High Resolution and Automatable Cytogenetic Biodosimetry Using In Situ Telomere and Centromere Hybridization for the Accurate Detection of DNA Damage: An Overview
Abstract
:1. Introduction
2. Cytogenetic Markers of Irradiation
- (1)
- Direct molecular consequences such as single and double-strand breaks in the DNA molecule detected by fluorochrome-labeled antibodies specific for, e.g., γH2AX, MR11 proteins, or p53-binding protein (53BP1) that are markers of double-strand breaks (DSB) [19,20]. The preparation and analysis of the immunostainings can be automated [4,21]. The proteins are useful markers of DSB (Table 1) in analyses of putative biological effects of low doses of irradiation [22] and for the triage of exposed populations [23,24]. In addition, immunostaining can be used to assess interindividual sensitivity to radiation [25]. However, the specificity of the abovementioned markers to irradiation, the lack of stability of the fluorescent signals over time, the standardization of the technique, and the interindividual variation constitute the main drawbacks for their use in biological dosimetry [26,27].
- (2)
- The kinetics of DNA break repair is monitored in prematurely condensed chromosomes (PCCs) in the form of single-stranded filaments. PCCs can be observed by ordinary light microscopy or by fluorescence microscopy after staining with appropriate DNA dyes [28,29]; however, the techniques are subject to significant constraints and low stability of the acentric breaks over time, which limit their use in particular investigations.
- (3)
- The consequences of DNA misrepair on the integrity of chromosomes at the time of the first postirradiation cell division or during the second interphase. Such failures resulting in more or less stable dicentrics, translocations, acentric fragments, or micronuclei can be visualized microscopically by the employment of relevant cytogenetic techniques of high sensitivity, specificity, and accuracy. Moreover, the techniques are automatable and easy to implement.
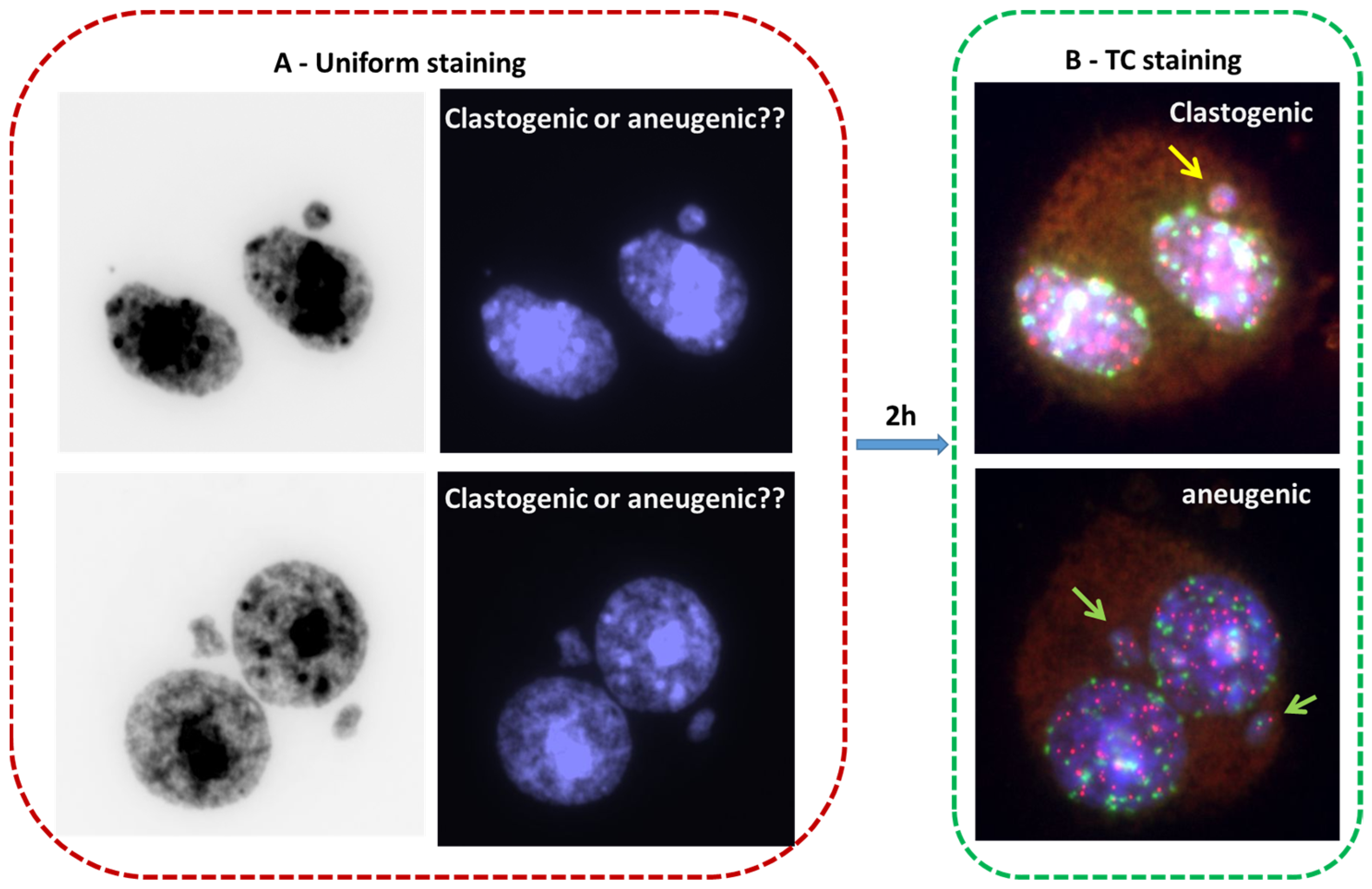
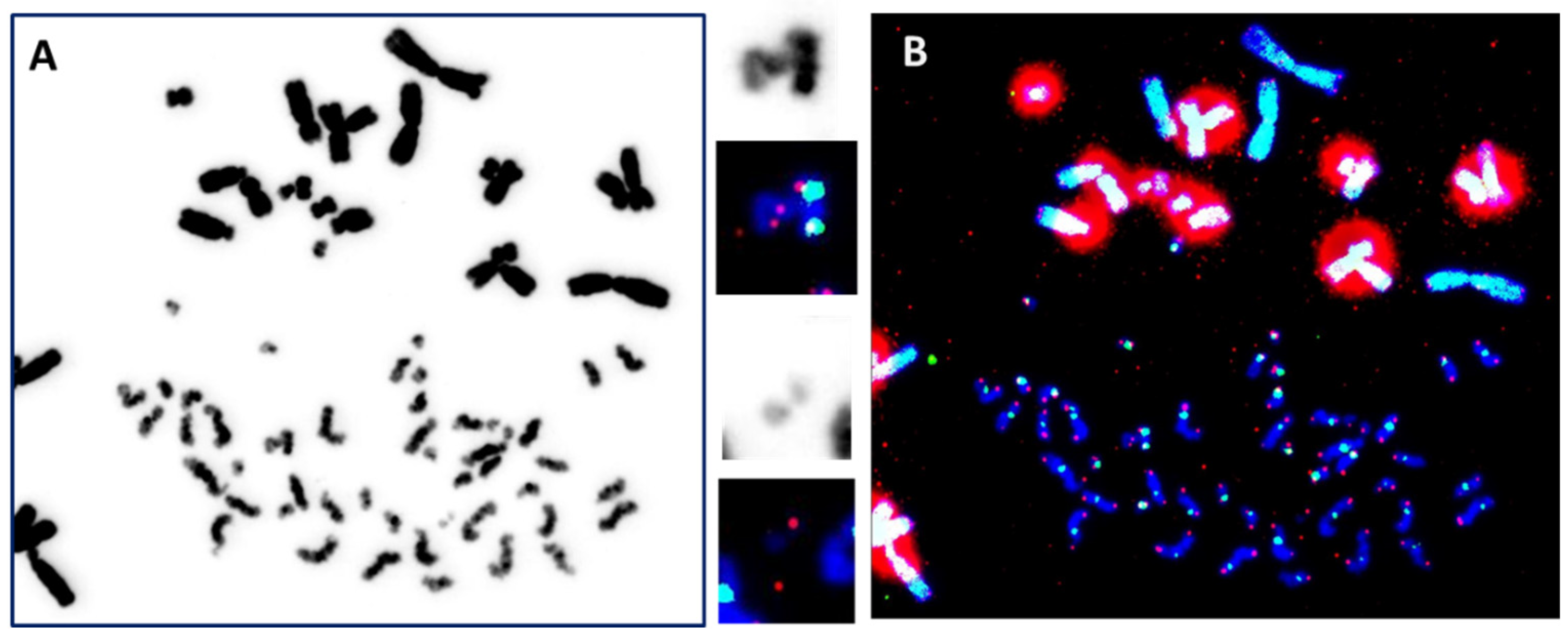
3. TC Staining Allows Insight into Mechanisms of Formation of Chromosome Aberrations
4. Employment of TC Staining Adds Distinctive Value to Commonly Used Cytogenetic Techniques
4.1. The “Gold Standard” Technique
4.2. Detection of Translocations
4.3. The Cytokinesis-Blocked Micronucleus Assay (CBMN)
4.4. Premature Chromosome Condensation (PCC) Assay
5. Automation of Biological Dosimetry Methods
6. Application of Cytogenetic Tools in a Wider Spectrum of Fields in Medicine and Biology
7. Advantages and Limitations of Current Cytogenetic Biomarkers of Ionizing Radiation
7.1. Specificity of Cytogenetic Biodosimetry Markers
7.2. Challenges in Dose Estimation
- The spontaneous rate of chromosomal aberrations in the general population is an important factor in cytogenetic biodosimetry because it can influence the interpretation of data. Thus, DNA damage can also be induced by occupational activities, lifestyle, and environmental factors [91]. Furthermore, there are variations in natural, terrestrial, and cosmic radioactivity, which differ from region to region and from country to country [92]. Finally, the worldwide increase in exposure to magnetic fields such as the use of mobile phones and the multiplicity of the employment of ionizing radiation in industry and medicine may have contributed to significant variations [93]. The latest evaluation of the frequencies of spontaneous chromosome aberrations dates back several decades. Since then, a vast amount of insight has accumulated into the causes and frequencies of chromosome aberrations. Moreover, significant technical improvements in their detection have been achieved. Therefore, the re-examination of the frequency of spontaneous chromosomal aberrations in the general population is urgently needed. For that purpose, an automated TC+M-FISH approach would be most relevant.
- The distribution of aberrations according to age and sex is not clear and differs from one study to another. Recently, several studies have demonstrated the difference in genotoxic stress response according to sex [94,95,96]. Notably, the used dose-response curves did not take into account the age or sex of the exposed population.
- The interpretation of complex chromosomal rearrangements in the estimation of the absorbed dose has always been challenging in biological dosimetry. A significant correlation has been found between the formation of complex chromosomal rearrangements and the clinical outcome of patients treated with radiotherapy [97,98]. The presence of these kinds of aberrations has also been correlated with interindividual radiation sensitivity and genomic instability [99]. The lack of analysis of complex chromosomal rearrangement in a large cohort of an exposed population using a sensitive technique did not make it possible to advance our knowledge regarding their formations and their interpretations.
- The interpretation of “Rogue cells” in the analysis of chromosomal aberrations and the estimation of the dose after exposure is still unclear. “Rogue cells” are cells with multiple and complex chromosomal aberrations (e.g., dicentric, tricentric, translocations, insertions, deletions, and acentric chromosomes) related to the activation of viral infection [100,101]. A significant increase in induced chromosomal aberrations has been detected in the presence of rogue cells [102,103,104,105]. Further studies are needed to investigate the role of viral infection in the formation of radiation-induced chromosomal aberrations.
7.3. Relevant Questions for Cytogenetic Biological Dosimetry Assays
- -
- How can we validly relate lymphocyte lifetime and (re)circulation to partial exposure and thus introduce useful correction factors in the estimation of an absorbed dose of ionizing radiation?
- -
- What value can be attributed to translocation analysis in the concept of dose, especially decades or years after a potential exposure?
- -
- How do we coordinate all of the biological and biophysical markers available to the dosimetrist into a coherent entity, integrating the concept of multiparameter analyses?
- -
- How do we integrate new developments (genomics, proteomics, and transcriptomics) that, without renovating the current biotechnological landscape, make it possible to associate physiopathology more globally with genomic instability?
- -
- How do we integrate modulation phenomena, such as cellular and tissue radiosensitivity, radiation adaptation, and abscopal or bystander effects, that are still incompletely understood?
7.4. Internal Exposure
8. New Challenges for the Use of Biological Dosimetry in Detecting Carcinogenesis Susceptibility
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IAEA. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies: A Manual; IAEA: Vienna, Austria, 2013. [Google Scholar]
- Sproull, M.; Camphausen, K. State-of-the-Art Advances in Radiation Biodosimetry for Mass Casualty Events Involving Radiation Exposure. Radiat. Res. 2016, 186, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, J.M.; Prasanna, P.G.; Grace, M.B.; Wathen, L.K.; Wallace, R.L.; Koerner, J.F.; Coleman, C.N. Assessment of biodosimetry methods for a mass-casualty radiological incident: Medical response and management considerations. Health Phys. 2013, 105, 540–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsbury, E.A.; Moquet, J. The future of biological dosimetry in mass casualty radiation emergency response, personalized radiation risk estimation and space radiation protection. Int. J. Radiat. Biol. 2022, 98, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Pernot, E.; Hall, J.; Baatout, S.; Benotmane, M.A.; Blanchardon, E.; Bouffler, S.; El Saghire, H.; Gomolka, M.; Guertler, A.; Harms-Ringdahl, M.; et al. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat. Res. 2012, 751, 258–286. [Google Scholar] [CrossRef]
- Edwards, A.; Voisin, P.; Sorokine-Durm, I.; Maznik, N.; Vinnikov, V.; Mikhalevich, L.; Moquet, J.; Lloyd, D.; Delbos, M.; Durand, V. Biological estimates of dose to inhabitants of Belarus and Ukraine following the Chernobyl accident. Radiat. Prot. Dosim. 2004, 111, 211–219. [Google Scholar] [CrossRef]
- IAEA. Cytogenetic dosimetry applications in preparedness for and response to radiation emergencies. In EPR-Biodose; IAEA: Vienna, Austria, 2011. [Google Scholar]
- IAEA. Biological Dosimetry: Chromosomal aberration analysis for dose assessment. In Technical Report series N°260; IAEA: Vienna, Austria, 1986. [Google Scholar]
- IAEA. Cytogenetic Analysis for Radiation Dose Assessment. In Technical Report Series; IAEA: Vienna, Austria, 2001. [Google Scholar]
- Shi, L.; Fujioka, K.; Sun, J.; Kinomura, A.; Inaba, T.; Ikura, T.; Ohtaki, M.; Yoshida, M.; Kodama, Y.; Livingston, G.K.; et al. A modified system for analyzing ionizing radiation-induced chromosome abnormalities. Radiat. Res. 2012, 177, 533–538. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Maalouf, E.E.; Ricoul, M.; Heidingsfelder, L.; Laplagne, E.; Cuceu, C.; Hempel, W.M.; Colicchio, B.; Dieterlen, A.; Sabatier, L. New tool for biological dosimetry: Reevaluation and automation of the gold standard method following telomere and centromere staining. Mutat. Res. 2014, 770, 45–53. [Google Scholar] [CrossRef]
- M’Kacher, R.; El Maalouf, E.; Terzoudi, G.; Ricoul, M.; Heidingsfelder, L.; Karachristou, I.; Laplagne, E.; Hempel, W.M.; Colicchio, B.; Dieterlen, A.; et al. Detection and automated scoring of dicentric chromosomes in nonstimulated lymphocyte prematurely condensed chromosomes after telomere and centromere staining. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 640–649. [Google Scholar] [CrossRef]
- Gotoh, E. G2 Premature Chromosome Condensation/Chromosome Aberration Assay: Drug-Induced Premature Chromosome Condensation (PCC) Protocols and Cytogenetic Approaches in Mitotic Chromosome and Interphase Chromatin for Radiation Biology. Methods Mol Biol. 2019, 1984, 47–60. [Google Scholar] [CrossRef]
- Zaguia, N.; Laplagne, E.; Colicchio, B.; Cariou, O.; Al Jawhari, M.; Heidingsfelder, L.; Hempel, W.M.; Jrad, B.B.H.; Jeandidier, E.; Dieterlen, A.; et al. A new tool for genotoxic risk assessment: Reevaluation of the cytokinesis-block micronucleus assay using semi-automated scoring following telomere and centromere staining. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2020, 850–851, 503143. [Google Scholar] [CrossRef] [PubMed]
- Soumboundou, M.; Dossou, J.; Kalaga, Y.; Nkengurutse, I.; Faye, I.; Guingani, A.; Gadji, M.; Yameogo, K.J.; Zongo, H.; Mbaye, G.; et al. Is Response to Genotoxic Stress Similar in Populations of African and European Ancestry? A Study of Dose-Response After in vitro Irradiation. Front. Genet. 2021, 12, 657999. [Google Scholar] [CrossRef]
- Soumboundou, M.; Nkengurutse, I.; Dossou, J.; Colicchio, B.; Djebou, C.; Gadji, M.; Houenon, G.; Dem, A.; Dedjan, A.; Diarra, M.; et al. Biological Dosimetry Network in Africa: Establishment of a Dose-Response Curve Using Telomere and Centromere Staining. Health Phys. 2019, 117, 618–624. [Google Scholar] [CrossRef]
- M’Kacher, R.; Colicchio, B.; Borie, C.; Junker, S.; Marquet, V.; Heidingsfelder, L.; Soehnlen, K.; Najar, W.; Hempel, W.M.; Oudrhiri, N.; et al. Telomere and Centromere Staining Followed by M-FISH Improves Diagnosis of Chromosomal Instability and Its Clinical Utility. Genes 2020, 11, 475. [Google Scholar] [CrossRef]
- M’Kacher, R.; Miguet, M.; Maillard, P.Y. A Central Role of Telomere Dysfunction in the Formation of a Unique Translocation within the Sub-Telomere Region Resulting in Duplication and Partial Trisomy. Genes 2020, 13, 1762. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, R.K.; Bhat, N.N.; Gaur, N.; Shirsath, K.B.; Desai, U.N.; Sapra, B.K. Establishment and multiparametric-cytogenetic validation of (60)Co-gamma-ray induced, phospho-gamma-H2AX calibration curve for rapid biodosimetry and triage management during radiological emergencies. Mutat. Res. Toxicol. Environ. Mutagen. 2021, 866, 503354. [Google Scholar] [CrossRef]
- Blakely, W.F.; Port, M.; Abend, M. Early-response multiple-parameter biodosimetry and dosimetry: Risk predictions. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2021, 41, R152–R175. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Cairncross, S.; Miles, X.; Engelbrecht, M.; du Plessis, P.; Bolcaen, J.; Fisher, R.; Ndimba, R.; Cunningham, C.; Martínez-López, W.; et al. An Automated Microscopic Scoring Method for the γ-H2AX Foci Assay in Human Peripheral Blood Lymphocytes. J. Vis. Exp. 2021, 178. [Google Scholar] [CrossRef]
- Jakl, L.; Marková, E.; Koláriková, L.; Belyaev, I. Biodosimetry of Low Dose Ionizing Radiation Using DNA Repair Foci in Human Lymphocytes. Genes 2020, 11, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raavi, V.; Perumal, V.; Paul, S.F. Potential application of γ-H2AX as a biodosimetry tool for radiation triage. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108350. [Google Scholar] [CrossRef]
- Viau, M.; Testard, I.; Shim, G.; Morat, L.; Normil, M.D.; Hempel, W.M.; Sabatier, L. Global quantification of γH2AX as a triage tool for the rapid estimation of received dose in the event of accidental radiation exposure. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 793, 123–131. [Google Scholar] [CrossRef]
- Penninckx, S.; Pariset, E.; Cekanaviciute, E. Quantification of radiation-induced DNA double strand break repair foci to evaluate and predict biological responses to ionizing radiation. NAR Cancer 2021, 3, zcab046. [Google Scholar] [CrossRef]
- Moquet, J.; Barnard, S.; Staynova, A.; Lindholm, C.; Monteiro Gil, O.; Martins, V.; Rößler, U.; Vral, A.; Vandevoorde, C.; Wojewódzka, M.; et al. The second gamma-H2AX assay inter-comparison exercise carried out in the framework of the European biodosimetry network (RENEB). Int. J. Radiat. Biol. 2017, 93, 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valdiglesias, V.; Giunta, S.; Fenech, M.; Neri, M.; Bonassi, S. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat. Res. 2013, 753, 24–40. [Google Scholar] [CrossRef]
- Pantelias, G.E.; Maillie, H.D. The measurement of immediate and persistent radiation-induced chromosome damage in rodent primary cells using premature chromosome condensation. Health Phys. 1985, 49, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hatzi, V.I.; Terzoudi, G.I.; Paraskevopoulou, C.; Makropoulos, V.; Matthopoulos, D.P.; Pantelias, G.E. The use of premature chromosome condensation to study in interphase cells the influence of environmental factors on human genetic material. Sci. World J. 2006, 6, 1174–1190. [Google Scholar] [CrossRef] [Green Version]
- Awa, A.A. Persistent chromosome aberrations in the somatic cells of A-bomb survivors, Hiroshima and Nagasaki. J. Radiat. Res. 1991, 32 (Suppl. S1), 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Dorp, W.; Haupt, R.; Anderson, R.A.; Mulder, R.L.; van den Heuvel-Eibrink, M.M.; van Dulmen-den Broeder, E.; Su, H.I.; Winther, J.F.; Hudson, M.M.; Levine, J.M.; et al. Reproductive Function and Outcomes in Female Survivors of Childhood, Adolescent, and Young Adult Cancer: A Review. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, O.; Boyd, H.A.; Giwercman, A.; Lindholm, M.; Jensen, A.; Kjær, S.K.; Anderson, H.; Cavallin-Ståhl, E.; Rylander, L. Risk of birth abnormalities in the offspring of men with a history of cancer: A cohort study using Danish and Swedish national registries. J. Natl. Cancer Inst. 2011, 103, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Signorello, L.B.; Mulvihill, J.J.; Green, D.M.; Munro, H.M.; Stovall, M.; Weathers, R.E.; Mertens, A.C.; Whitton, J.A.; Robison, L.L.; Boice, J.D., Jr. Congenital anomalies in the children of cancer survivors: A report from the childhood cancer survivor study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 239–245. [Google Scholar] [CrossRef]
- Reulen, R.C.; Zeegers, M.P.; Wallace, W.H.; Frobisher, C.; Taylor, A.J.; Lancashire, E.R.; Winter, D.L.; Hawkins, M.M. Pregnancy outcomes among adult survivors of childhood cancer in the British Childhood Cancer Survivor Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2239–2247. [Google Scholar] [CrossRef] [Green Version]
- Little, M.P.; Goodhead, D.T.; Bridges, B.A.; Bouffler, S.D. Evidence relevant to untargeted and transgenerational effects in the offspring of irradiated parents. Mutat. Res. 2013, 753, 50–67. [Google Scholar] [CrossRef] [Green Version]
- Jordan, B.R. The Hiroshima/Nagasaki Survivor Studies: Discrepancies Between Results and General Perception. Genetics 2016, 203, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T. Transgenerational carcinogenesis: Induction and transmission of genetic alterations and mechanisms of carcinogenesis. Mutat. Res. 2003, 544, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Straume, T. A radiobiological basis for setting neutron radiation safety standards. Health Phys. 1985, 49, 883–896. [Google Scholar] [CrossRef]
- Doloy, M.T.; Malarbet, J.L.; Guedeney, G.; Bourguignon, M.; Leroy, A.; Reillaudou, M.; Masse, R. Use of unstable chromosome aberrations for biological dosimetry after the first postirradiation mitosis. Radiat. Res. 1991, 125, 141–151. [Google Scholar] [CrossRef]
- Guedeney, G.; Rigaud, O.; Duranton, I.; Malarbet, J.L.; Doloy, M.T.; Magdelenat, H. Chromosomal aberrations and DNA repair ability of in vitro irradiated white blood cells of monkeys previously exposed to total body irradiation. Mutat. Res. 1989, 212, 159–166. [Google Scholar] [CrossRef]
- ISO21243; Radiation Protection—Performance Criteria for Laboratories Performing Cytogenetic Triage for Assessment of Mass Casualties in Radiological or Nuclear Emergencies-General Principles and Application to Dicentric Assay. ISO: Genève, Switzerland, 2008.
- ISO19238; Radiological Protection-Performance Criteria for Service Laboratories Performing Biological Dosimetry by Cytogenetics. ISO: Geneva, Switzerland, 2014.
- ISO17099; Radiological Protection-Performance Criteria for Laboratories Using the Cytokinesis-Block Micronucleus (CBMN) Assay in Peripheral Blood Lymphocytes for Biological Dosimetry. ISO: Geneva, Switzerland, 2014.
- Kaddour, A.; Colicchio, B.; Buron, D.; El Maalouf, E.; Laplagne, E.; Borie, C.; Ricoul, M.; Lenain, A.; Hempel, W.M.; Morat, L.; et al. Transmission of Induced Chromosomal Aberrations through Successive Mitotic Divisions in Human Lymphocytes after In Vitro and In Vivo Radiation. Sci. Rep. 2017, 7, 3291. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Knoll, J.H.; Wilkins, R.C.; Flegal, F.N.; Rogan, P.K. Automated discrimination of dicentric and monocentric chromosomes by machine learning-based image processing. Microsc. Res. Tech. 2016, 79, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Romm, H.; Ainsbury, E.A.; Barquinero, J.F.; Barrios, L.; Beinke, C.; Cucu, A.; Domene, M.M.; Filippi, S.; Monteiro Gil, O.; Gregoire, E.; et al. Web based scoring is useful for validation and harmonisation of scoring criteria within RENEB. Int. J. Radiat. Biol. 2017, 93, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuryak, I.; Royba, E.; Repin, M.; Turner, H.C.; Garty, G.; Deoli, N.; Brenner, D.J. A machine learning method for improving the accuracy of radiation biodosimetry by combining data from the dicentric chromosomes and micronucleus assays. Sci. Rep. 2022, 12, 21077. [Google Scholar] [CrossRef]
- Royba, E.; Repin, M.; Balajee, A.S.; Shuryak, I.; Pampou, S.; Karan, C.; Brenner, D.J.; Garty, G. The RABiT-II DCA in the Rhesus Macaque Model. Radiat. Res. 2020, 196, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Garty, G.; Bigelow, A.W.; Repin, M.; Turner, H.C.; Bian, D.; Balajee, A.S.; Lyulko, O.V.; Taveras, M.; Yao, Y.L.; Brenner, D.J. An automated imaging system for radiation biodosimetry. Microsc. Res. Tech. 2015, 78, 587–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roukos, V.; Burman, B.; Misteli, T. The cellular etiology of chromosome translocations. Curr. Opin. Cell Biol. 2013, 25, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Tucker, J.D. Reflections on the development and application of FISH whole chromosome painting. Mutat. Res. Rev. Mutat. Res. 2015, 763, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.L.; Bailey, S.M.; Beck, H.L.; Boice, J.D.; Bouville, A.; Brill, A.B.; Cornforth, M.N.; Inskip, P.D.; McKenna, M.J.; Mumma, M.T.; et al. Estimation of Radiation Doses to U.S. Military Test Participants from Nuclear Testing: A Comparison of Historical Film-Badge Measurements, Dose Reconstruction and Retrospective Biodosimetry. Radiat. Res. 2019, 191, 297–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, G.K.; Khvostunov, I.K. Cytogenetic effects of radioiodine therapy: A 20-year follow-up study. Radiat. Environ. Biophys. 2016, 55, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Goh, V.S.T.; Fujishima, Y.; Abe, Y.; Sakai, A.; Yoshida, M.A.; Ariyoshi, K.; Kasai, K.; Wilkins, R.C.; Blakely, W.F.; Miura, T. Construction of fluorescence in situ hybridization (FISH) translocation dose-response calibration curve with multiple donor data sets using R, based on ISO 20046:2019 recommendations. Int. J. Radiat. Biol. 2019, 95, 1668–1684. [Google Scholar] [CrossRef]
- Gregoire, E.; Barquinero, J.F.; Shi, L.; Tashiro, S.; Sotnik, N.V.; Azizova, T.V.; Darroudi, F.; Ainsbury, E.A.; Moquet, J.E.; Fomina, J.; et al. Verification by the FISH translocation assay of historic doses to Mayak workers from external gamma radiation. Radiat. Environ. Biophys. 2015, 54, 445–451. [Google Scholar]
- Andreassi, M.G.; Little, M.P.; Wakeford, R.; Hatch, M.; Ainsbury, E.A.; Tawn, E.J. Chromosome Aberrations in a Group of People Exposed to Radioactive Releases from the Three Mile Island Nuclear Accident and Inferences for Radiation Effects. Int. J. Mol. Sci. 2021, 22, 7504. [Google Scholar] [CrossRef]
- Sorokine-Durm, I.; Durand, V.; Le Roy, A.; Paillole, N.; Roy, L.; Voisin, P. Is FISH painting an appropriate biological marker for dose estimates of suspected accidental radiation overexposure? A review of cases investigated in France from 1995 to 1996. Environ. Health Perspect. 1997, 105 (Suppl. S6), 1427–1432. [Google Scholar] [CrossRef]
- Buckton, K.E.; Jacobs, P.A.; Court Brown, W.M.; Doll, R. A study of the chromosome damage persisting after x-ray therapy for ankylosing spondylitis. Lancet 1962, 2, 676–682. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Holland, N.; Bonassi, S.; Kirsch-Volders, M. Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108342. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Bonassi, S. The effect of age, gender, diet and lifestyle on DNA damage measured using micronucleus frequency in human peripheral blood lymphocytes. Mutagenesis 2011, 26, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M.; Morley, A.A. Measurement of micronuclei in lymphocytes. Mutat. Res. 1985, 147, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M. Micronuclei and their association with sperm abnormalities, infertility, pregnancy loss, pre-eclampsia and intra-uterine growth restriction in humans. Mutagenesis 2011, 26, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Vral, A.; Fenech, M.; Thierens, H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis 2011, 26, 11–17. [Google Scholar] [CrossRef]
- Depuydt, J.; Baert, A.; Vandersickel, V.; Thierens, H.; Vral, A. Relative biological effectiveness of mammography X-rays at the level of DNA and chromosomes in lymphocytes. Int. J. Radiat. Biol. 2013, 89, 532–538. [Google Scholar] [CrossRef]
- Vral, A.; Decorte, V.; Depuydt, J.; Wambersie, A.; Thierens, H. A semi-automated FISH-based micronucleus-centromere assay for biomonitoring of hospital workers exposed to low doses of ionizing radiation. Mol. Med. Rep. 2016, 14, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Pantelias, A.; Terzoudi, G.I. Development of an automatable micro-PCC biodosimetry assay for rapid individualized risk assessment in large-scale radiological emergencies. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 65–71. [Google Scholar] [CrossRef]
- Sun, M.; Moquet, J.; Barnard, S.; Lloyd, D.; Ainsbury, E. A Simplified Calyculin A-Induced Premature Chromosome Condensation (PCC) Protocol for the Biodosimetric Analysis of High-Dose Exposure to Gamma Radiation. Radiat. Res. 2020, 193, 560–568. [Google Scholar] [CrossRef]
- Puig, R.; Barrios, L.; Pujol, M.; Caballín, M.R.; Barquinero, J.F. Suitability of scoring PCC rings and fragments for dose assessment after high-dose exposures to ionizing radiation. Mutat. Res. 2013, 757, 1–7. [Google Scholar] [CrossRef]
- Durante, M.; George, K.; Yang, T.C. Biodosimetry of ionizing radiation by selective painting of prematurely condensed chromosomes in human lymphocytes. Radiat. Res. 1997, 148, S45–S50. [Google Scholar] [CrossRef] [Green Version]
- Karachristou, I.; Karakosta, M.; Pantelias, A.; Hatzi, V.I.; Karaiskos, P.; Dimitriou, P.; Pantelias, G.; Terzoudi, G.I. Triage biodosimetry using centromeric/telomeric PNA probes and Giemsa staining to score dicentrics or excess fragments in non-stimulated lymphocyte prematurely condensed chromosomes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 793, 107–114. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Rossnerova, A.; Sram, R.; Romm, H.; Bolognesi, C.; Ramakumar, A.; Soussaline, F.; Schunck, C.; Elhajouji, A.; et al. HUMN project initiative and review of validation, quality control and prospects for further development of automated micronucleus assays using image cytometry systems. Int. J. Hyg. Environ. Health 2013, 216, 541–552. [Google Scholar] [CrossRef]
- Schunck, C.; Johannes, T.; Varga, D.; Lörch, T.; Plesch, A. New developments in automated cytogenetic imaging: Unattended scoring of dicentric chromosomes, micronuclei, single cell gel electrophoresis, and fluorescence signals. Cytogenet. Genome Res. 2004, 104, 383–389. [Google Scholar] [CrossRef]
- Wang, Q.; Rodrigues, M.A.; Repin, M.; Pampou, S.; Beaton-Green, L.A.; Perrier, J.; Garty, G.; Brenner, D.J.; Turner, H.C.; Wilkins, R.C. Automated Triage Radiation Biodosimetry: Integrating Imaging Flow Cytometry with High-Throughput Robotics to Perform the Cytokinesis-Block Micronucleus Assay. Radiat. Res. 2019, 191, 342–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capaccio, C.; Perrier, J.R.; Cunha, L.; Mahnke, R.C.; Lörch, T.; Porter, M.; Smith, C.L.; Damer, K.; Bourland, J.D.; Frizzell, B.; et al. CytoRADx: A High-Throughput, Standardized Biodosimetry Diagnostic System Based on the Cytokinesis-Block Micronucleus Assay. Radiat. Res. 2021, 196, 523–534. [Google Scholar] [CrossRef]
- Repin, M.; Pampou, S.; Karan, C.; Brenner, D.J.; Garty, G. RABiT-II: Implementation of a High-Throughput Micronucleus Biodosimetry Assay on Commercial Biotech Robotic Systems. Radiat. Res. 2017, 187, 492–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M. Cytokinesis-Block Micronucleus Cytome Assay Evolution into a More Comprehensive Method to Measure Chromosomal Instability. Genes 2020, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Romm, H.; Ainsbury, E.; Barnard, S.; Barrios, L.; Barquinero, J.F.; Beinke, C.; Deperas, M.; Gregoire, E.; Koivistoinen, A.; Lindholm, C.; et al. Automatic scoring of dicentric chromosomes as a tool in large scale radiation accidents. Mutat. Res. 2013, 756, 174–183. [Google Scholar] [CrossRef]
- Alsbeih, G.A.; Al-Hadyan, K.S.; Al-Harbi, N.M.; Bin Judia, S.S.; Moftah, B.A. Establishing a Reference Dose-Response Calibration Curve for Dicentric Chromosome Aberrations to Assess Accidental Radiation Exposure in Saudi Arabia. Front. Public Health 2020, 8, 599194. [Google Scholar] [CrossRef]
- Oestreicher, U.; Samaga, D.; Ainsbury, E.; Antunes, A.C.; Baeyens, A.; Barrios, L.; Beinke, C.; Beukes, P.; Blakely, W.F.; Cucu, A.; et al. RENEB intercomparisons applying the conventional Dicentric Chromosome Assay (DCA). Int. J. Radiat. Biol. 2017, 93, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Royba, E.; Repin, M.; Pampou, S.; Karan, C.; Brenner, D.J.; Garty, G. RABiT-II-DCA: A Fully-automated Dicentric Chromosome Assay in Multiwell Plates. Radiat. Res. 2019, 192, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Herate, C.; Brochard, P.; De Vathaire, F.; Ricoul, M.; Martins, B.; Laurier, L.; Deverre, J.R.; Thirion, B.; Hertz-Pannier, L.; Sabatier, L. The effects of repeated brain MRI on chromosomal damage. Eur. Radiol. Exp. 2022, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Oudrhiri, N.; M’Kacher, R. Patient-Derived iPSCs Reveal Evidence of Telomere Instability and DNA Repair Deficiency in Coats Plus Syndrome. Genes 2022, 13, 1395. [Google Scholar] [CrossRef]
- Sumption, N.; Goodhead, D.T.; Anderson, R.M. Alpha-Particle-Induced Complex Chromosome Exchanges Transmitted through Extra-Thymic Lymphopoiesis In Vitro Show Evidence of Emerging Genomic Instability. PLoS ONE 2015, 10, e0134046. [Google Scholar] [CrossRef] [Green Version]
- Nieri, D.; Berardinelli, F.; Antoccia, A.; Tanzarella, C.; Sgura, A. Comparison between two FISH techniques in the in vitro study of cytogenetic markers for low-dose X-ray exposure in human primary fibroblasts. Front. Genet. 2013, 4, 141. [Google Scholar] [CrossRef] [Green Version]
- Kohda, A.; Toyokawa, T.; Umino, T.; Ayabe, Y.; Tanaka, I.B.; Komura, J.I. Frequencies of Chromosome Aberrations are Lower in Splenic Lymphocytes from Mice Continuously Exposed to Very Low-Dose-Rate Gamma Rays Compared with Non-Irradiated Control Mice. Radiat. Res. 2022, 198, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.A.; Estrada-Girona, G.; Themis, M.; Garimberti, E.; Hill, M.A.; Bridger, J.M.; Anderson, R.M. Relative proximity of chromosome territories influences chromosome exchange partners in radiation-induced chromosome rearrangements in primary human bronchial epithelial cells. Mutat. Res. 2013, 756, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Bastiani, I.; McMahon, S.J.; Turner, P.; Redmond, K.M.; McGarry, C.K.; Cole, A.; O’Sullivan, J.M.; Prise, K.M.; Ainsbury, L.; Anderson, R. Dose estimation after a mixed field exposure: Radium-223 and intensity modulated radiotherapy. Nucl. Med. Biol. 2022, 106–107, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Balajee, A.S.; Bertucci, A.; Taveras, M.; Brenner, D.J. Multicolour FISH analysis of ionising radiation induced micronucleus formation in human lymphocytes. Mutagenesis 2014, 29, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuceu, C.; Colicchio, B.; Jeandidier, E. Independent Mechanisms Lead to Genomic Instability in Hodgkin Lymphoma: Microsatellite or Chromosomal Instability (dagger). Cancers 2018, 10, 233. [Google Scholar] [CrossRef] [Green Version]
- Gascoigne, K.E.; Cheeseman, I.M. Induced dicentric chromosome formation promotes genomic rearrangements and tumorigenesis. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 2013, 21, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Vaurijoux, A.; Gruel, G.; Pouzoulet, F.; Grégoire, E.; Martin, C.; Roch-Lefèvre, S.; Voisin, P.; Voisin, P.; Roy, L. Strategy for population triage based on dicentric analysis. Radiat. Res. 2009, 171, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bauchinger, M. Quantification of low-level radiation exposure by conventional chromosome aberration analysis. Mutat. Res. 1995, 339, 177–189. [Google Scholar] [CrossRef]
- Lopes, J.; Baudin, C.; Leuraud, K.; Klokov, D.; Bernier, M.O. Ionizing radiation exposure during adulthood and risk of developing central nervous system tumors: Systematic review and meta-analysis. Sci. Rep. 2022, 12, 16209. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Slama, P.; Roychoudhury, S. COVID-19, oxidative stress, and male reproductive dysfunctions: Is vitamin C a potential remedy? Physiol. Res. 2022, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Hazrati, L.N. Evidence of sex differences in cellular senescence. Neurobiol. Aging 2022, 120, 88–104. [Google Scholar] [CrossRef]
- Kananen, L.; Hurme, M.; Bürkle, A.; Moreno-Villanueva, M.; Bernhardt, J.; Debacq-Chainiaux, F.; Grubeck-Loebenstein, B.; Malavolta, M.; Basso, A.; Piacenza, F.; et al. Circulating cell-free DNA in health and disease-the relationship to health behaviours, ageing phenotypes and metabolomics. GeroScience 2023, 45, 85–103. [Google Scholar] [CrossRef]
- M’Kacher, R.; Bennaceur-Griscelli, A.; Girinsky, T.; Koscielny, S.; Delhommeau, F.; Dossou, J.; Violot, D.; Leclercq, E.; Courtier, M.H.; Beron-Gaillard, N.; et al. Telomere shortening and associated chromosomal instability in peripheral blood lymphocytes of patients with Hodgkin’s lymphoma prior to any treatment are predictive of second cancers. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 465–471. [Google Scholar] [CrossRef]
- M’Kacher, R.; Girinsky, T.; Koscielny, S.; Dossou, J.; Violot, D.; Beron-Gaillard, N.; Ribrag, V.; Bourhis, J.; Bernheim, A.; Parmentier, C.; et al. Baseline and treatment-induced chromosomal abnormalities in peripheral blood lymphocytes of Hodgkin’s lymphoma patients. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA Damage: A Route to Radiation-Induced Genomic Instability and Carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Lazutka, J.R.; Neel, J.V.; Major, E.O.; Dedonyte, V.; Mierauskine, J.; Slapsyte, G.; Kesminiene, A. High titers of antibodies to two human polyomaviruses, JCV and BKV, correlate with increased frequency of chromosomal damage in human lymphocytes. Cancer Lett. 1996, 109, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Neel, J.V.; Major, E.O.; Awa, A.A.; Glover, T.; Burgess, A.; Traub, R.; Curfman, B.; Satoh, C. Hypothesis: “Rogue cell”-type chromosomal damage in lymphocytes is associated with infection with the JC human polyoma virus and has implications for oncopenesis. Proc. Natl. Acad. Sci. USA 1996, 93, 2690–2695. [Google Scholar] [CrossRef] [Green Version]
- Neel, J.V. An association, in adult Japanese, between the occurrence of rogue cells among cultured lymphocytes (JC virus activity) and the frequency of “simple” chromosomal damage among the lymphocytes of persons exhibiting these rogue cells. Am. J. Hum. Genet. 1998, 63, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- M’Kacher, R.; Andreoletti, L.; Flamant, S.; Milliat, F.; Girinsky, T.; Dossou, J.; Violot, D.; Assaf, E.; Clausse, B.; Koscielny, S.; et al. JC human polyomavirus is associated to chromosomal instability in peripheral blood lymphocytes of Hodgkin’s lymphoma patients and poor clinical outcome. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 826–832. [Google Scholar] [CrossRef]
- Testard, I.; Ricoul, M.; Hoffschir, F.; Flury-Herard, A.; Dutrillaux, B.; Fedorenko, B.; Gerasimenko, V.; Sabatier, L. Radiation-induced chromosome damage in astronauts’ lymphocytes. Int. J. Radiat. Biol. 1996, 70, 403–411. [Google Scholar] [CrossRef]
- Mustonen, R.; Lindholm, C.; Tawn, E.J.; Sabatier, L.; Salomaa, S. The incidence of cytogenetically abnormal rogue cells in peripheral blood. Int. J. Radiat. Biol. 1998, 74, 781–785. [Google Scholar] [CrossRef]
- Pujol-Canadell, M.; Perrier, J.R.; Cunha, L.; Shuryak, I.; Harken, A.; Garty, G.; Brenner, D.J. Cytogenetically-based biodosimetry after high doses of radiation. PLoS ONE 2020, 15, e0228350. [Google Scholar] [CrossRef] [Green Version]
- Abend, M.; Pfeiffer, R.M.; Port, M.; Hatch, M.; Bogdanova, T.; Tronko, M.D.; Mabuchi, K.; Azizova, T.; Unger, K.; Braselmann, H.; et al. Utility of gene expression studies in relation to radiation exposure and clinical outcomes: Thyroid cancer in the Ukrainian-American cohort and late health effects in a MAYAK worker cohort. Int. J. Radiat. Biol. 2021, 97, 12–18. [Google Scholar] [CrossRef]
- Tichy, A.; Kabacik, S.; O’Brien, G.; Pejchal, J.; Sinkorova, Z.; Kmochova, A.; Sirak, I.; Malkova, A.; Beltran, C.G.; Gonzalez, J.R.; et al. The first in vivo multiparametric comparison of different radiation exposure biomarkers in human blood. PLoS ONE 2018, 13, e0193412. [Google Scholar] [CrossRef] [PubMed]
- Moquet, J.; Higueras, M.; Donovan, E.; Boyle, S.; Barnard, S.; Bricknell, C.; Sun, M.; Gothard, L.; O’Brien, G.; Cruz-Garcia, L.; et al. Dicentric Dose Estimates for Patients Undergoing Radiotherapy in the RTGene Study to Assess Blood Dosimetric Models and the New Bayesian Method for Gradient Exposure. Radiat. Res. 2018, 190, 596–604. [Google Scholar] [CrossRef]
- Cruz-Garcia, L.; O’Brien, G.; Donovan, E.; Gothard, L.; Boyle, S.; Laval, A.; Testard, I.; Ponge, L.; Woźniak, G.; Miszczyk, L.; et al. Influence of Confounding Factors on Radiation Dose Estimation Using In Vivo Validated Transcriptional Biomarkers. Int. J. Radiat. Biol. 2018, 115, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Palma, V.; Patrono, C. A Novel Biological Dosimetry Assay as a Potential Tool for Triage Dose Assessment in Case of Large-Scale Radiological Emergency. Radiat. Prot. Dosim. 2019, 186, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Testa, A.; Palma, V.; Patrono, C. Dicentric Chromosome Assay (DCA) and Cytokinesis-Block Micronucleus (CBMN) Assay in the Field of Biological Dosimetry. Methods Mol. Biol. (Clifton N.J.) 2019, 2031, 105–119. [Google Scholar] [CrossRef]
- Moquet, J.; Ellender, M.; Bouffler, S.; Badie, C.; Baldwin-Cleland, R.; Monahan, K.; Latchford, A.; Lloyd, D.; Clark, S.; Anyamene, N.A.; et al. Transcriptional Dynamics of DNA Damage Responsive Genes in Circulating Leukocytes during Radiotherapy. Fam. Cancer 2022, 14, 2649. [Google Scholar] [CrossRef]
- Cruz-Garcia, L.; Badie, C.; Anbalagan, S.; Moquet, J.; Gothard, L.; O’Brien, G.; Somaiah, N.; Ainsbury, E.A. An ionising radiation-induced specific transcriptional signature of inflammation-associated genes in whole blood from radiotherapy patients: A pilot study. Radiat. Oncol. 2021, 16, 83. [Google Scholar] [CrossRef]
- Abend, M.; Amundson, S.A.; Badie, C.; Brzoska, K.; Hargitai, R.; Kriehuber, R.; Schüle, S.; Kis, E.; Ghandhi, S.A.; Lumniczky, K.; et al. Inter-laboratory comparison of gene expression biodosimetry for protracted radiation exposures as part of the RENEB and EURADOS WG10 2019 exercise. Cancers 2021, 11, 9756. [Google Scholar] [CrossRef]
- Tichy, A.; Ricobonno, D.; Cary, L.H.; Badie, C. Editorial: Recent advances in radiation medical countermeasures. Front. Pharmacol. 2022, 13, 983702. [Google Scholar] [CrossRef]





| Techniques | Types of Exposure to Ionizing Radiation | Sensitivity | |||
|---|---|---|---|---|---|
| Recent and Homogeneous Events | Recent and Heterogeneous Events | Past Event | Large-Scale Event | Sensitivity | |
| Dicentric and centric rings | YES | YES | NO | YES | 0.1 Gy |
| Micronuclei | YES | YES | NO | YES | 0.3 Gy |
| Translocations | YES | YES | YES | NO | 0.25–0.3 Gy |
| PCC—CHO | YES | YES | NO | YES | 0.1 Gy |
| PCC—ring | YES | ? | NO | ? | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’Kacher, R.; Colicchio, B.; Junker, S.; El Maalouf, E.; Heidingsfelder, L.; Plesch, A.; Dieterlen, A.; Jeandidier, E.; Carde, P.; Voisin, P. High Resolution and Automatable Cytogenetic Biodosimetry Using In Situ Telomere and Centromere Hybridization for the Accurate Detection of DNA Damage: An Overview. Int. J. Mol. Sci. 2023, 24, 5699. https://doi.org/10.3390/ijms24065699
M’Kacher R, Colicchio B, Junker S, El Maalouf E, Heidingsfelder L, Plesch A, Dieterlen A, Jeandidier E, Carde P, Voisin P. High Resolution and Automatable Cytogenetic Biodosimetry Using In Situ Telomere and Centromere Hybridization for the Accurate Detection of DNA Damage: An Overview. International Journal of Molecular Sciences. 2023; 24(6):5699. https://doi.org/10.3390/ijms24065699
Chicago/Turabian StyleM’Kacher, Radhia, Bruno Colicchio, Steffen Junker, Elie El Maalouf, Leonhard Heidingsfelder, Andreas Plesch, Alain Dieterlen, Eric Jeandidier, Patrice Carde, and Philippe Voisin. 2023. "High Resolution and Automatable Cytogenetic Biodosimetry Using In Situ Telomere and Centromere Hybridization for the Accurate Detection of DNA Damage: An Overview" International Journal of Molecular Sciences 24, no. 6: 5699. https://doi.org/10.3390/ijms24065699
APA StyleM’Kacher, R., Colicchio, B., Junker, S., El Maalouf, E., Heidingsfelder, L., Plesch, A., Dieterlen, A., Jeandidier, E., Carde, P., & Voisin, P. (2023). High Resolution and Automatable Cytogenetic Biodosimetry Using In Situ Telomere and Centromere Hybridization for the Accurate Detection of DNA Damage: An Overview. International Journal of Molecular Sciences, 24(6), 5699. https://doi.org/10.3390/ijms24065699












