Endogenous Neural Stem Cell Activation after Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation
Abstract
1. Introduction
2. Results
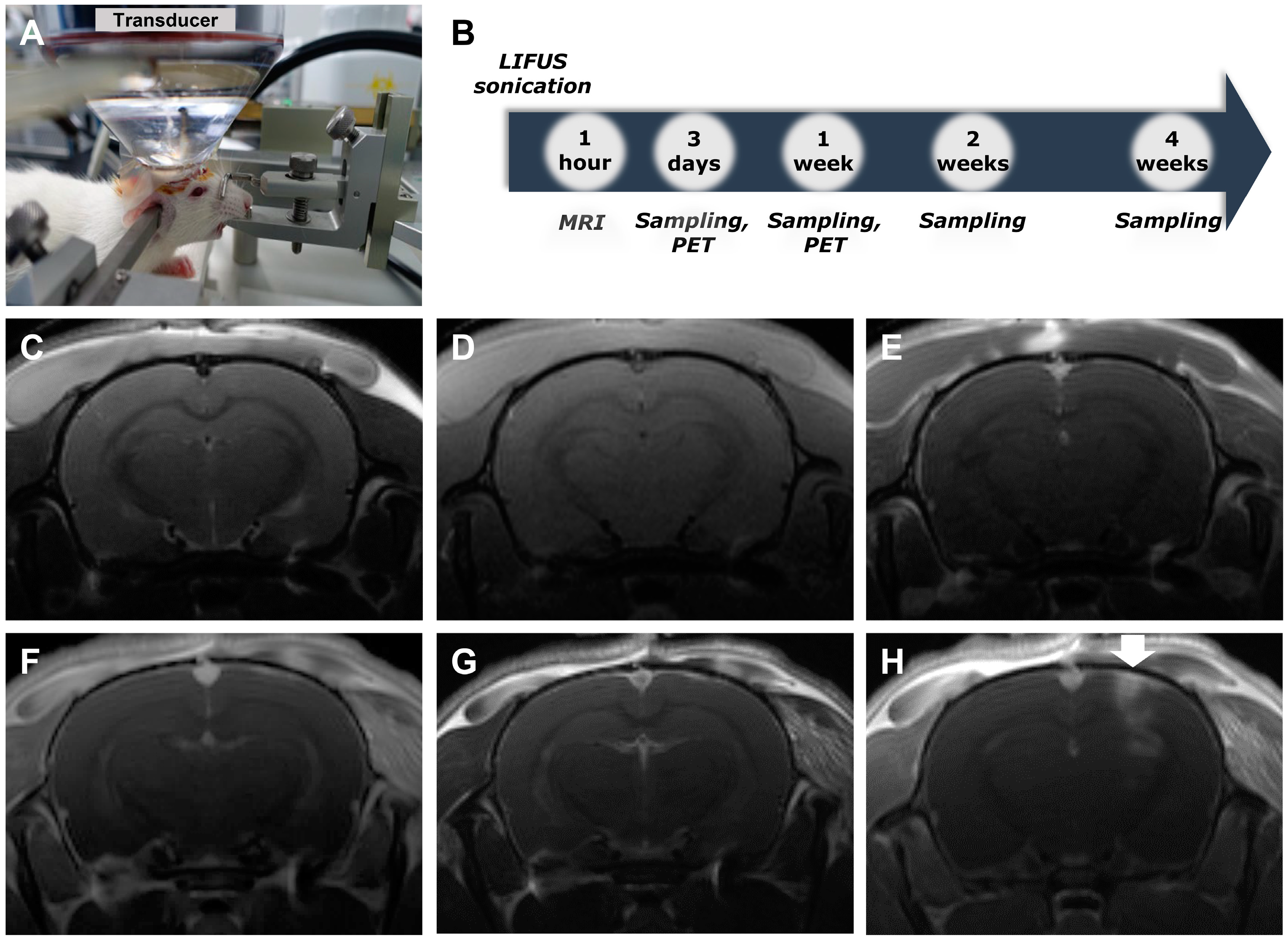
2.1. Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation
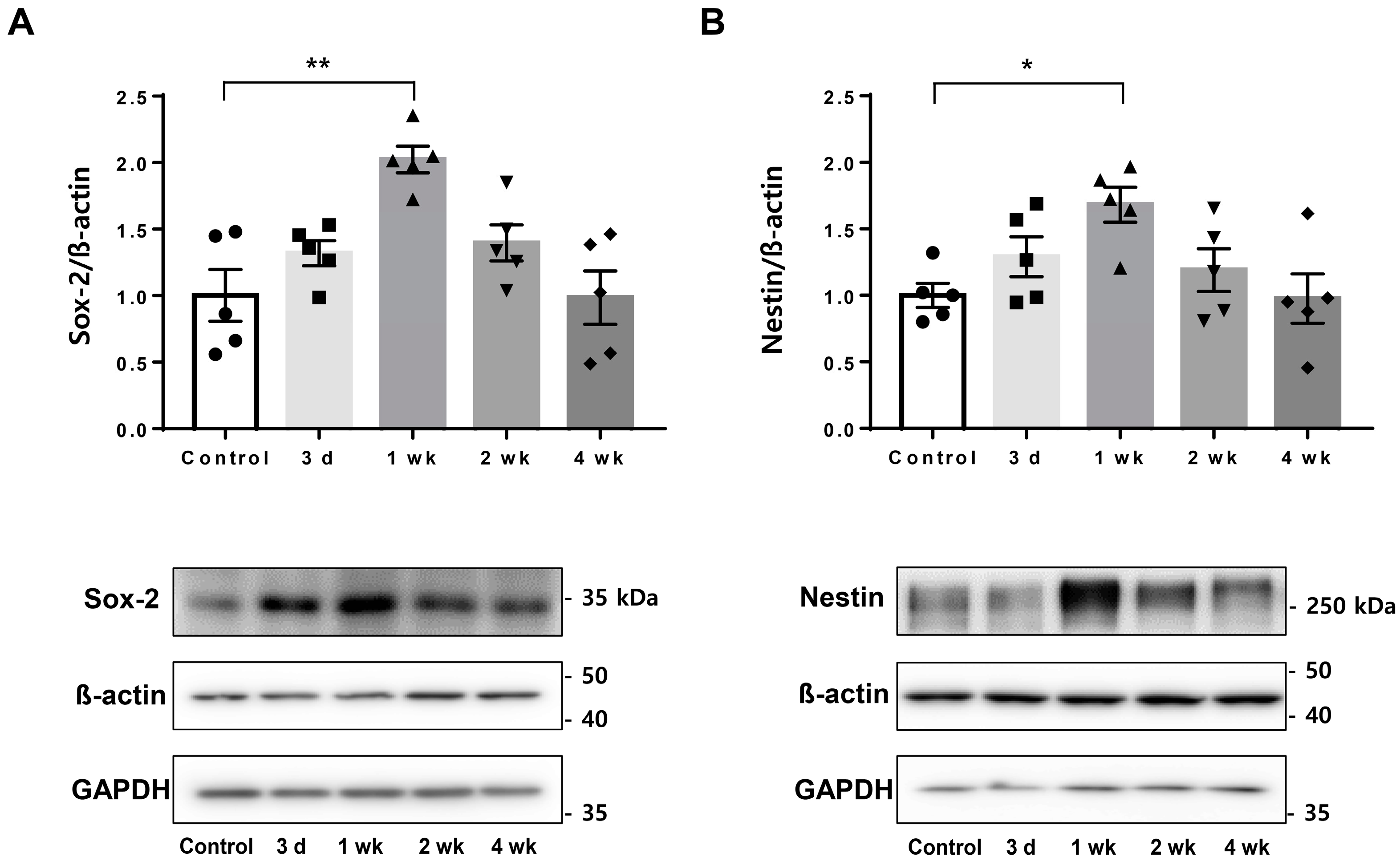
2.2. Upregulated Endogenous Neural Stem Cell Markers after Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation
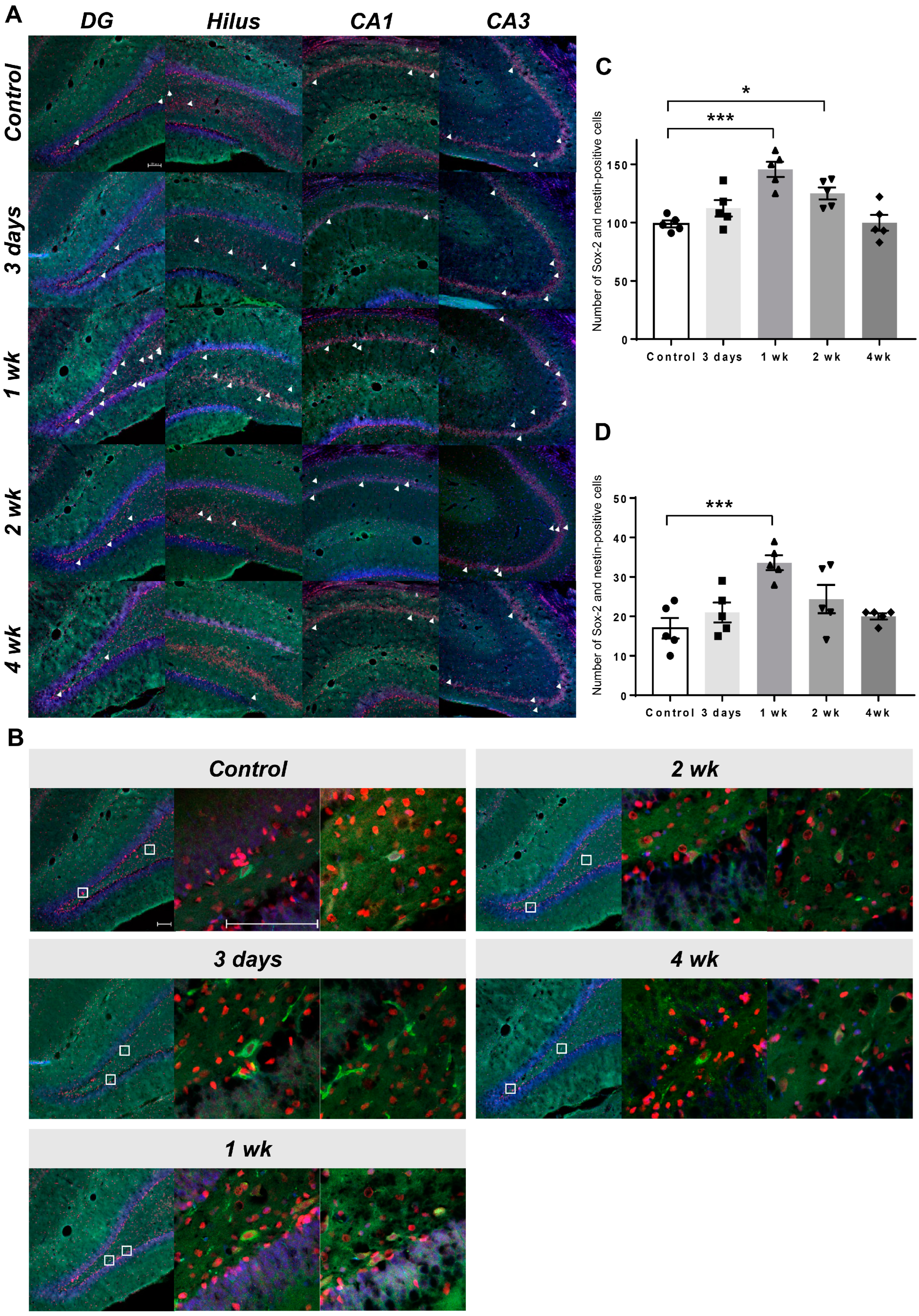
2.3. Co-Expression of Endogenous Neural Stem Cell Markers
2.4. Visualization of Upregulated Endogenous Neural Stem Cell Activation Using [18F] Fluoro-L-Thymidine Positron Emission Tomography
3. Discussion
3.1. Low-Intensity Focused Ultrasound-Induced Blood–brain Barrier Modulation
3.2. Endogenous Neural Stem Cell-Induced Neurogenesis
3.3. Endogenous Neural Stem Cell Activation after Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation
4. Materials and Methods
4.1. Animals
4.2. Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation
4.3. Immunohistochemistry
4.4. Western Blot Analysis
4.5. Positron Emission Tomography and Image Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rueger, M.A.; Backes, H.; Walberer, M.; Neumaier, B.; Ullrich, R.; Simard, M.L.; Emig, B.; Fink, G.R.; Hoehn, M.; Graf, R.; et al. Noninvasive imaging of endogenous neural stem cell mobilization in vivo using positron emission tomography. J. Neurosci. 2010, 30, 6454–6460. [Google Scholar] [CrossRef] [PubMed]
- van den Berge, S.A.; van Strien, M.E.; Korecka, J.A.; Dijkstra, A.A.; Sluijs, J.A.; Kooijman, L.; Eggers, R.; De Filippis, L.; Vescovi, A.L.; Verhaagen, J.; et al. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain 2011, 134, 3249–3263. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Temple, S.; Alvarez-Buylla, A. Stem cells in the adult mammalian central nervous system. Curr. Opin. Neurobiol. 1999, 9, 135–141. [Google Scholar] [CrossRef]
- Clarke, D.L.; Johansson, C.B.; Wilbertz, J.; Veress, B.; Nilsson, E.; Karlström, H.; Lendahl, U.; Frisén, J. Generalized potential of adult neural stem cells. Science 2000, 288, 1660–1663. [Google Scholar] [CrossRef] [PubMed]
- Momma, S.; Johansson, C.B.; Frisén, J. Get to know your stem cells. Curr. Opin. Neurobiol. 2000, 10, 45–49. [Google Scholar] [CrossRef]
- Snyder, E.Y.; Deitcher, D.L.; Walsh, C.; Arnold-Aldea, S.; Hartwieg, E.A.; Cepko, C.L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 1992, 68, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.M.; Abou-Elkacem, L.; Lee, T.; Dahl, J.; Lutz, A.M. Ultrasound and microbubble mediated therapeutic delivery: Underlying mechanisms and future outlook. J. Control. Release 2020, 326, 75–90. [Google Scholar] [CrossRef]
- Ilovitsh, T.; Ilovitsh, A.; Foiret, J.; Caskey, C.F.; Kusunose, J.; Fite, B.Z.; Zhang, H.; Mahakian, L.M.; Tam, S.; Butts-Pauly, K.; et al. Enhanced microbubble contrast agent oscillation following 250 kHz insonation. Sci. Rep. 2018, 8, 16347. [Google Scholar] [CrossRef]
- Kong, C.; Yang, E.-J.; Shin, J.; Park, J.; Kim, S.-H.; Park, S.-W.; Chang, W.S.; Lee, C.-H.; Kim, H.; Kim, H.-S.; et al. Enhanced delivery of a low dose of aducanumab via FUS in 5×FAD mice, an AD model. Transl. Neurodegener. 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Leinenga, G.; Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra33. [Google Scholar] [CrossRef]
- Kovacs, Z.I.; Kim, S.; Jikaria, N.; Qureshi, F.; Milo, B.; Lewis, B.K.; Bresler, M.; Burks, S.R.; Frank, J.A. Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc. Natl. Acad. Sci. USA 2017, 114, E75–E84. [Google Scholar] [CrossRef]
- Evans, K.D.; Weiss, B.; Knopp, M. High-intensity focused ultrasound (HIFU) for specific therapeutic treatments: A literature review. J. Diagn. Med. Sonogr. 2007, 23, 319–327. [Google Scholar] [CrossRef]
- Darrow, D.P. Focused ultrasound for neuromodulation. Neurotherapeutics 2019, 16, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.J.; Pernot, M.; Small, S.A.; Konofagou, E.E. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 2007, 33, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.S.; Vlachos, F.; Feshitan, J.A.; Borden, M.A.; Konofagou, E.E. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J. Acoust. Soc. Am. 2011, 130, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kong, C.; Cho, J.S.; Lee, J.; Koh, C.S.; Yoon, M.S.; Na, Y.C.; Chang, W.S.; Chang, J.W. Focused ultrasound-mediated noninvasive blood-brain barrier modulation: Preclinical examination of efficacy and safety in various sonication parameters. Neurosurg. Focus 2018, 44, E15. [Google Scholar] [CrossRef]
- Shin, J.; Kong, C.; Lee, J.; Choi, B.Y.; Sim, J.; Koh, C.S.; Park, M.; Na, Y.C.; Suh, S.W.; Chang, W.S.; et al. Focused ultrasound-induced blood-brain barrier opening improves adult hippocampal neurogenesis and cognitive function in a cholinergic degeneration dementia rat model. Alzheimers Res. Ther. 2019, 11, 110. [Google Scholar] [CrossRef]
- Lee, J.; Chang, W.S.; Shin, J.; Seo, Y.; Kong, C.; Song, B.W.; Na, Y.C.; Kim, B.S.; Chang, J.W. Non-invasively enhanced intracranial transplantation of mesenchymal stem cells using focused ultrasound mediated by overexpression of cell-adhesion molecules. Stem Cell Res. 2020, 43, 101726. [Google Scholar] [CrossRef]
- Scarcelli, T.; Jordão, J.F.; O’Reilly, M.A.; Ellens, N.; Hynynen, K.; Aubert, I. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014, 7, 304–307. [Google Scholar] [CrossRef]
- Song, S.; Ma, D.; Xu, L.; Wang, Q.; Liu, L.; Tong, X.; Yan, H. Low-intensity pulsed ultrasound-generated singlet oxygen induces telomere damage leading to glioma stem cell awakening from quiescence. iScience 2021, 25, 103558. [Google Scholar] [CrossRef]
- Mooney, S.J.; Shah, K.; Yeung, S.; Burgess, A.; Aubert, I.; Hynynen, K. Focused ultrasound-induced neurogenesis requires an increase in blood-brain barrier permeability. PLoS ONE 2016, 11, e0159892. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Temple, S. The development of neural stem cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef]
- Parent, J.M.; Vexler, Z.S.; Gong, C.; Derugin, N.; Ferriero, D.M. Forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002, 52, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Gabriel-Salazar, M.; Lei, T.; Grayston, A.; Costa, C.; Medina-Gutiérrez, E.; Comabella, M.; Montaner, J.; Rosell, A. Angiogenin in the neurogenic subventricular zone after stroke. Front. Neurol. 2021, 12, 662235. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.S.; Malik, S.C.; Conforti, P.; Lin, J.D.; Chu, Y.H.; Nath, S.; Greulich, F.; Dumbach, M.A.; Uhlenhaut, N.H.; Schachtrup, C. P75 neurotrophin receptor controls subventricular zone neural stem cell migration after stroke. Cell Tissue Res. 2022, 387, 415–431. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef]
- Pencea, V.; Bingaman, K.D.; Wiegand, S.J.; Luskin, M.B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001, 21, 6706–6717. [Google Scholar] [CrossRef]
- Nakatomi, H.; Kuriu, T.; Okabe, S.; Yamamoto, S.; Hatano, O.; Kawahara, N.; Tamura, A.; Kirino, T.; Nakafuku, M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 2002, 110, 429–441. [Google Scholar] [CrossRef]
- Burks, S.R.; Ziadloo, A.; Kim, S.J.; Nguyen, B.A.; Frank, J.A. Noninvasive pulsed focused ultrasound allows spatiotemporal control of targeted homing for multiple stem cell types in murine skeletal muscle and the magnitude of cell homing can be increased through repeated applications. Stem Cells 2013, 31, 2551–2560. [Google Scholar] [CrossRef]
- Crowley, N.A.; Medina, S.H. Targeted and transient opening of the blood brain barrier in discrete neurocircuits and brain regions. Neuropsychopharmacology 2023, 48, 253–254. [Google Scholar] [CrossRef]
- Petro, M.; Jaffer, H.; Yang, J.; Kabu, S.; Morris, V.B.; Labhasetwar, V. Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 2016, 81, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Pagin, M.; Pernebrink, M.; Giubbolini, S.; Barone, C.; Sambruni, G.; Zhu, Y.; Chiara, M.; Ottolenghi, S.; Pavesi, G.; Wei, C.L.; et al. Sox2 controls neural stem cell self-renewal through a Fos-centered gene regulatory network. Stem Cells 2021, 39, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Sirko, S.; Beckers, J.; Irmler, M. Reactive astrocytes as neural stem or progenitor cells: In vivo lineage, In vitro potential, and Genome-wide expression analysis. Glia 2015, 63, 1452–1468. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.; Han, S.; Song, B.-W.; Chang, J.W.; Na, Y.C.; Chang, W.S. Endogenous Neural Stem Cell Activation after Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation. Int. J. Mol. Sci. 2023, 24, 5712. https://doi.org/10.3390/ijms24065712
Seo Y, Han S, Song B-W, Chang JW, Na YC, Chang WS. Endogenous Neural Stem Cell Activation after Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation. International Journal of Molecular Sciences. 2023; 24(6):5712. https://doi.org/10.3390/ijms24065712
Chicago/Turabian StyleSeo, Younghee, Sangheon Han, Byeong-Wook Song, Jin Woo Chang, Young Cheol Na, and Won Seok Chang. 2023. "Endogenous Neural Stem Cell Activation after Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation" International Journal of Molecular Sciences 24, no. 6: 5712. https://doi.org/10.3390/ijms24065712
APA StyleSeo, Y., Han, S., Song, B.-W., Chang, J. W., Na, Y. C., & Chang, W. S. (2023). Endogenous Neural Stem Cell Activation after Low-Intensity Focused Ultrasound-Induced Blood–Brain Barrier Modulation. International Journal of Molecular Sciences, 24(6), 5712. https://doi.org/10.3390/ijms24065712





