The Mechanism of the Channel Opening in Channelrhodopsin-2: A Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Results and Discussion
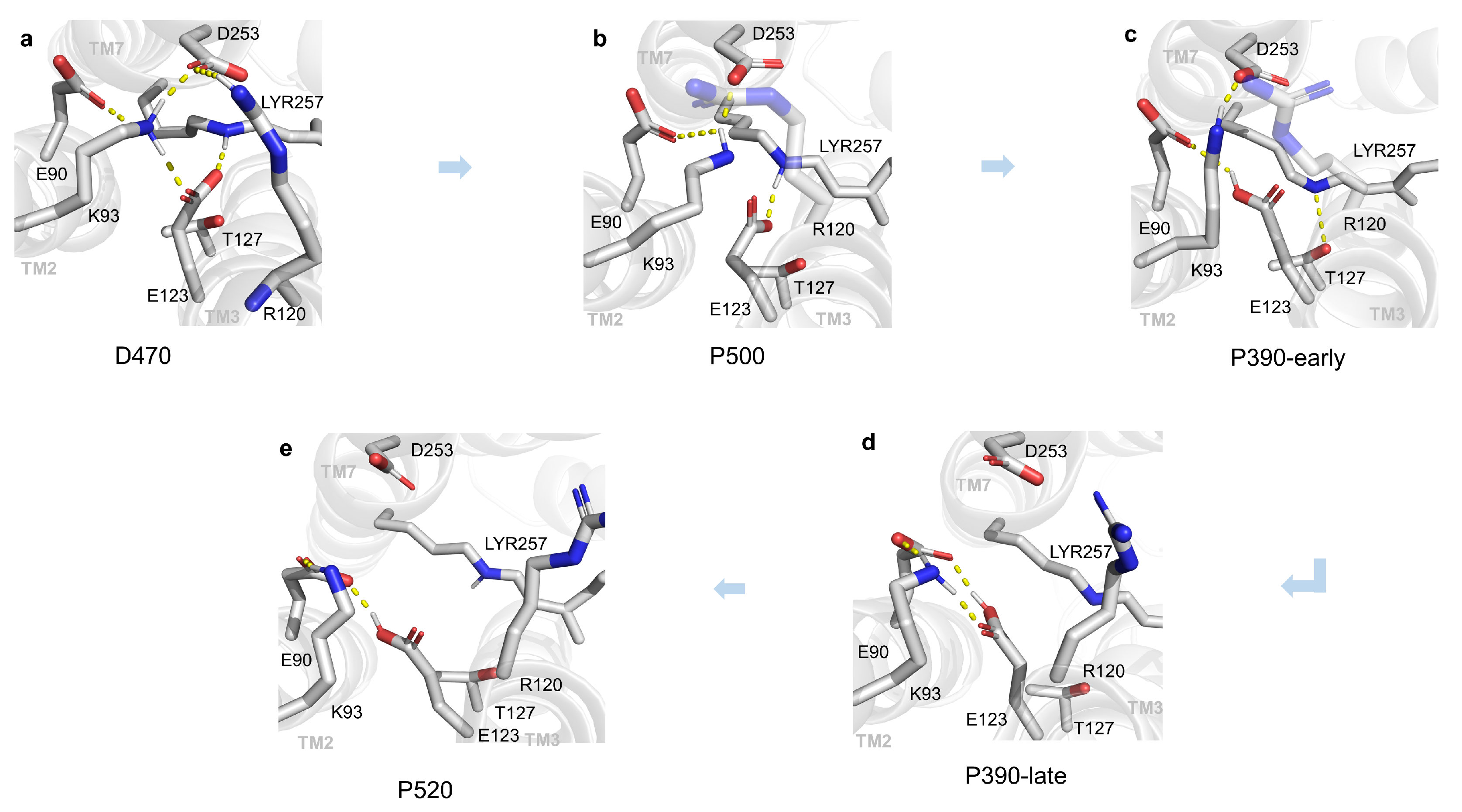
2.1. Structural Changes during the Opening of the Ion Channel
2.2. Compared with C1C2 Crystal Structures
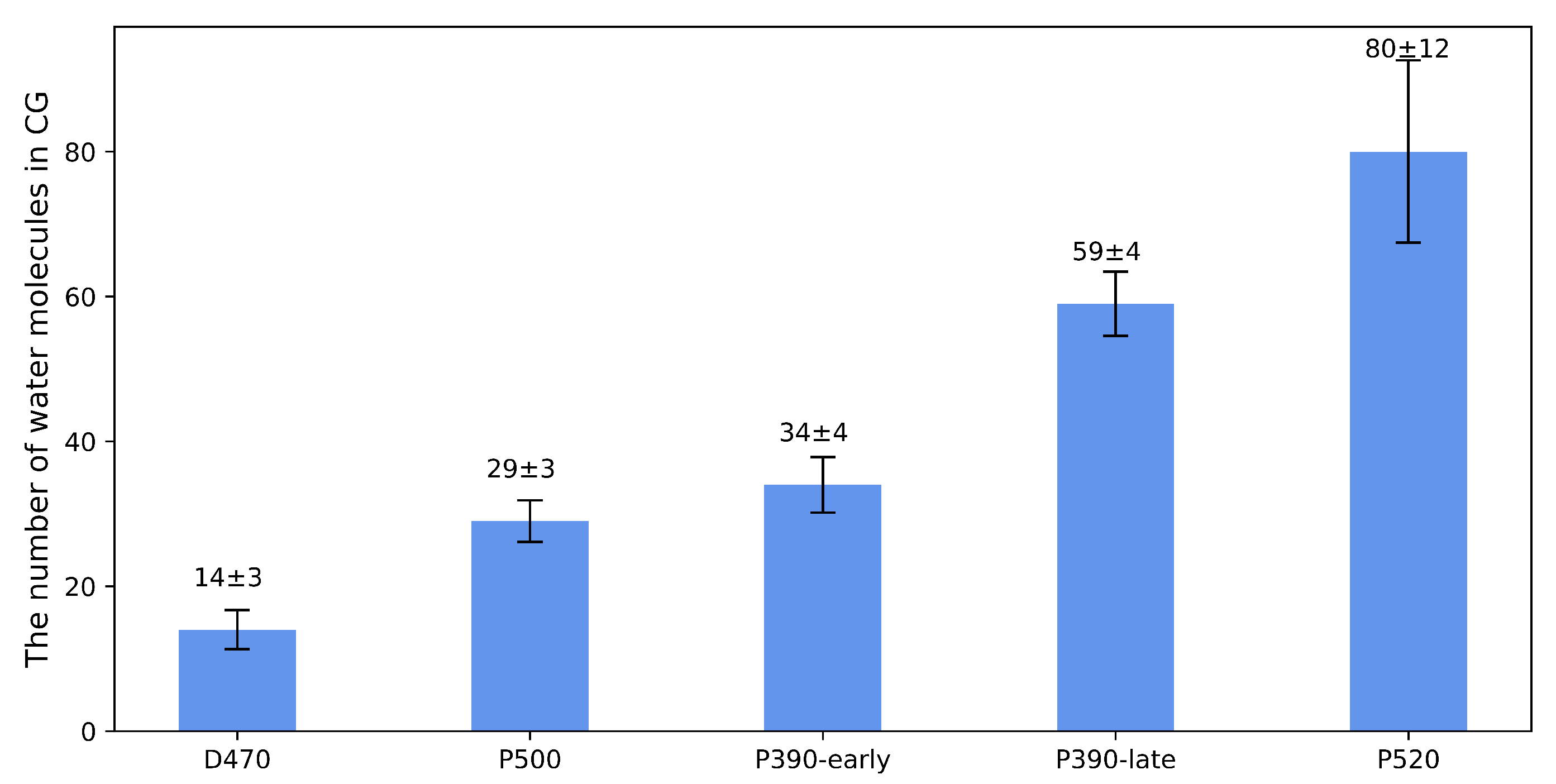
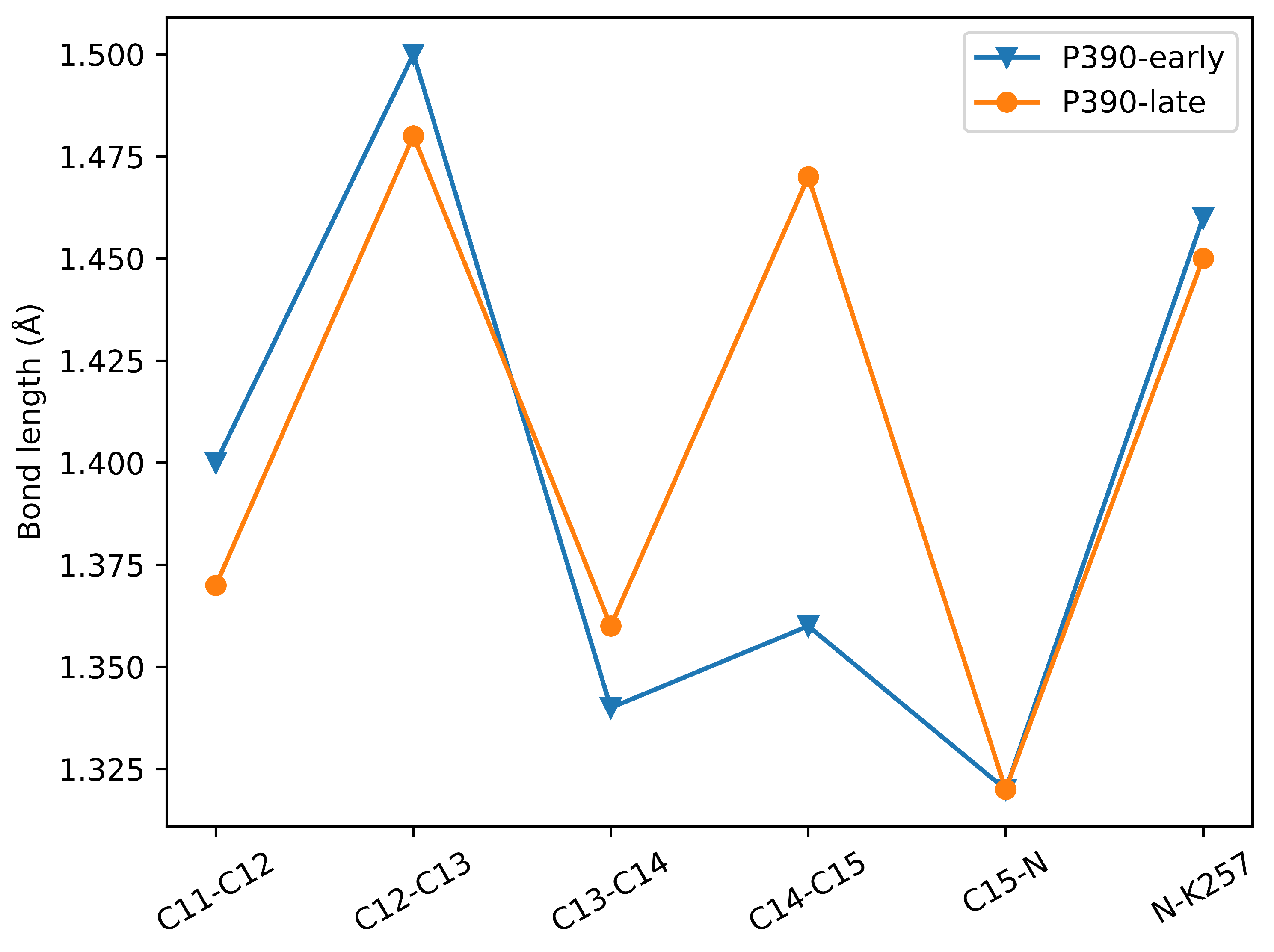
2.3. P390-Early and P390-Late
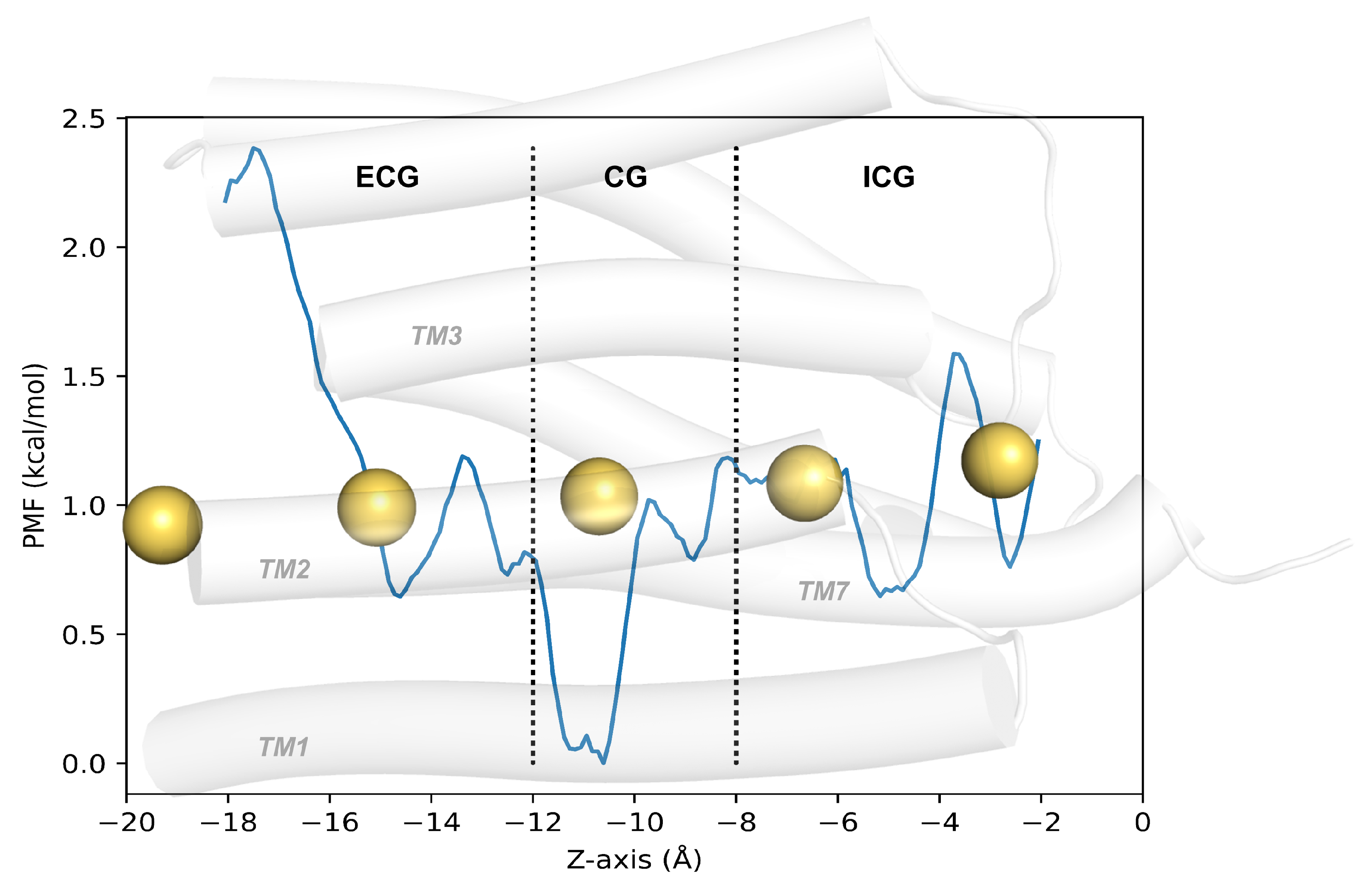
2.4. Verification of Channel Openness
3. Materials and Methods
3.1. MD Simulations
3.2. Modeling D470
3.3. Modeling P500
3.4. Modeling P390 and P520
3.5. Calculate Maximum Absorption Wavelength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ChR2 | Channelrhodopsin-2 |
| ChRs | Channelrhodopsins |
| MD | Molecular Dynamics |
| SMD | Steered Molecular Dynamics |
| TM | Transmembrane helix |
| ECG | Extracellular Gate |
| CG | Central Gate |
| ICG | Intracellular Gate |
| RSBH | Protonated Retinal Schiff Base |
| RMSD | Root Mean Square Deviation |
| PMF | Potential of Mean Force |
References
- Deisseroth, K. Optogenetics. Nat. Methods 2011, 8, 26–29. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Volkov, O.; Kovalev, K.; Polovinkin, V.; Borshchevskiy, V.; Bamann, C.; Astashkin, R.; Marin, E.; Popov, A.; Balandin, T.; Willbold, D.; et al. Structural insights into ion conduction by channelrhodopsin 2. Science 2017, 358, eaan8862. [Google Scholar] [CrossRef] [PubMed]
- Ernst, O.P.; Murcia, P.A.S.; Daldrop, P.; Tsunoda, S.P.; Kateriya, S.; Hegemann, P. Photoactivation of channelrhodopsin. J. Biol. Chem. 2008, 283, 1637–1643. [Google Scholar] [CrossRef] [Green Version]
- Stehfest, K.; Hegemann, P. Evolution of the channelrhodopsin photocycle model. ChemPhysChem 2010, 11, 1120–1126. [Google Scholar] [CrossRef]
- Nikolic, K.; Grossman, N.; Grubb, M.S.; Burrone, J.; Toumazou, C.; Degenaar, P. Photocycles of channelrhodopsin-2. Photochem. Photobiol. 2009, 85, 400–411. [Google Scholar] [CrossRef]
- Ardevol, A.; Hummer, G. Retinal isomerization and water-pore formation in channelrhodopsin-2. Proc. Natl. Acad. Sci. USA 2018, 115, 3557–3562. [Google Scholar] [CrossRef] [Green Version]
- Kuhne, J.; Vierock, J.; Tennigkeit, S.A.; Dreier, M.A.; Wietek, J.; Petersen, D.; Gavriljuk, K.; El-Mashtoly, S.F.; Hegemann, P.; Gerwert, K. Unifying photocycle model for light adaptation and temporal evolution of cation conductance in channelrhodopsin-2. Proc. Natl. Acad. Sci. USA 2019, 116, 9380–9389. [Google Scholar] [CrossRef] [Green Version]
- Lórenz-Fonfría, V.A.; Bamann, C.; Resler, T.; Schlesinger, R.; Bamberg, E.; Heberle, J. Temporal evolution of helix hydration in a light-gated ion channel correlates with ion conductance. Proc. Natl. Acad. Sci. USA 2015, 112, E5796–E5804. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.G.; Sun, B.Q.; Bizounok, M.; Hatcher, M.E.; Lansing, J.C.; Raap, J.; Verdegem, P.J.; Lugtenburg, J.; Griffin, R.G.; Herzfeld, J. Early and late M intermediates in the bacteriorhodopsin photocycle: A solid-state NMR study. Biochemistry 1998, 37, 8088–8096. [Google Scholar] [CrossRef]
- Schneider, F.; Grimm, C.; Hegemann, P. Biophysics of channelrhodopsin. Annu. Rev. Biophys. 2015, 44, 167–186. [Google Scholar] [CrossRef] [Green Version]
- Becker-Baldus, J.; Leeder, A.; Brown, L.J.; Brown, R.C.; Bamann, C.; Glaubitz, C. The Desensitized Channelrhodopsin-2 Photointermediate Contains 13-cis, 15-syn Retinal Schiff Base. Angew. Chem. 2021, 133, 16578–16583. [Google Scholar] [CrossRef]
- Subramaniam, S.; Henderson, R. Molecular mechanism of vectorial proton translocation by bacteriorhodopsin. Nature 2000, 406, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Hosaka, T.; Kojima, K.; Nishimura, Y.; Nakajima, Y.; Kimura-Someya, T.; Shirouzu, M.; Sudo, Y.; Yoshizawa, S. A unique clade of light-driven proton-pumping rhodopsins evolved in the cyanobacterial lineage. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wietek, J.; Wiegert, J.S.; Adeishvili, N.; Schneider, F.; Watanabe, H.; Tsunoda, S.P.; Vogt, A.; Elstner, M.; Oertner, T.G.; Hegemann, P. Conversion of channelrhodopsin into a light-gated chloride channel. Science 2014, 344, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, J.; Eisenhauer, K.; Ritter, E.; Hegemann, P.; Gerwert, K.; Bartl, F. Early Formation of the Ion-Conducting Pore in Channelrhodopsin-2. Angew. Chem. Int. Ed. 2015, 54, 4953–4957. [Google Scholar] [CrossRef]
- Kato, H.E.; Zhang, F.; Yizhar, O.; Ramakrishnan, C.; Nishizawa, T.; Hirata, K.; Ito, J.; Aita, Y.; Tsukazaki, T.; Hayashi, S.; et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 2012, 482, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Zhang, W.; Zhou, S.; Ran, X.; Shang, Y.; Lo, G.V.; Dou, Y.; Yuan, S. The effect on ion channel of different protonation states of E90 in channelrhodopsin-2: A molecular dynamics simulation. RSC Adv. 2021, 11, 14542–14551. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [Green Version]
- Lórenz-Fonfría, V.A.; Resler, T.; Krause, N.; Nack, M.; Gossing, M.; Fischer von Mollard, G.; Bamann, C.; Bamberg, E.; Schlesinger, R.; Heberle, J. Transient protonation changes in channelrhodopsin-2 and their relevance to channel gating. Proc. Natl. Acad. Sci. USA 2013, 110, E1273–E1281. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Andersen, O.S.; Roux, B. Energetics of double-ion occupancy in the gramicidin A channel. J. Phys. Chem. B 2010, 114, 13881–13888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.Y.; Lin, M.Z.; Steinbach, P.; Tsien, R.Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 2009, 96, 1803–1814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oda, K.; Nomura, T.; Nakane, T.; Yamashita, K.; Inoue, K.; Ito, S.; Vierock, J.; Hirata, K.; Maturana, A.D.; Katayama, K.; et al. Time-resolved serial femtosecond crystallography reveals early structural changes in channelrhodopsin. Elife 2021, 10, e62389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.C.; Zhou, Y.; Cao, C. Proton transfer during class-A GPCR activation: Do the CWxP motif and the membrane potential act in concert? Biophys. Rep. 2018, 4, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Rappleye, M.; Berndt, A. Structural basis for ion selectivity and engineering in channelrhodopsins. Curr. Opin. Struct. Biol. 2019, 57, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhang, W.; Cheng, J.; Nie, Y.; Xin, Q.; Yuan, S.; Dou, Y. Formation Mechanism of Ion Channel in Channelrhodopsin-2: Molecular Dynamics Simulation and Steering Molecular Dynamics Simulations. Int. J. Mol. Sci. 2019, 20, 3780. [Google Scholar] [CrossRef] [Green Version]
- Izrailev, S.; Stepaniants, S.; Isralewitz, B.; Kosztin, D.; Lu, H.; Molnar, F.; Wriggers, W.; Schulten, K. Steered molecular dynamics. In Computational Molecular Dynamics: Challenges, Methods, Ideas; Springer: Berlin/Heidelberg, Germany, 1999; pp. 39–65. [Google Scholar]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Brown, M.F.; Feller, S.E. Retinal conformation governs p K a of protonated Schiff base in rhodopsin activation. J. Am. Chem. Soc. 2013, 135, 9391–9398. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.E.; Kim, Y.S.; Paggi, J.M.; Evans, K.E.; Allen, W.E.; Richardson, C.; Inoue, K.; Ito, S.; Ramakrishnan, C.; Fenno, L.E.; et al. Structural mechanisms of selectivity and gating in anion channelrhodopsins. Nature 2018, 561, 349–354. [Google Scholar] [CrossRef]
- Hammonds, K.D.; Ryckaert, J.P. On the convergence of the SHAKE algorithm. Comput. Phys. Commun. 1991, 62, 336–351. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Kästner, J. Umbrella sampling. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 932–942. [Google Scholar] [CrossRef]
- Case, D.; Ben-Shalom, I.; Brozell, S.; Cerutti, D.; Cheatham III, T.; Cruzeiro, V.; Darden, T.; Duke, R.; Ghoreishi, D.; Gilson, M.; et al. AMBER; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Goddard, T.D.; Huang, C.C.; Ferrin, T.E. Software extensions to UCSF chimera for interactive visualization of large molecular assemblies. Structure 2005, 13, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Bamann, C.; Gueta, R.; Kleinlogel, S.; Nagel, G.; Bamberg, E. Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry 2010, 49, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Ritter, E.; Stehfest, K.; Berndt, A.; Hegemann, P.; Bartl, F.J. Monitoring light-induced structural changes of Channelrhodopsin-2 by UV-visible and Fourier transform infrared spectroscopy. J. Biol. Chem. 2008, 283, 35033–35041. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.H.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical p K a predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision D.01.; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adam, S.; Wiebeler, C.; Schapiro, I. Structural factors determining the absorption spectrum of channelrhodopsins: A Case study of the chimera C1C2. J. Chem. Theory Comput. 2021, 17, 6302–6313. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, Q.; Zhang, W.; Yuan, S. The Mechanism of the Channel Opening in Channelrhodopsin-2: A Molecular Dynamics Simulation. Int. J. Mol. Sci. 2023, 24, 5667. https://doi.org/10.3390/ijms24065667
Xin Q, Zhang W, Yuan S. The Mechanism of the Channel Opening in Channelrhodopsin-2: A Molecular Dynamics Simulation. International Journal of Molecular Sciences. 2023; 24(6):5667. https://doi.org/10.3390/ijms24065667
Chicago/Turabian StyleXin, Qi, Wenying Zhang, and Shuai Yuan. 2023. "The Mechanism of the Channel Opening in Channelrhodopsin-2: A Molecular Dynamics Simulation" International Journal of Molecular Sciences 24, no. 6: 5667. https://doi.org/10.3390/ijms24065667
APA StyleXin, Q., Zhang, W., & Yuan, S. (2023). The Mechanism of the Channel Opening in Channelrhodopsin-2: A Molecular Dynamics Simulation. International Journal of Molecular Sciences, 24(6), 5667. https://doi.org/10.3390/ijms24065667






