PLDLA/TPU Matrix Enriched with Cyclosporine A as a Therapeutic Platform for Immune-Mediated Keratitis (IMMK) in Horses
Abstract
:1. Introduction
2. Results
2.1. Dynamic Contact Angle Measurement of TPU/PLDLA Blends
2.2. The In Vitro Degradation Analysis
2.3. The CsA In Vitro Release Analysis
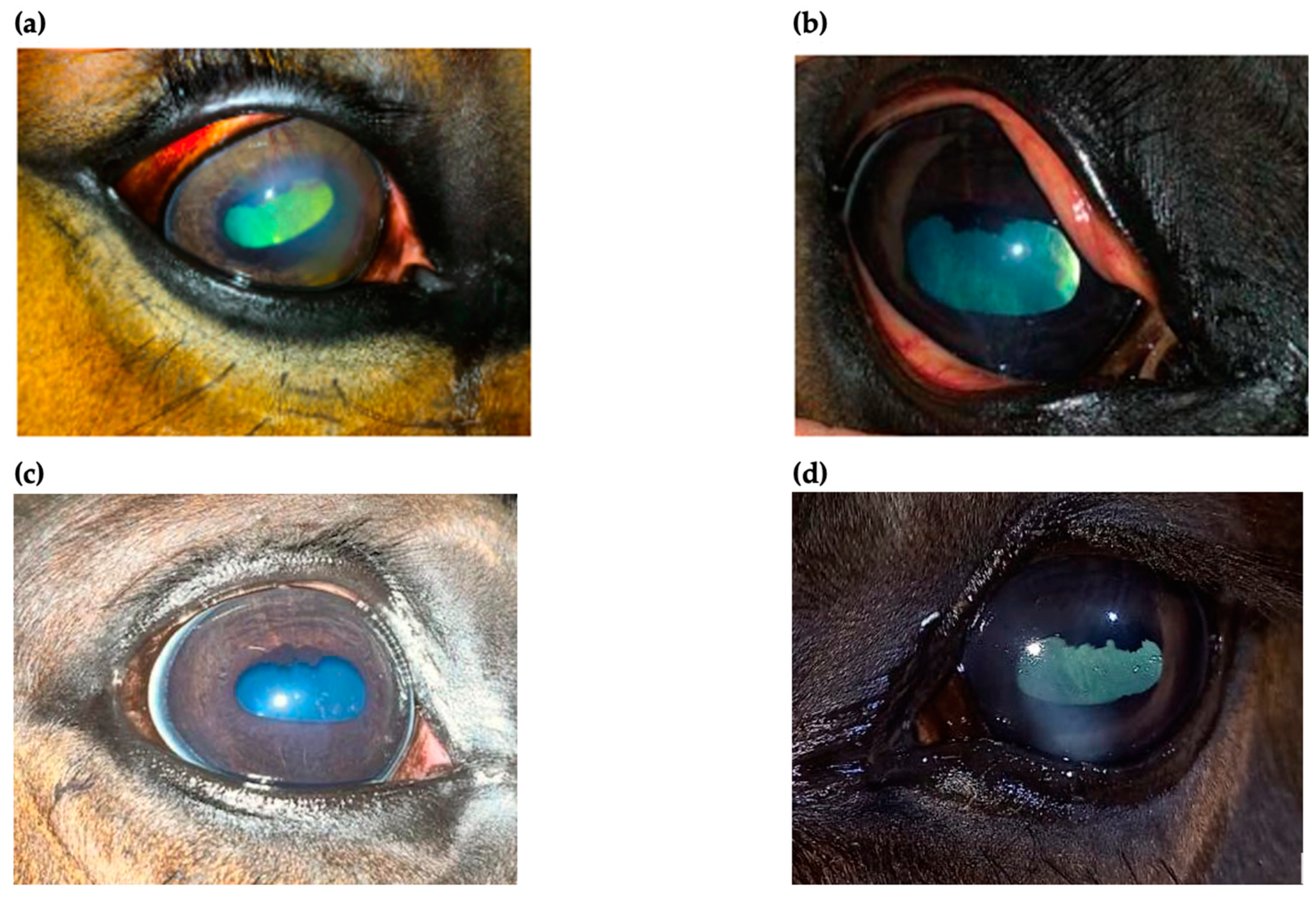
2.4. The Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of TPU/ PLDLA Blends
4.3. Contact Angle Measurements
4.4. In Vitro Release Test
4.5. In Vitro Degradation Measurements in STF
4.6. Evaluation of the Records of Horses Diagnosed with IMMK
4.7. The Clinical Procedures
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matthews, A.G. Nonulcerative Keratopathies in the Horse. Equine Vet. Educ. 2000, 12, 271–278. [Google Scholar] [CrossRef]
- Matthews, A.; Gilger, B.C. Equine Immune-Mediated Keratopathies. Vet. Ophthalmol. 2009, 12, 10–16. [Google Scholar] [CrossRef]
- Pate, D.O.; Clode, A.B.; Olivry, T.; Cullen, J.M.; Salmon, J.H.; Gilger, B.C. Immunohistochemical and Immunopathologic Characterization of Superficial Stromal Immune-Mediated Keratitis in Horses. Am. J. Vet. Res. 2012, 73, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.R.; Whitcup, S.M. Pharmacologic and Clinical Profile of Dexamethasone Intravitreal Implant. Expert Rev. Clin. Pharmacol. 2012, 5, 629–647. [Google Scholar] [CrossRef]
- Ledbetter, E.C.; Irby, N.L. Laser Scanning in Vivo Confocal Microscopic Characterization of Equine Immune-Mediated Keratitis. Vet. Ophthalmol. 2020, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Michau, T.M.; Salmon, J.H. Immune-Mediated Keratitis in Horses: 19 Cases (1998–2004). Vet. Ophthalmol. 2005, 8, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Gilger, B.C.; Stoppini, R.; Wilkie, D.A.; Clode, A.B.; Pinto, N.H.; Hempstead, J.; Gerding, J.; Salmon, J.H. Treatment of Immune-Mediated Keratitis in Horses with Episcleral Silicone Matrix Cyclosporine Delivery Devices. Vet. Ophthalmol. 2014, 17, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.G. Cyclosporine A and the Equine Cornea. Equine Vet. J. 1995, 27, 320–321. [Google Scholar] [CrossRef]
- Gratzek, A.T.; Kaswan, R.L.; Martin, C.L.; Champagne, E.S.; White, S.L. Ophthalmic Cyclosporine in Equine Keratitis and Keratouveitis: 11 Cases. Equine Vet. J. 1995, 27, 327–333. [Google Scholar] [CrossRef]
- Padjasek, M.; Qasem, B.; Cisło-Pakuluk, A.; Marycz, K. Cyclosporine A Delivery Platform for Veterinary Ophthalmology-A New Concept for Advanced Ophthalmology. Biomolecules 2022, 12, 1525. [Google Scholar] [CrossRef]
- Faulds, D.; Goa, K.L.; Benfield, P. Cyclosporin. A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Use in Immunoregulatory Disorders. Drugs 1993, 45, 953–1040. [Google Scholar] [CrossRef]
- Patocka, J.; Nepovimova, E.; Kuca, K.; Wu, W. Cyclosporine A: Chemistry and Toxicity—A Review. Curr. Med. Chem. 2021, 28, 3925–3934. [Google Scholar] [CrossRef]
- Chang-Lin, J.-E.; Burke, J.A.; Peng, Q.; Lin, T.; Orilla, W.C.; Ghosn, C.R.; Zhang, K.-M.; Kuppermann, B.D.; Robinson, M.R.; Whitcup, S.M.; et al. Pharmacokinetics of a Sustained-Release Dexamethasone Intravitreal Implant in Vitrectomized and Nonvitrectomized Eyes. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4605–4609. [Google Scholar] [CrossRef] [Green Version]
- Ghosn, C.R.; Li, Y.; Orilla, W.C.; Lin, T.; Wheeler, L.; Burke, J.A.; Robinson, M.R.; Whitcup, S.M. Treatment of Experimental Anterior and Intermediate Uveitis by a Dexamethasone Intravitreal Implant. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2917–2923. [Google Scholar] [CrossRef] [Green Version]
- Lowder, C.; Belfort, R., Jr.; Lightman, S.; Foster, C.S.; Robinson, M.R.; Schiffman, R.M.; Li, X.-Y.; Cui, H.; Whitcup, S.M.; Ozurdex HURON Study Group. Dexamethasone Intravitreal Implant for Noninfectious Intermediate or Posterior Uveitis. Arch. Ophthalmol. 2011, 129, 545–553. [Google Scholar] [CrossRef]
- Braus, B.K.; Miller, I.; Kummer, S.; Kleinwort, K.J.H.; Hirmer, S.; Hauck, S.M.; McMullen, R.J.; Kerschbaumer, M.; Deeg, C.A. Investigation of Corneal Autoantibodies in Horses with Immune Mediated Keratitis (IMMK). Vet. Immunol. Immunopathol. 2017, 187, 48–54. [Google Scholar] [CrossRef]
- Patel, D.; Wairkar, S. Recent Advances in Cyclosporine Drug Delivery: Challenges and Opportunities. Drug Deliv. Transl. Res. 2019, 9, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Kaswan, R.L.; Salisbury, M.A.; Ward, D.A. Spontaneous Canine Keratoconjunctivitis Sicca. A Useful Model for Human Keratoconjunctivitis Sicca: Treatment with Cyclosporine Eye Drops. Arch Ophthalmol 1989, 107, 1210–1216. [Google Scholar] [CrossRef]
- Kaswan, R.L.; Salisbury, M.A. A New Perspective on Canine Keratoconjunctivitis Sicca: Treatment with Ophthalmic Cyclosporine. Vet. Clin. North Am. Small Anim. Pract. 1990, 20, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Hoey, A.J.; Smitherman, P. Comparison of Topical Cyclosporin and Dexamethasone for the Treatment of Chronic Superficial Keratitis in Dogs. Vet. Rec. 1995, 137, 635–639. [Google Scholar] [PubMed]
- McMullen, R.J.; Fischer, B.M. Medical and Surgical Management of Equine Recurrent Uveitis. Vet. Clin. N. Am. Equine Pract. 2017, 33, 465–481. [Google Scholar] [CrossRef]
- Bauer, B.S. Ocular Pathology. Vet. Clin. N. Am. Equine Pract. 2015, 31, 425–448. [Google Scholar] [CrossRef]
- Robin, M. Immune-Mediated Disorders of the Equine Eye: Part 1—The Cornea. UK-Vet. Equine 2020, 4, 176–182. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Characterization of Thermoplastic Polyurethane/Polylactic Acid (TPU/PLA) Tissue Engineering Scaffolds Fabricated by Microcellular Injection Molding. Mater. Sci. Eng. Mater. Biol. Appl. 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentschel, T.; Münstedt, H. Thermoplastic Polyurethane—The Material Used for the Erlanger Silver Catheter. Infection 1999, 27, S43–S45. [Google Scholar] [CrossRef]
- Diao, H.; Si, Y.; Zhu, A.; Ji, L.; Shi, H. Surface Modified Nano-Hydroxyapatite/Poly(Lactide Acid) Composite and Its Osteocyte Compatibility. Mater. Sci. Eng. C 2012, 32, 1796–1801. [Google Scholar] [CrossRef]
- Kong, Y.; Yuan, J.; Qiu, J. Preparation and Characterization of Aligned Carbon Nanotubes/Polylactic Acid Composite Fibers. Phys. B Condens. Matter 2012, 407, 2451–2457. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Qi, N. Controllable Structure, Properties, and Degradation of the Electrospun PLGA/PLA-Blended Nanofibrous Scaffolds. J. Appl. Polym. Sci. 2012, 125, E468–E476. [Google Scholar] [CrossRef]
- Haroosh, H.J.; Chaudhary, D.S.; Dong, Y. Electrospun PLA/PCL Fibers with Tubular Nanoclay: Morphological and Structural Analysis. J. Appl. Polym. Sci. 2012, 124, 3930–3939. [Google Scholar] [CrossRef]
- Liu, X.; Huang, C.; Feng, Y.; Liang, J.; Fan, Y.; Gu, Z.; Zhang, X. Reinforcement of a Porous Collagen Scaffold with Surface-Activated PLA Fibers. J. Biomater. Sci. Polym. Ed. 2010, 21, 963–977. [Google Scholar] [CrossRef] [PubMed]
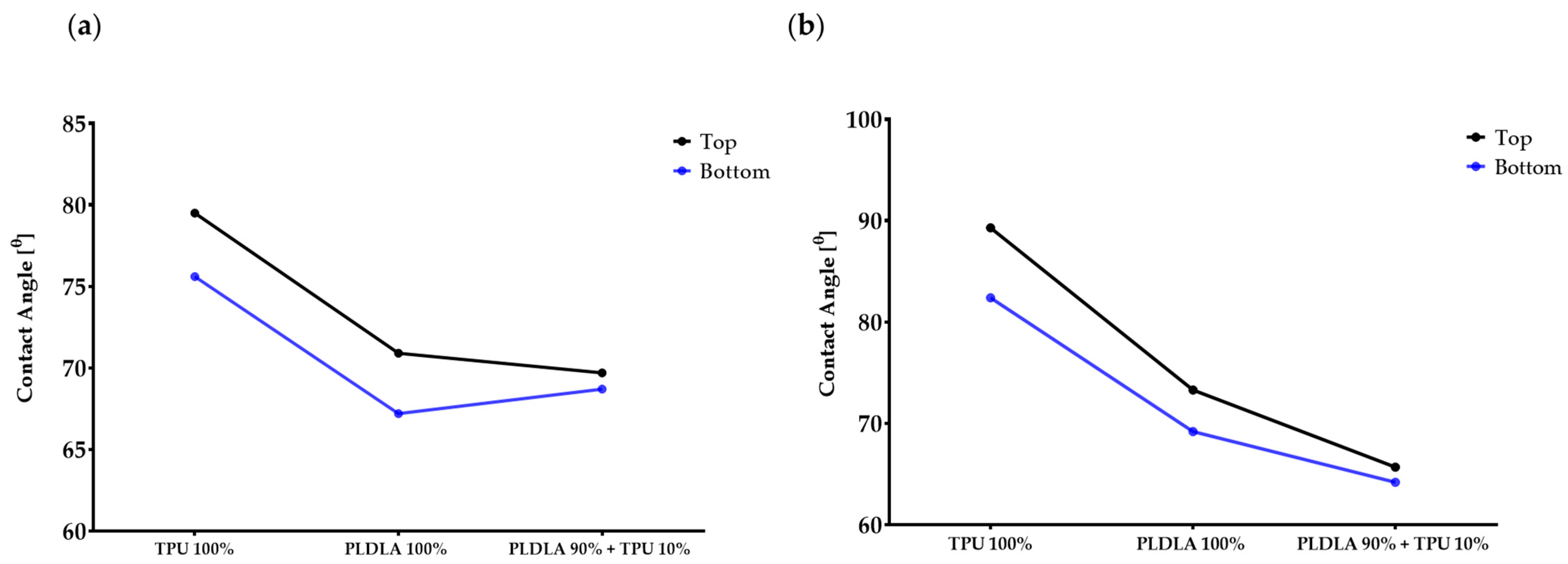





| Polymer Types | Water Contact Angle, N = 10 | Diiodomethane Contact Angle, N = 10 | ||
|---|---|---|---|---|
| Top * | Bottom ** | Top * | Bottom ** | |
| TPU 100% | 79.5 ± 2.5° | 75.6 ± 2.3° | 89.3 ± 1.1° | 82.4 ± 0.9° |
| PLDLA 100% | 70.9 ± 2.1° | 67.2 ± 1.7° | 73.3 ± 0.9° | 69.2 ± 1.0° |
| TPU 10% + PLDLA 90% | 69.7 ± 1.1° | 68.7 ± 0.8° | 65.7 ± 0.7° | 64.2 ± 1.2° |
| Time | PLDLA (100%) | TPU (100%) | TPU + PLDLA (90%/10%) | |||
|---|---|---|---|---|---|---|
| Mass% | pH (7.4) | Mass% | pH (7.4) | Mass% | pH (7.4) | |
| Week 1 | 82.2 | 6.08 | 98.4 | 7.08 | 80.2 | 6.37 |
| Week 2 | 76.4 | 6.01 | 96.4 | 6.84 | 76.2 | 6.11 |
| Week 3 | 61.6 | 5.88 | 93.4 | 6.84 | 71.2 | 5.61 |
| Week 4 | 53.4 | 5.80 | 90.8 | 6.71 | 67.4 | 5.79 |
| Week 5 | 48.2 | 5.74 | 86.8 | 6.46 | 63.6 | 5.87 |
| Week 6 | 44.4 | 5.61 | 82.8 | 6.43 | 57.8 | 5.93 |
| Week 7 | 41.2 | 5.52 | 79.6 | 6.50 | 45.8 | 5.99 |
| Time | Mean ± SD |
|---|---|
| Week 1 | 0.122 ± 0.027 |
| Week 2 | 0.162 ± 0.019 |
| Week 3 | 0.198 ± 0.037 |
| Week 4 | 0.218 ± 0.019 |
| Week 5 | 0.356 ± 0.062 |
| Week 6 | 0.896 ± 0.142 |
| Week 7 | 1.074 ± 0.071 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padjasek, M.; Cisło-Sankowska, A.; Lis-Bartos, A.; Qasem, B.; Marycz, K. PLDLA/TPU Matrix Enriched with Cyclosporine A as a Therapeutic Platform for Immune-Mediated Keratitis (IMMK) in Horses. Int. J. Mol. Sci. 2023, 24, 5735. https://doi.org/10.3390/ijms24065735
Padjasek M, Cisło-Sankowska A, Lis-Bartos A, Qasem B, Marycz K. PLDLA/TPU Matrix Enriched with Cyclosporine A as a Therapeutic Platform for Immune-Mediated Keratitis (IMMK) in Horses. International Journal of Molecular Sciences. 2023; 24(6):5735. https://doi.org/10.3390/ijms24065735
Chicago/Turabian StylePadjasek, Martyna, Anna Cisło-Sankowska, Anna Lis-Bartos, Badr Qasem, and Krzysztof Marycz. 2023. "PLDLA/TPU Matrix Enriched with Cyclosporine A as a Therapeutic Platform for Immune-Mediated Keratitis (IMMK) in Horses" International Journal of Molecular Sciences 24, no. 6: 5735. https://doi.org/10.3390/ijms24065735





