC50 (µM) | |||||
|---|---|---|---|---|---|
| Molecule | Dark | 640 nm | 583 nm | 457 nm | 254 nm |
| Methylene blue | 10.9 | 3.8 | 5.0 | 6.2 | 4.3 |
| Acridine orange | 11.5 | 6.6 | 4.1 | 0.4 | 7.1 |
| Gentian violet | 2.9 | 1.8 | 1.0 | 2.7 | 1.5 |
| Quercetin | 230 | 340 | 269 | 288 | 205 |
| Curcumin | 15.5 | 11.0 | 11.5 | 10.7 | 9.3 |
| Epigallocatechin gallate | 191 | 224 | 211 | 222 | 193 |
| Neocuproine | - | - | - | - | - |
| 1,10-phenanthroline | 7.4 | 4.8 | 6.8 | 4.9 | 6.0 |
| 2,2′-bipyridyl | 228 | 202 | 190 | 171 | 225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivetta, T.P.; Vieira, T.; Silva, J.C.; Ribeiro, P.A.; Raposo, M. Phototoxic Potential of Different DNA Intercalators for Skin Cancer Therapy: In Vitro Screening. Int. J. Mol. Sci. 2023, 24, 5602. https://doi.org/10.3390/ijms24065602
Pivetta TP, Vieira T, Silva JC, Ribeiro PA, Raposo M. Phototoxic Potential of Different DNA Intercalators for Skin Cancer Therapy: In Vitro Screening. International Journal of Molecular Sciences. 2023; 24(6):5602. https://doi.org/10.3390/ijms24065602
Chicago/Turabian StylePivetta, Thais P., Tânia Vieira, Jorge C. Silva, Paulo A. Ribeiro, and Maria Raposo. 2023. "Phototoxic Potential of Different DNA Intercalators for Skin Cancer Therapy: In Vitro Screening" International Journal of Molecular Sciences 24, no. 6: 5602. https://doi.org/10.3390/ijms24065602
APA StylePivetta, T. P., Vieira, T., Silva, J. C., Ribeiro, P. A., & Raposo, M. (2023). Phototoxic Potential of Different DNA Intercalators for Skin Cancer Therapy: In Vitro Screening. International Journal of Molecular Sciences, 24(6), 5602. https://doi.org/10.3390/ijms24065602










