The Influence of Prenatal Exposure to Methamphetamine on the Development of Dopaminergic Neurons in the Ventral Midbrain
Abstract
1. Introduction
2. Results
2.1. Methamphetamine Did Not Alter the Metabolic Activity of EVMNs
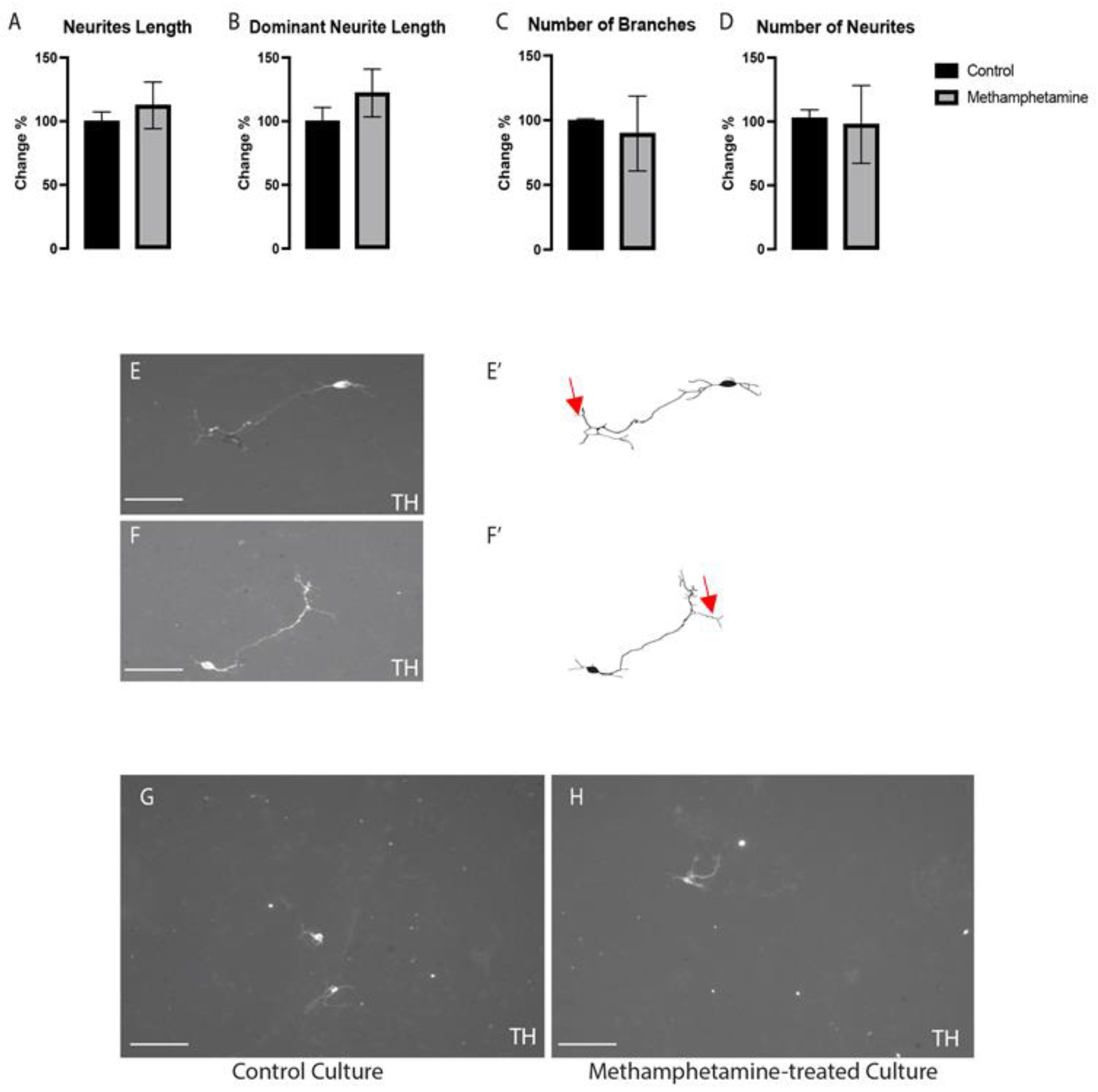
2.2. Methamphetamine Did Not Affect the Morphogenesis of VMDNs
2.3. Methamphetamine Did Not Change the Morphogenesis of Non-Dopaminergic Ventral Midbrain Neurons
2.4. Methamphetamine Altered the Expression of Dopaminergic-Related Genes in VMDNs
3. Discussion
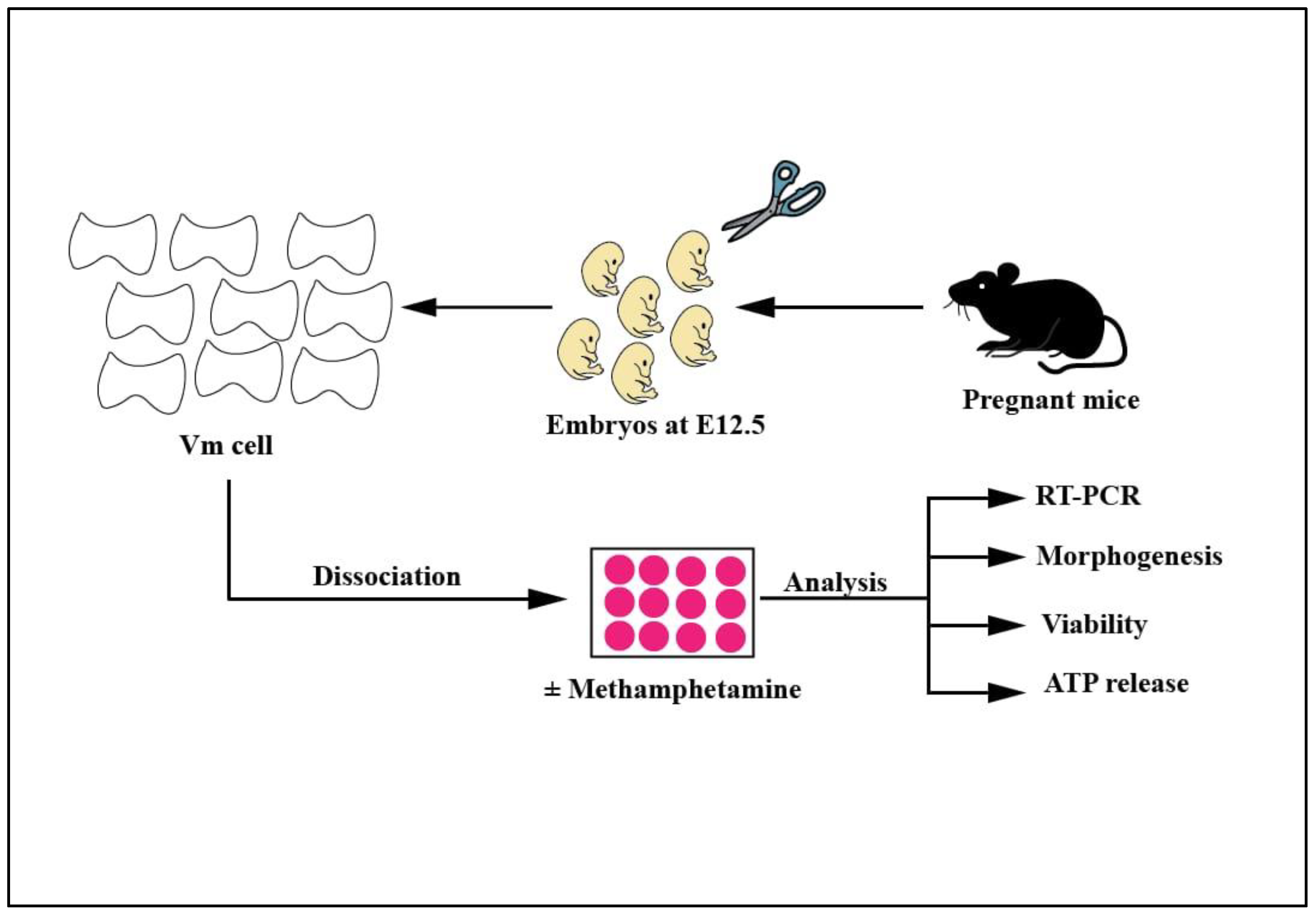
4. Materials and Methods
4.1. Isolation of Primary Mouse Embryonic Ventral Midbrain Neurons
4.2. Three-Dimensional (3D) Neuronal Cell Culture and Methamphetamine Treatment
4.3. Assessment of the Viability of EVMNs and ATP Release
4.4. Immunocytochemistry
4.5. Morphogenetic Analysis
4.6. Quantitative PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNODC. World Drug Report 2013; United Nations: New York, NY, USA, 2014. [Google Scholar]
- Krasnova, I.N.; Cadet, J.L. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009, 60, 379–407. [Google Scholar] [CrossRef]
- McCann, U.D.; Wong, D.F.; Yokoi, F.; Villemagne, V.; Dannals, R.F.; Ricaurte, G.A. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: Evidence from positron emission tomography studies with [11C] WIN-35,428. J. Neurosci. 1998, 18, 8417–8422. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Chang, L.; Wang, G.-J.; Fowler, J.S.; Franceschi, D.; Sedler, M.; Gatley, S.J.; Miller, E.; Hitzemann, R.; Ding, Y.-S. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J. Neurosci. 2001, 21, 9414–9418. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.S.; Kobeissy, F.H.; Wang, K.K.; Merlo, L.J.; Bruijnzeel, A.W.; Krasnova, I.N.; Cadet, J.L. Methamphetamine-and trauma-induced brain injuries: Comparative cellular and molecular neurobiological substrates. Biol. Psychiatry 2009, 66, 118–127. [Google Scholar] [CrossRef]
- Callaghan, R.C.; Cunningham, J.K.; Sajeev, G.; Kish, S.J. Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov. Disord. 2010, 25, 2333–2339. [Google Scholar] [CrossRef]
- Chang, L.; Alicata, D.; Ernst, T.; Volkow, N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 2007, 102, 16–32. [Google Scholar] [CrossRef]
- Marwick, C. NIDA seeking data on effect of fetal exposure to methamphetamine. Jama 2000, 283, 2225–2226. [Google Scholar] [CrossRef]
- Zoubková, H.; Tomášková, A.; Nohejlová, K.; Černá, M.; Šlamberová, R. Prenatal exposure to methamphetamine: Up-regulation of brain receptor genes. Front. Neurosci. 2019, 13, 771. [Google Scholar] [CrossRef]
- Vavrinková, B.; Binder, T.; Zivný, J. Characteristics of a population of drug dependent pregnant women in the Czech Republic. Cěeská Gynekol. 2001, 66, 285–291. [Google Scholar]
- Šlamberová, R. Drugs in pregnancy: The effects on mother and her progeny. Physiol. Res. 2012, 61, S123–S135. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Liu, J.-L.; Zhang, K.-K.; Chen, L.-J.; Xu, J.-T.; Xie, X.-L. The adverse effects of prenatal METH exposure on the offspring: A review. Front. Pharmacol. 2021, 12, 715176. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-H.; Zhang, K.-K.; Xu, J.-T.; Qu, D.; Chen, L.-J.; Li, J.-H.; Wang, Q.; Wang, H.-J.; Xie, X.-L. Luteolin alleviates methamphetamine-induced neurotoxicity by suppressing PI3K/Akt pathway-modulated apoptosis and autophagy in rats. Food Chem. Toxicol. 2020, 137, 111179. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yun, J.; Park, B. Methamphetamine-induced neuronal damage: Neurotoxicity and neuroinflammation. Biomol. Ther. 2020, 28, 381. [Google Scholar] [CrossRef]
- Xue, Y.; He, J.-T.; Zhang, K.-K.; Chen, L.-J.; Wang, Q.; Xie, X.-L. Methamphetamine reduces expressions of tight junction proteins, rearranges F-actin cytoskeleton and increases the blood brain barrier permeability via the RhoA/ROCK-dependent pathway. Biochem. Biophys. Res. Commun. 2019, 509, 395–401. [Google Scholar] [CrossRef]
- Rudnick, G.; Clark, J. From synapse to vesicle: The reuptake and storage of biogenic amine neurotransmitters. Biochim. Biophys. Acta BBA-Bioenerg. 1993, 1144, 249–263. [Google Scholar] [CrossRef]
- Amara, S.G.; Kuhar, M.J. Neurotransmitter transporters: Recent progress. Annu. Rev. Neurosci. 1993, 16, 73–93. [Google Scholar] [CrossRef]
- Wright, T.E.; Schuetter, R.; Tellei, J.; Sauvage, L. Methamphetamines and pregnancy outcomes. J. Addict. Med. 2015, 9, 111–117. [Google Scholar] [CrossRef]
- Ganapathy, V.; Prasad, P.D.; Ganapathy, M.E.; Leibach, F.H. Drugs of abuse and placental transport. Adv. Drug Deliv. Rev. 1999, 38, 99–110. [Google Scholar] [CrossRef]
- Ganapathy, V.; Ramamoorthy, S.; Leibach, F.H. Transport and metabolism of monoamines in the human placenta: A review. Placenta 1993, 14, 35–51. [Google Scholar] [CrossRef]
- Bottalico, B.; Larsson, I.; Brodszki, J.; Hernandez-Andrade, E.; Casslén, B.; Marsal, K.; Hansson, S. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta 2004, 25, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, V. Drugs of abuse and human placenta. Life Sci. 2011, 88, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, S.V.; Sullivan, A.M.; O’keeffe, G.W. Midbrain dopaminergic neurons: A review of the molecular circuitry that regulates their development. Dev. Biol. 2013, 379, 123–138. [Google Scholar] [CrossRef]
- Abdelrahman, S.; Alsanie, W.F.; Khan, Z.N.; Albalawi, H.I.; Felimban, R.I.; Moretti, M.; Steiner, N.; Chaudhary, A.G.; Hauser, C.A. A Parkinson’s disease model composed of 3D bioprinted dopaminergic neurons within a biomimetic peptide scaffold. Biofabrication 2022, 14, 044103. [Google Scholar] [CrossRef] [PubMed]
- Susapto, H.H.; Alhattab, D.; Abdelrahman, S.; Khan, Z.; Alshehri, S.; Kahin, K.; Ge, R.; Moretti, M.; Emwas, A.-H.; Hauser, C.A. Ultrashort peptide bioinks support automated printing of large-scale constructs assuring long-term survival of printed tissue constructs. Nano Lett. 2021, 21, 2719–2729. [Google Scholar] [CrossRef]
- Johnson, M.M.; Michelhaugh, S.K.; Bouhamdan, M.; Schmidt, C.J.; Bannon, M.J. The transcription factor NURR1 exerts concentration-dependent effects on target genes mediating distinct biological processes. Front. Neurosci. 2011, 5, 135. [Google Scholar] [CrossRef]
- Kadkhodaei, B.; Alvarsson, A.; Schintu, N.; Ramsköld, D.; Volakakis, N.; Joodmardi, E.; Yoshitake, T.; Kehr, J.; Decressac, M.; Björklund, A. Transcription factor Nurr1 maintains fiber integrity and nuclear-encoded mitochondrial gene expression in dopamine neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 2360–2365. [Google Scholar] [CrossRef]
- Torretta, S.; Rampino, A.; Basso, M.; Pergola, G.; Di Carlo, P.; Shin, J.H.; Kleinman, J.E.; Hyde, T.M.; Weinberger, D.R.; Masellis, R. NURR1 and ERR1 modulate the expression of genes of a DRD2 coexpression network enriched for schizophrenia risk. J. Neurosci. 2020, 40, 932–941. [Google Scholar] [CrossRef]
- Alsanie, W.F.; Abdelrahman, S.; Alhomrani, M.; Gaber, A.; Alosimi, E.A.; Habeeballah, H.; Alkhatabi, H.A.; Felimban, R.I.; Hauser, C.A.; Tayeb, H.H. The Influence of Prenatal Exposure to Quetiapine Fumarate on the Development of Dopaminergic Neurons in the Ventral Midbrain of Mouse Embryos. Int. J. Mol. Sci. 2022, 23, 12352. [Google Scholar] [CrossRef]
- Van den Heuvel, D.M.; Pasterkamp, R.J. Getting connected in the dopamine system. Prog. Neurobiol. 2008, 85, 75–93. [Google Scholar] [CrossRef]
- Fernando, C.V.; Kele, J.; Bye, C.R.; Niclis, J.C.; Alsanie, W.; Blakely, B.D.; Stenman, J.; Turner, B.J.; Parish, C.L. Diverse roles for Wnt7a in ventral midbrain neurogenesis and dopaminergic axon morphogenesis. Stem Cells Dev. 2014, 23, 1991–2003. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.L.; Thompson, L.H. Modulating Wnt signaling to improve cell replacement therapy for Parkinson’s disease. J. Mol. Cell Biol. 2014, 6, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Alsanie, W.F.; Abdelrahman, S.; Alhomrani, M.; Gaber, A.; Habeeballah, H.; Alkhatabi, H.A.; Felimban, R.I.; Hauser, C.A.; Tayeb, H.H.; Alamri, A.S. Prenatal Exposure to Gabapentin Alters the Development of Ventral Midbrain Dopaminergic Neurons. Front. Pharmacol. 2022, 13, 2706. [Google Scholar] [CrossRef] [PubMed]
- Hobert, O.; Westphal, H. Functions of LIM-homeobox genes. Trends Genet. 2000, 16, 75–83. [Google Scholar] [CrossRef]
- Doucet-Beaupré, H.; Gilbert, C.; Profes, M.S.; Chabrat, A.; Pacelli, C.; Giguère, N.; Rioux, V.; Charest, J.; Deng, Q.; Laguna, A. Lmx1a and Lmx1b regulate mitochondrial functions and survival of adult midbrain dopaminergic neurons. Proc. Natl. Acad. Sci. USA 2016, 113, E4387–E4396. [Google Scholar] [CrossRef]
- Caiazzo, M.; Dell’Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011, 476, 224–227. [Google Scholar] [CrossRef]
- Doucet-Beaupré, H.; Lévesque, M. The role of developmental transcription factors in adult midbrain dopaminergic neurons. OA Neurosci 2013, 1, 3. [Google Scholar]
- Bergman, O.; Håkansson, A.; Westberg, L.; Belin, A.C.; Sydow, O.; Olson, L.; Holmberg, B.; Fratiglioni, L.; Bäckman, L.; Eriksson, E. Do polymorphisms in transcription factors LMX1A and LMX1B influence the risk for Parkinson’s disease? J. Neural Transm. 2009, 116, 333–338. [Google Scholar] [CrossRef]
- Hoekstra, E.J.; von Oerthel, L.; van der Heide, L.P.; Kouwenhoven, W.M.; Veenvliet, J.V.; Wever, I.; Jin, Y.-R.; Yoon, J.K.; van der Linden, A.J.; Holstege, F.C. Lmx1a encodes a rostral set of mesodiencephalic dopaminergic neurons marked by the Wnt/B-catenin signaling activator R-spondin 2. PLoS ONE 2013, 8, e74049. [Google Scholar] [CrossRef]
- Alsanie, W.; Penna, V.; Schachner, M.; Thompson, L.; Parish, C. Homophilic binding of the neural cell adhesion molecule CHL1 regulates development of ventral midbrain dopaminergic pathways. Sci. Rep. 2017, 7, 9368. [Google Scholar] [CrossRef]
- Krasnova, I.N.; Ladenheim, B.; Hodges, A.B.; Volkow, N.D.; Cadet, J.L. Chronic methamphetamine administration causes differential regulation of transcription factors in the rat midbrain. PLoS ONE 2011, 6, e19179. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Liu, S.; Yin, Y.; Li, S.; Li, Z.; Wu, X.; Zhang, B.; Ang, S.-L.; Ding, Y.; Zhou, J. Ventral mesencephalon-enriched genes that regulate the development of dopaminergic neurons in vivo. J. Neurosci. 2009, 29, 5170–5182. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, F.; De Gregorio, R.; Pulcrano, S.; Perrone-Capano, C.; di Porzio, U.; Bellenchi, G.C. Direct regulation of Pitx3 expression by Nurr1 in culture and in developing mouse midbrain. PLoS ONE 2012, 7, e30661. [Google Scholar] [CrossRef] [PubMed]
- -Rahman, A.-u.; Khalid, A.; Sultana, N.; Nabeel Ghayur, M.; Ahmed Mesaik, M.; Riaz Khan, M.; Gilani, A.H.; Iqbal Choudhary, M. New natural cholinesterase inhibiting and calcium channel blocking quinoline alkaloids. J. Enzym. Inhib. Med. Chem. 2006, 21, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.H.; Parish, C.L. Transplantation of fetal midbrain dopamine progenitors into a rodent model of Parkinson’s disease. Neural Progenit. Cells Methods Protoc. 2013, 1059, 169–180. [Google Scholar] [CrossRef]
- Alsanie, W.F.; Bahri, O.A.; Habeeballah, H.H.; Alhomrani, M.; Almehmadi, M.M.; Alsharif, K.; Felemban, E.M.; Althobaiti, Y.S.; Almalki, A.H.; Alsaab, H.O. Generating homogenous cortical preplate and deep-layer neurons using a combination of 2D and 3D differentiation cultures. Sci. Rep. 2020, 10, 6272. [Google Scholar] [CrossRef] [PubMed]
- Gygi, M.P.; Gygi, S.P.; Johnson, M.; Wilkins, D.G.; Gibb, J.W.; Hanson, G.R. Mechanisms for tolerance to methamphetamine effects. Neuropharmacology 1996, 35, 751–757. [Google Scholar] [CrossRef]
- Melega, W.P.; Cho, A.K.; Harvey, D.; Laćan, G. Methamphetamine blood concentrations in human abusers: Application to pharmacokinetic modeling. Synapse 2007, 61, 216–220. [Google Scholar] [CrossRef]
- McIntyre, I.M.; Nelson, C.L.; Schaber, B.; Hamm, C.E. Antemortem and postmortem methamphetamine blood concentrations: Three case reports. J. Anal. Toxicol. 2013, 37, 386–389. [Google Scholar] [CrossRef]
- Schulz, M.; Schmoldt, A. Therapeutic and toxic blood concentrations of more than 800 drugs and other xenobiotics. Die Pharm.-Int. J. Pharm. Sci. 2003, 58, 447–474. [Google Scholar]



| Gene Name | Primer Sequence 5 to 3 | |
|---|---|---|
| Gapdh | Forward primer: Reverse primer: | TGA AGG TCG GAG TCA ACG GA CCA ATT GAT GAC AAG CTT CCC G |
| Th | Forward primer: Reverse primer: | TGA AGG AAC GGA CTG GCT TC GAG TGC ATA GGT GAG GAG GC |
| Nurr1 | Forward primer: Reverse primer: | GAC CAG GAC CTG CTT TTT GA ACC CCA TTG CAA AAG ATG AG |
| Lmx1a | Forward primer: Reverse primer: | GAG ACC ACC TGC TTC TAC CG GCA CGC ATG ACA AAC TCA TT |
| En1 | Forward primer: Reverse primer: | TCA CAG CAA CCC CTA GTG TG CGC TTG TCT TCC TTC TCG TT |
| Pitx3 | Forward primer: Reverse primer: | CAT GGA GTT TGG GCT GCT TG CCT TCT CCG AGT CAC TGT GC |
| Chl1 | Forward primer: Reverse primer: | TGG AAT TGC CAT TAT GTG GA CAC CTG CAC GTA TGA CTG CT |
| Dat | Forward primer: Reverse primer: | TTG CAG CTG GCA CAT CTA TC ATG CTG ACC ACG ACC ACA TA |
| Drd2 | Forward primer: Reverse primer: | CTC AAC AAC ACA GAC CAG AAT GAA CGA GAC GAT GGA GGA |
| Bdnf | Forward primer: Reverse primer: | ACT ATG GTT ATT TCA TAC TTC GGT T CCA TTC ACG CTC TCC AGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsanie, W.F.; Abdelrahman, S.; Felimban, R.I.; Alkhatabi, H.A.; Gaber, A.; Alosimi, E.A.; Alhomrani, M.; Habeeballah, H.; Hauser, C.A.E.; S. Alamri, A.; et al. The Influence of Prenatal Exposure to Methamphetamine on the Development of Dopaminergic Neurons in the Ventral Midbrain. Int. J. Mol. Sci. 2023, 24, 5668. https://doi.org/10.3390/ijms24065668
Alsanie WF, Abdelrahman S, Felimban RI, Alkhatabi HA, Gaber A, Alosimi EA, Alhomrani M, Habeeballah H, Hauser CAE, S. Alamri A, et al. The Influence of Prenatal Exposure to Methamphetamine on the Development of Dopaminergic Neurons in the Ventral Midbrain. International Journal of Molecular Sciences. 2023; 24(6):5668. https://doi.org/10.3390/ijms24065668
Chicago/Turabian StyleAlsanie, Walaa F., Sherin Abdelrahman, Raed I. Felimban, Heba A. Alkhatabi, Ahmed Gaber, Ebtisam Abdulah Alosimi, Majid Alhomrani, Hamza Habeeballah, Charlotte A. E. Hauser, Abdulhakeem S. Alamri, and et al. 2023. "The Influence of Prenatal Exposure to Methamphetamine on the Development of Dopaminergic Neurons in the Ventral Midbrain" International Journal of Molecular Sciences 24, no. 6: 5668. https://doi.org/10.3390/ijms24065668
APA StyleAlsanie, W. F., Abdelrahman, S., Felimban, R. I., Alkhatabi, H. A., Gaber, A., Alosimi, E. A., Alhomrani, M., Habeeballah, H., Hauser, C. A. E., S. Alamri, A., Althobaiti, A., Alsharif, A., Alzahrani, A. S., Al-Ghamdi, M. S., Raafat, B. M., Alswat, K. A., Althobaiti, Y. S., & Asiri, Y. A. (2023). The Influence of Prenatal Exposure to Methamphetamine on the Development of Dopaminergic Neurons in the Ventral Midbrain. International Journal of Molecular Sciences, 24(6), 5668. https://doi.org/10.3390/ijms24065668







