The Role of the Insular Cortex in Pain
Abstract
:1. Introduction
2. Insular Cortex Function
3. Association of the Insular Cortex Function and Pain in Human Studies
| Subject Condition | Brain Region | Experimental Procedure | Measure | Nociceptive Stimulus | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Healthy | Anterior and/or posterior insula | PET scan | Cerebral blood flow | Thermal | Activation | [47,50] |
| Healthy | Anterior and/or posterior insula | fMRI | BOLD | Thermal | Activation | [51,53,69,75] |
| Healthy | Anterior or posterior insula | fMRI | BOLD | Electrical pain | Activation | [52,74] |
| Healthy | Anterior and posterior insula | fMRI | BOLD | Muscle and cutaneous hypertonic solution injection | Activation | [68] |
| Healthy | Posterior insula | fMRI | BOLD | 1% capsaicin cream | Activation | [71] |
| Healthy | Anterior and posterior insula | fMRI | BOLD | Electrical pain and empathic pain | Activation | [76] |
| Healthy | Anterior insula | EEG | EEG potentials | Thermal (laser) | Activation | [73] |
| Healthy | Posterior insula | TMS | Patient rating | Thermal | Pain threshold increase | [80] |
| Epileptic | Posterior and anterior insula | Electrical recording | Evoked cortical potentials | Thermal (laser) | Insula potentials | [46,49] |
| Epileptic | Posterior and anterior insula | Electrical recording | Evoked cortical potentials | Thermal (laser) | Insula potentials and enhanced gamma band oscillations | [54,55] |
| Epileptic | Posterior insula | Electrical stimulation | Behavioral pain responses or patient rating | Intracortical stimulation | Pain sensation | [17,56,70] |
| Epileptic | Anterior and posterior insula | Electrical recording | Intracortical activity | Empathic pain | Activity increase | [78] |
| Epileptic | Anterior insula | Electrical stimulation | Patient rating | Thermal | Pain thresholds increase | [83] |
| Insula lesion | Posterior insula | No procedure | Patient rating | Thermal | Spontaneous pain and dissociated sensory loss | [18,57] |
| Insula lesion | Posterior insula | No procedure | Patient rating | Thermal | Higher pain intensity ratings | [58] |
| Insula lesion | Anterior and/or posterior insula | No procedure | Behavioral motor response | Thermal, pinprick, heavy pressure | Pain asymbolia | [59] |
| Insula lesion | Anterior and posterior | No procedure | Patient rating | Thermal, mechanical | Lesion location dependent pain sensitivity alterations | [72] |
| Insula lesion | Anterior insula | No procedure | Patient rating | Empathic pain | Altered empathic pain perception | [77] |
| Insular lesion and epilepsy | Posterior insula | Electrical recording and stimulation | Behavioral pain responses | Spontaneous and intracortical stimulation | Pain sensation during ictal discharges and after stimulation | [60] |
| Neuropathic pain/painful mononeuropathy | Anterior insula | PET | Cerebral blood flow | No stimulation | activation | [62] |
| Neuropathic pain (trigeminal) | Anterior and posterior insula | MRI | Voxel-based morphometry | No stimulation | Gray matter volume changes | [63] |
| Chronic low back pain | Anterior insula | fMRI | BOLD | Physical maneuver-induced pain exacerbation | Increased insular functional connectivity to S1 | [64] |
| Chronic back pain | ND | fMRI | BOLD | No stimulation | Increased insular functional connectivity to mPFC | [65] |
| Chronic low back pain | Anterior and mid insula | MRI | DTI | No stimulation | Decreased insular connectivity to dlPFC, increased connectivity after pharmacological treatment | [66] |
| Back pain (chronic or subacute) | Anterior Insula | fMRI | BOLD | Spontaneous pain | Activation | [79] |
| Fibromyalgia | Posterior insula | MRI | H-MRS | No stimulation | Increased glutamate levels | [67] |
| Chronic central neuropathic pain | Posterior insula | TMS | Patient rating | Thermal | Increased threshold | [81] |
| Peripheral neuropathic pain | Posterior insula | TMS | Patient rating | No stimulus | Pain score improvements | [82] |
4. Preclinical Studies on the Role of the Insula in Pain
| Subregion | Species | Pain Model | Insula Manipulation | Pain Measure | Noxious Stimulus | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Posterior Insula | Mouse | Naïve | In vivo calcium imaging | Cell activity | Electric tail shock | Activation | [27] |
| Posterior Insula | Mouse | Naïve | Optogenetic Excitation of MCC inputs activation | Paw withdrawal | Mechanical | Increased sensitivity | [84] |
| Capsaicin | Optogenetic inhibition of pyramidal neurons | Decreased sensitivity | |||||
| Posterior Insula | Mouse | Sham & 6-OHDA | Optogenetic excitation of ACC inputs | Paw withdrawal | Mechanical & Thermal | Increased sensitivity | [85] |
| Optogenetic inhibition of ACC inputs | Decreased sensitivity | ||||||
| Posterior Insula | Rat | Neuropathic | Lesion | Paw withdrawal | Mechanical | Reduced sensitivity | [85] |
| Posterior Insula | Macaque | Naive | Pharmacologic inactivation (muscimol) | Hand withdrawal | Thermal | Decreased sensitivity | [87] |
| Anterior Insula | Rat | Naive | Pharmacologic & genetic inactivation | Paw withdrawal | Thermal | Decreased sensitivity | [89] |
| Anterior Insula | Rat | Naive | Pharmacologic inactivation (morphine) | Formalin score | Chemical (Formalin) | Reduced score | [95] |
| Spinal cFos | Decrease in expression | ||||||
| Spinal electro-physiology | Decrease of firing | ||||||
| Anterior Insula | Rat | Naive | Pharmacologic modulation dopaminergic | Formalin score | Chemical (Formalin) | Pharmacologic agent dependent | [96] |
| Spinal cFos | |||||||
| Spinal electro-physiology | |||||||
| Paw withdrawal | Thermal | ||||||
| Anterior Insula | Rat | Sciatic de-nervation | Pharmacologic modulation dopaminergic | Autotomy score | No stimulus | Decreased score | [97] |
| Anterior Insula | Rat | Naive | Formalin score | Chemical (formalin) | Decreased score | [97] | |
| Anterior Insula | Rat | Inflam-matory and neuropathic | Lesion | Paw withdrawal | Thermal and Mechanical | Reduced sensitivity | [90] |
| Anterior and Posterior Insula | Rat | Chronic Pancreatitis | No manipulation | cFos staining | No Stimulus | Increased expression | [91] |
| Pharmacologic (CNQX, APV) and chemogenetic inhibition | Abdomen withdrawal | Mechanical | Reduced sensitivity | ||||
| Anterior Insula | Mouse | Neuropathic (nerve ligation) | Pharmacologic inhibition (CNQX) | Paw withdrawal | Mechanical | Reduced sensitivity | [92] |
| Anterior Insula | Rat | Neuropathic (SNI) | fMRI | BOLD signal | No stimulus | Enhanced activation | [94] |
| Anterior Insula | Mouse | Social transfer of pain | Pyramidal or interneuron cell ablation | Paw withdrawal | Mechanical | Increased and decreased sensitivity respectively | [99] |
| Anterior Insula | Mouse | Social transfer of pain | CoCl2 inactivation | Writhing test | Chemical | Decreased writhing | [100] |
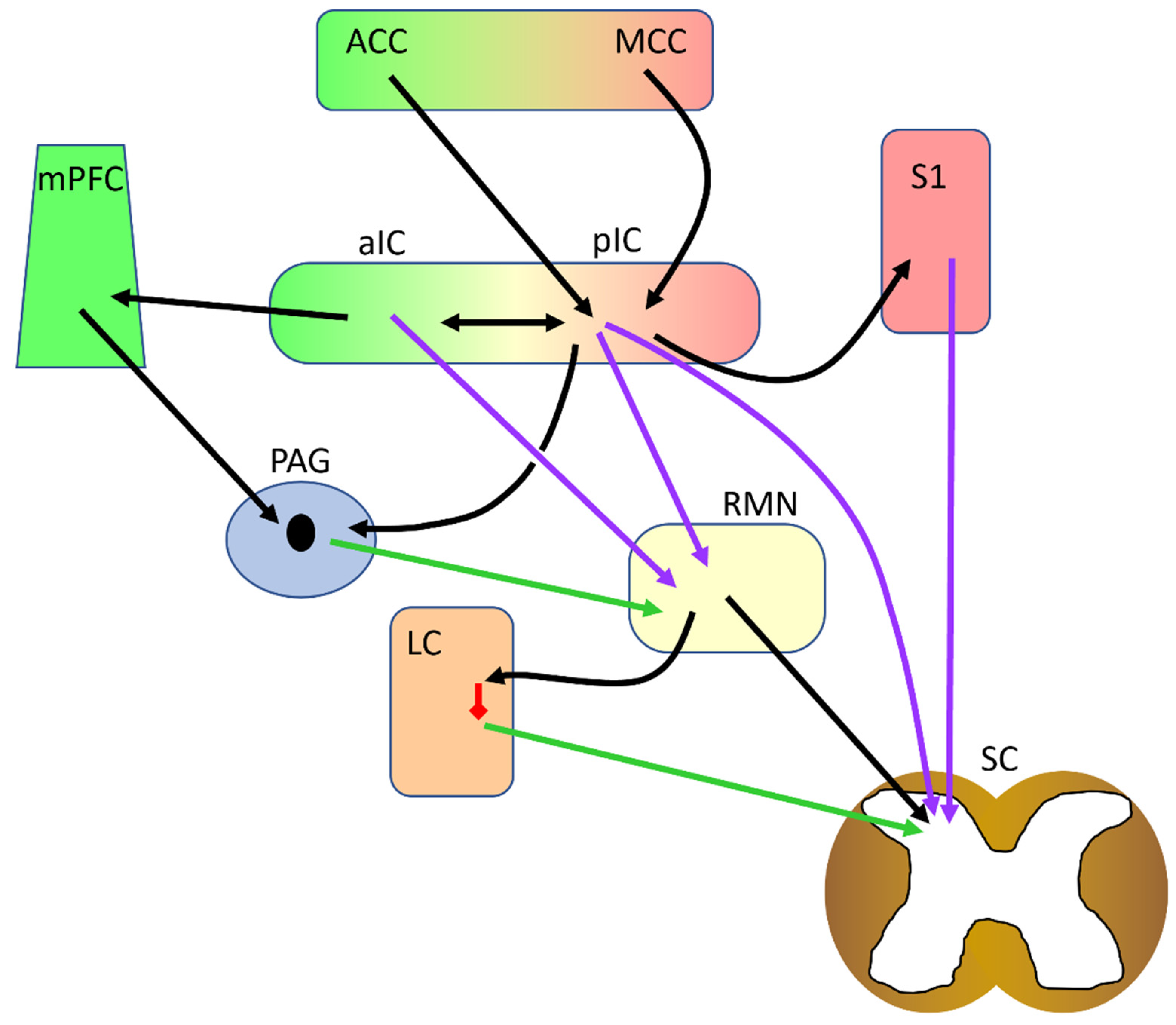
5. Insular Connectivity to and from Other Pain-Related Brain Regions
6. Discussion and Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Treede, R.D.; Kenshalo, D.R.; Gracely, R.H.; Jones, A.K.P. The Cortical Representation of Pain. Pain 1999, 79, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Apkarian, A.V. Nociception, Pain, Negative Moods, and Behavior Selection. Neuron 2015, 87, 474–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apkarian, A.V.; Bushnell, M.C.; Treede, R.D.; Zubieta, J.K. Human Brain Mechanisms of Pain Perception and Regulation in Health and Disease. Eur. J. Pain 2005, 9, 463–484. [Google Scholar] [CrossRef]
- Ong, W.; Stohler, C.S.; Herr, D.R. Role of the Prefrontal Cortex in Pain Processing. Mol. Neurobiol. 2019, 56, 1137–1166. [Google Scholar] [CrossRef] [Green Version]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The Effect of Pain on Cognitive Function: A Review of Clinical and Preclinical Research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denk, F.; McMahon, S.B.; Tracey, I. Pain Vulnerability: A Neurobiological Perspective. Nat. Neurosci. 2014, 17, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Apkarian, V.A.; Hashmi, J.A.; Baliki, M.N. Pain and the Brain: Specificity and Plasticity of the Brain in Clinical Chronic Pain. Pain 2011, 152, S49–S64. [Google Scholar] [CrossRef] [Green Version]
- Martucci, K.T.; Ng, P.; MacKey, S. Neuroimaging Chronic Pain: What Have We Learned and Where Are We Going? Future Neurol. 2014, 9, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Cichon, J.; Blanck, T.J.J.; Gan, W.B.; Yang, G. Activation of Cortical Somatostatin Interneurons Prevents the Development of Neuropathic Pain. Nat. Neurosci. 2017, 20, 1122–1132. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Nabekura, J. Rapid Synaptic Remodeling in the Adult Somatosensory Cortex Following Peripheral Nerve Injury and Its Association with Neuropathic Pain. J. Neurosci. 2011, 31, 5477–5482. [Google Scholar] [CrossRef] [Green Version]
- Eto, K.; Wake, H.; Watanabe, M.; Ishibashi, H.; Noda, M.; Yanagawa, Y.; Nabekura, J. Inter-Regional Contribution of Enhanced Activity of the Primary Somatosensory Cortex to the Anterior Cingulate Cortex Accelerates Chronic Pain Behavior. J. Neurosci. 2011, 31, 7631–7636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santello, M.; Nevian, T. Dysfunction of Cortical Dendritic Integration in Neuropathic Pain Reversed by Serotoninergic Neuromodulation. Neuron 2015, 86, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-Y.; Wang, N.; Wang, Y.-J.; Zuo, Z.-X.; Koga, K.; Luo, F.; Zhuo, M.; Wang, N.; Luo, F.; Zuo, Z.-X.; et al. Long-Term Temporal Imprecision of Information Coding in the Anterior Cingulate Cortex of Mice with Peripheral Inflammation or Nerve Injury. J. Neurosci. 2014, 34, 10675–10687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metz, A.E.; Yau, H.J.H.-J.; Centeno, M.V.; Apkarian, A.V.; Martina, M. Morphological and Functional Reorganization of Rat Medial Prefrontal Cortex in Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2009, 106, 2423–2428. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Larrea, L. The Posterior Insular-Opercular Region and the Search of a Primary Cortex for Pain. Neurophysiol. Clin. 2012, 42, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.C.W.; Tracey, I. The Insula: A Multidimensional Integration Site for Pain. Pain 2007, 128, 3–5. [Google Scholar] [CrossRef]
- Mazzola, L.; Isnard, J.; Peyron, R.; Guénot, M.; Mauguière, F. Somatotopic Organization of Pain Responses to Direct Electrical Stimulation of the Human Insular Cortex. Pain 2009, 146, 99–104. [Google Scholar] [CrossRef]
- Garcia-Larrea, L.; Perchet, C.; Creac’H, C.; Convers, P.; Peyron, R.; Laurent, B.; Mauguire, F.; Magnin, M. Operculo-Insular Pain (Parasylvian Pain): A Distinct Central Pain Syndrome. Brain 2010, 133, 2528–2539. [Google Scholar] [CrossRef] [Green Version]
- Gogolla, N. The Insular Cortex. Curr. Biol. 2017, 27, R580–R586. [Google Scholar] [CrossRef] [Green Version]
- Gehrlach, D.A.; Weiand, C.; Gaitanos, T.N.; Cho, E.; Klein, A.S.; Hennrich, A.A.; Conzelmann, K.-K.K.; Gogolla, N. A Whole-Brain Connectivity Map of Mouse Insular Cortex. eLife 2020, 9, 1–78. [Google Scholar] [CrossRef]
- Chen, X.; Gabitto, M.; Peng, Y.; Ryba, N.J.P.; Zuker, C.S. A Gustotopic Map of Taste Qualities in the Mammalian Brain. Science 2011, 333, 1262–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, A.D.; Chen, K.; Bandy, D.; Reiman, E.M. Thermosensory Activation of Insular Cortex. Nat. Neurosci. 2000, 3, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B. Visceral Sensation and Visceral Sensory Disorders. Continuum 2007, 13, 204–214. [Google Scholar] [CrossRef]
- Craig, A.D. How Do You Feel? Interoception: The Sense of the Physiological Condition of the Body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D.B. How Do You Feel—Now? The Anterior Insula and Human Awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Devue, C.; Collette, F.; Balteau, E.; Degueldre, C.; Luxen, A.; Maquet, P.; Brédart, S. Here I Am: The Cortical Correlates of Visual Self-Recognition. Brain Res. 2007, 1143, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrlach, D.A.; Dolensek, N.; Klein, A.S.; Roy Chowdhury, R.; Matthys, A.; Junghänel, M.; Gaitanos, T.N.; Podgornik, A.; Black, T.D.; Reddy Vaka, N.; et al. Aversive State Processing in the Posterior Insular Cortex. Nat. Neurosci. 2019, 22, 1424–1437. [Google Scholar] [CrossRef]
- Klein, A.S.; Dolensek, N.; Weiand, C.; Gogolla, N. Fear Balance Is Maintained by Bodily Feedback to the Insular Cortex in Mice. Science 2021, 374, 1010–1015. [Google Scholar] [CrossRef]
- Méndez-Ruette, M.; Linsambarth, S.; Moraga-Amaro, R.; Quintana-Donoso, D.; Méndez, L.; Tamburini, G.; Cornejo, F.; Torres, R.F.; Stehberg, J. The Role of the Rodent Insula in Anxiety. Front. Physiol. 2019, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Paulus, M.P.; Stein, M.B. An Insular View of Anxiety. Biol. Psychiatry 2006, 60, 383–387. [Google Scholar] [CrossRef]
- Contreras, M.; Billeke, P.; Vicencio, S.; Madrid, C.; Perdomo, G.; González, M.; Torrealba, F. A Role for the Insular Cortex in Long-Term Memory for Context-Evoked Drug Craving in Rats. Neuropsychopharmacology 2012, 37, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Rattoni, F.; Okuda, S.; Roozendaal, B.; McGaugh, J.L. Insular Cortex Is Involved in Consolidation of Object Recognition Memory. Learn. Mem. 2005, 12, 447–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Prats, A.; Paradiso, E.; Castaldi, F.; Sadeghi, M.; Mir, M.Y.; Hörtnagl, H.; Göbel, G.; Ferraguti, F. VIP-Expressing Interneurons in the Anterior Insular Cortex Contribute to Sensory Processing to Regulate Adaptive Behavior. Cell Rep. 2022, 39, 110893. [Google Scholar] [CrossRef] [PubMed]
- Kargl, D.; Kaczanowska, J.; Ulonska, S.; Groessl, F.; Piszczek, L.; Lazovic, J.; Buehler, K.; Haubensak, W. The Amygdala Instructs Insular Feedback for Affective Learning. Elife 2020, 9, e55585. [Google Scholar] [CrossRef]
- Uddin, L.Q. Salience Processing and Insular Cortical Function and Dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Livneh, Y.; Sugden, A.U.; Madara, J.C.; Essner, R.A.; Flores, V.I.; Sugden, L.A.; Resch, J.M.; Lowell, B.B.; Andermann, M.L. Estimation of Current and Future Physiological States in Insular Cortex. Neuron 2020, 105, 1094–1111.e10. [Google Scholar] [CrossRef]
- Barrett, L.F.; Simmons, W.K. Interoceptive Predictions in the Brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Namkung, H.; Kim, S.H.; Sawa, A. The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci. 2017, 40, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Critchley, H.D.; Harrison, N.A. Visceral Influences on Brain and Behavior. Neuron 2013, 77, 624–638. [Google Scholar] [CrossRef] [Green Version]
- Mutschler, I.; Hänggi, J.; Frei, M.; Lieb, R.; Holforth, M.G.; Seifritz, E.; Spinelli, S. Insular Volume Reductions in Patients with Major Depressive Disorder. Neurol. Psychiatry Brain Res. 2019, 33, 39–47. [Google Scholar] [CrossRef]
- Avery, J.A.; Drevets, W.C.; Moseman, S.E.; Bodurka, J.; Barcalow, J.C.; Simmons, W.K. Major Depressive Disorder Is Associated with Abnormal Interoceptive Activity and Functional Connectivity in the Insula. Biol. Psychiatry 2014, 76, 258–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, L.Q.; Menon, V. The Anterior Insula in Autism: Under-Connected and under-Examined. Neurosci. Biobehav. Rev. 2009, 33, 1198–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylie, K.P.; Tregellas, J.R. The Role of the Insula in Schizophrenia. Schizophr. Res. 2010, 123, 93–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naqvi, N.H.; Gaznick, N.; Tranel, D.; Bechara, A. The Insula: A Critical Neural Substrate for Craving and Drug Seeking under Conflict and Risk. Ann. N. Y. Acad. Sci. 2014, 1316, 53–70. [Google Scholar] [CrossRef] [Green Version]
- Droutman, V.; Read, S.J.; Bechara, A. Revisiting the Role of the Insula in Addiction. Trends Cogn. Sci. 2015, 19, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Frot, M.; Faillenot, I.; Mauguière, F. Processing of Nociceptive Input from Posterior to Anterior Insula in Humans. Hum. Brain Mapp. 2014, 35, 5486–5499. [Google Scholar] [CrossRef]
- Coghill, R.C.; Talbot, J.D.; Evans, A.C.; Meyer, E.; Gjedde, A.; Bushnell, M.; Duncan, G.H. Distributed Processing of Pain and Vibration by the Human Brain. J. Neurosci. 1994, 14, 4095–4108. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Larrea, L.; Peyron, R. Pain Matrices and Neuropathic Pain Matrices: A Review. Pain 2013, 154, S29–S43. [Google Scholar] [CrossRef] [Green Version]
- Bastuji, H.; Frot, M.; Perchet, C.; Magnin, M.; Garcia-Larrea, L. Pain Networks from the inside: Spatiotemporal Analysis of Brain Responses Leading from Nociception to Conscious Perception. Hum. Brain Mapp. 2016, 37, 4301–4315. [Google Scholar] [CrossRef]
- Coghill, R.C.; Sang, C.N.; Maisog, J.M.; Misra, G.; Wang, W.-E.; Archer, D.B.; Roy, A.; Coombes, S.A.; Chien, J.H.; Iadarola, M.J.; et al. Pain Intensity Processing within the Human Brain: A Bilateral, Distributed Mechanism. J. Neurophysiol. 1999, 82, 1934–1943. [Google Scholar] [CrossRef]
- Oshiro, Y.; Quevedo, A.S.; McHaffie, J.G.; Kraft, R.A.; Coghill, R.C. Brain Mechanisms Supporting Discrimination of Sensory Features of Pain: A New Model. J. Neurosci. 2009, 29, 14924–14931. [Google Scholar] [CrossRef] [Green Version]
- Alkire, M.T.; White, N.S.; Hsieh, R.; Haier, R.J. Dissociable Brain Activation Responses to 5-Hz Electrical Pain Stimulation. Anesthesiology 2004, 100, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Baliki, M.N.; Geha, P.Y.; Apkarian, A.V. Parsing Pain Perception between Nociceptive Representation and Magnitude Estimation. J. Neurophysiol. 2009, 101, 875–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberati, G.; Mulders, D.; Algoet, M.; van den Broeke, E.N.; Santos, S.F.; Ribeiro Vaz, J.G.; Raftopoulos, C.; Mouraux, A. Insular Responses to Transient Painful and Non-Painful Thermal and Mechanical Spinothalamic Stimuli Recorded Using Intracerebral EEG. Sci. Rep. 2020, 10, 22319. [Google Scholar] [CrossRef] [PubMed]
- Liberati, G.; Klöcker, A.; Algoet, M.; Mulders, D.; Maia Safronova, M.; Ferrao Santos, S.; Ribeiro Vaz, J.-G.; Raftopoulos, C.; Mouraux, A. Gamma-Band Oscillations Preferential for Nociception Can Be Recorded in the Human Insula. Cereb. Cortex 2018, 28, 3650–3664. [Google Scholar] [CrossRef] [Green Version]
- Ostrowsky, K.; Magnin, M.; Ryvlin, P.; Isnard, J.; Guenot, M.; Mauguière, F. Representation of Pain and Somatic Sensation in the Human Insula: A Study of Responses to Direct Electrical Cortical Stimulation. Cereb. Cortex 2002, 12, 376–385. [Google Scholar] [CrossRef]
- Schmahmann, J.D.; Leifer, D. Parietal Pseudothalamic Pain Syndrome. Clinical Features and Anatomic Correlates. Arch. Neurol. 1992, 49, 1032–1037. [Google Scholar] [CrossRef]
- Starr, C.J.; Sawaki, L.; Wittenberg, G.F.; Burdette, J.H.; Oshiro, Y.; Quevedo, A.S.; Coghill, R.C. Roles of the Insular Cortex in the Modulation of Pain: Insights from Brain Lesions. J. Neurosci. 2009, 29, 2684–2694. [Google Scholar] [CrossRef] [Green Version]
- Berthier, M.; Starkstein, S.; Leiguarda, R. Asymbolia for Pain: A Sensory-limbic Disconnection Syndrome. Ann. Neurol. 1988, 24, 41–49. [Google Scholar] [CrossRef]
- Isnard, J.; Magnin, M.; Jung, J.; Mauguire, F.; Garcia-Larrea, L. Does the Insula Tell Our Brain That We Are in Pain? Pain 2011, 152, 946–951. [Google Scholar] [CrossRef]
- Boivie, J. Central Post-Stroke Pain. In Handbook of Clinical Neurology; Cervero, F., Jensen, T.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 81, pp. 715–730. ISBN 9780444519016. [Google Scholar]
- Hsieh, J.-C.; Belfrage, M.; Stone-Elander, S.; Hansson, P.; Ingvar, M. Central Representation of Chronic Ongoing Neuropathic Pain Studied by Positron Emission Tomography. Pain 1995, 63, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Gustin, S.M.; Peck, C.C.; Wilcox, S.L.; Nash, P.G.; Murray, G.M.; Henderson, L.A. Different Pain, Different Brain: Thalamic Anatomy in Neuropathic and Non-Neuropathic Chronic Pain Syndromes. J. Neurosci. 2011, 31, 5956–5964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Mawla, I.; Kong, J.; Lee, J.; Gerber, J.; Ortiz, A.; Kim, H.; Chan, S.T.; Loggia, M.L.; Wasan, A.D.; et al. Somatotopically Specific Primary Somatosensory Connectivity to Salience and Default Mode Networks Encodes Clinical Pain. Pain 2019, 160, 1594–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliki, M.N.; Baria, A.T.; Apkarian, A.V. The Cortical Rhythms of Chronic Back Pain. J. Neurosci. 2011, 31, 13981–13990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čeko, M.; Shir, Y.; Ouellet, J.A.; Ware, M.A.; Stone, L.S.; Seminowicz, D.A. Partial Recovery of Abnormal Insula and Dorsolateral Prefrontal Connectivity to Cognitive Networks in Chronic Low Back Pain after Treatment. Hum. Brain Mapp. 2015, 36, 2075–2092. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.E.; Sundgren, P.C.; Craig, A.D.; Kirshenbaum, E.; Sen, A.; Napadow, V.; Clauw, D.J. Elevated Insular Glutamate in Fibromyalgia Is Associated with Experimental Pain. Arthritis Rheum. 2009, 60, 3146–3152. [Google Scholar] [CrossRef] [Green Version]
- Henderson, L.A.; Gandevia, S.C.; Macefield, V.G. Somatotopic Organization of the Processing of Muscle and Cutaneous Pain in the Left and Right Insula Cortex: A Single-Trial FMRI Study. Pain 2007, 128, 20–30. [Google Scholar] [CrossRef]
- Brooks, J.C.W.; Zambreanu, L.; Godinez, A.; Craig, A.D.; Tracey, I. Somatotopic Organisation of the Human Insula to Painful Heat Studied with High Resolution Functional Imaging. Neuroimage 2005, 27, 201–209. [Google Scholar] [CrossRef]
- Mazzola, L.; Isnard, J.; Mauguière, F. Somatosensory and Pain Responses to Stimulation of the Second Somatosensory Area (SII) in Humans. A Comparison with SI and Insular Responses. Cereb. Cortex 2006, 16, 960–968. [Google Scholar] [CrossRef] [Green Version]
- Segerdahl, A.R.; Mezue, M.; Okell, T.W.; Farrar, J.T.; Tracey, I. The Dorsal Posterior Insula Subserves a Fundamental Role in Human Pain. Nat. Neurosci. 2015, 18, 499–500. [Google Scholar] [CrossRef]
- Greenspan, J.D.; Lee, R.R.; Lenz, F.A. Pain Sensitivity Alterations as a Function of Lesion Location in the Parasylvian Cortex. Pain 1999, 81, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Stancak, A.; Fallon, N. Emotional Modulation of Experimental Pain: A Source Imaging Study of Laser Evoked Potentials. Front. Hum. Neurosci. 2013, 7, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Piché, M.; Chen, J.-I.; Peretz, I.; Rainville, P. Cerebral and Spinal Modulation of Pain by Emotions. Proc. Natl. Acad. Sci. USA 2009, 106, 20900–20905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.; White, N.S.; Kwong, K.K.; Vangel, M.G.; Rosman, I.S.; Gracely, R.H.; Gollub, R.L. Using FMRI to Dissociate Sensory Encoding from Cognitive Evaluation of Heat Pain Intensity. Hum. Brain Mapp. 2006, 27, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for Pain Involves the Affective but Not Sensory Components of Pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Gao, Z.; Wang, X.; Liu, X.; Knight, R.T.; Hof, P.R.; Fan, J. Anterior Insular Cortex Is Necessary for Empathetic Pain Perception. Brain 2012, 135, 2726–2735. [Google Scholar] [CrossRef] [Green Version]
- Soyman, E.; Bruls, R.; Ioumpa, K.; Müller-Pinzler, L.; Gallo, S.; Qin, C.; van Straaten, E.C.; Self, M.W.; Peters, J.C.; Possel, J.K.; et al. Intracranial Human Recordings Reveal Association between Neural Activity and Perceived Intensity for the Pain of Others in the Insula. Elife 2022, 11, e75197. [Google Scholar] [CrossRef]
- Hashmi, J.A.; Baliki, M.N.; Huang, L.; Baria, A.T.; Torbey, S.; Hermann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Shape Shifting Pain: Chronification of Back Pain Shifts Brain Representation from Nociceptive to Emotional Circuits. Brain 2013, 136, 2751–2768. [Google Scholar] [CrossRef] [Green Version]
- Lenoir, C.; Algoet, M.; Mouraux, A. Deep Continuous Theta Burst Stimulation of the Operculo-Insular Cortex Selectively Affects Aδ-Fibre Heat Pain. J. Physiol. 2018, 596, 4767–4787. [Google Scholar] [CrossRef]
- Galhardoni, R.; Da Silva, V.A.; García-Larrea, L.; Dale, C.; Baptista, A.F.; Barbosa, L.M.; Bahia Menezes, L.M.; De Siqueira, S.R.D.T.; Valério, F.; Rosi, J.; et al. Insular and Anterior Cingulate Cortex Deep Stimulation for Central Neuropathic Pain Disassembling the Percept of Pain. Neurology 2019, 92, E2165–E2175. [Google Scholar] [CrossRef] [Green Version]
- Dongyang, L.; Fernandes, A.M.; da Cunha, P.H.M.; Tibes, R.; Sato, J.; Listik, C.; Dale, C.; Kubota, G.T.; Galhardoni, R.; Teixeira, M.J.; et al. Posterior-Superior Insular Deep Transcranial Magnetic Stimulation Alleviates Peripheral Neuropathic Pain—A Pilot Double-Blind, Randomized Cross-over Study. Neurophysiol. Clin. 2021, 51, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Moosa, S.; Quigg, M.; Elias, W.J. Anterior Insula Stimulation Increases Pain Threshold in Humans: A Pilot Study. J. Neurosurg. 2021, 135, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Pelzer, P.; Heinl, C.; Tang, W.; Gangadharan, V.; Flor, H.; Sprengel, R.; Kuner, T.; Kuner, R. A Pathway from Midcingulate Cortex to Posterior Insula Gates Nociceptive Hypersensitivity. Nat. Neurosci. 2017, 20, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Bouchatta, O.; Aby, F.; Sifeddine, W.; Bouali-Benazzouz, R.; Brochoire, L.; Manouze, H.; Fossat, P.; Ba M’Hamed, S.; Bennis, M.; Landry, M. Pain Hypersensitivity in a Pharmacological Mouse Model of Attention-Deficit/Hyperactivity Disorder. Proc. Natl. Acad. Sci. USA 2022, 119, e2114094119. [Google Scholar] [CrossRef]
- Benison, A.M.; Chumachenko, S.; Harrison, J.A.; Maier, S.F.; Falci, S.P.; Watkins, L.R.; Barth, D.S. Caudal Granular Insular Cortex Is Sufficient and Necessary for the Long-Term Maintenance of Allodynic Behavior in the Rat Attributable to Mononeuropathy. J. Neurosci. 2011, 31, 6317–6328. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, K.; Takashima, I.; Matsuda, K.; Higo, N. Pharmacological Inactivation of the Primate Posterior Insular/Secondary Somatosensory Cortices Attenuates Thermal Hyperalgesia. Eur. J. Pain 2022, 26, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, J.; Bayet-Robert, M.; Dalmann, R.; Guerrab, A.; Aissouni, Y.; Graveron-Demilly, D.; Chalus, M.; Pinguet, J.; Eschalier, A.; Richard, D.; et al. Cholinergic Neurotransmission in the Posterior Insular Cortex Is Altered in Preclinical Models of Neuropathic Pain: Key Role of Muscarinic M2 Receptors in Donepezil-Induced Antinociception. J. Neurosci. 2015, 35, 16418–16430. [Google Scholar] [CrossRef] [Green Version]
- Jasmin, L.; Rabkin, S.D.; Granato, A.; Boudah, A.; Ohara, P.T. Analgesia and Hyperalgesia from GABA-Mediated Modulation of the Cerebral Cortex. Nature 2003, 424, 316–320. [Google Scholar] [CrossRef]
- Coffeen, U.; Ortega-Legaspi, J.M.; López-Muñoz, F.J.; Simón-Arceo, K.; Jaimes, O.; Pellicer, F. Insular Cortex Lesion Diminishes Neuropathic and Inflammatory Pain-like Behaviours. Eur. J. Pain 2011, 15, 132–138. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, L.-T.; Chen, Y.-B.; Ren, D.; Chen, Y.-B.; Li, Y.-Q.; Sun, H.-K.; Qiu, X.-T.; Zhang, T.; Zhang, M.-M.; et al. Anterior Insular Cortex Mediates Hyperalgesia Induced by Chronic Pancreatitis in Rats. Mol. Brain 2019, 12, 76. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Zhang, M.; Liu, Y.; Guo, Y.; Zhao, H.; Song, Q.; Zhao, M.; Huganir, R.L.; Luo, J.; Xu, H.; et al. GluA1 Phosphorylation Contributes to Postsynaptic Amplification of Neuropathic Pain in the Insular Cortex. J. Neurosci. 2014, 34, 13505–13515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, S.; Chen, T.; Koga, K.; Guo, Y.Y.; Xu, H.; Song, Q.; Wang, J.J.; Descalzi, G.; Kaang, B.K.; Luo, J.H.; et al. An Increase in Synaptic NMDA Receptors in the Insular Cortex Contributes to Neuropathic Pain. Sci. Signal. 2013, 6, ra34. [Google Scholar] [CrossRef]
- Chao, T.-H.H.; Chen, J.-H.; Yen, C.-T. Plasticity Changes in Forebrain Activity and Functional Connectivity during Neuropathic Pain Development in Rats with Sciatic Spared Nerve Injury. Mol. Brain 2018, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Burkey, A.R.; Carstens, E.; Wenniger, J.J.; Tang, J.; Jasmin, L. An Opioidergic Cortical Antinociception Triggering Site in the Agranular Insular Cortex of the Rat That Contributes to Morphine Antinociception. J. Neurosci. 1996, 16, 6612–6623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkey, A.R.; Carstens, E.; Jasmin, L. Dopamine Reuptake Inhibition in the Rostral Agranular Insular Cortex Produces Antinociception. J. Neurosci. 1999, 19, 4169–4179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffeen, U.; López-Avila, A.; Ortega-Legaspi, J.M.; del Ángel, R.; López-Muñoz, F.J.; Pellicer, F. Dopamine Receptors in the Anterior Insular Cortex Modulate Long-Term Nociception in the Rat. Eur. J. Pain 2008, 12, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eltrabily, M.; Espinosa de Los Monteros-Zúñiga, A.; Manzano-García, A.; Martínez-Lorenzana, G.; Condés-Lara, M.; González-Hernández, A. The Rostral Agranular Insular Cortex, a New Site of Oxytocin to Induce Antinociception. J. Neurosci. 2020, 40, 5669–5680. [Google Scholar] [CrossRef]
- Zhang, M.-M.; Geng, A.-Q.; Chen, K.; Wang, J.; Wang, P.; Qiu, X.-T.; Gu, J.-X.; Fan, H.-W.; Zhu, D.-Y.; Yang, S.-M.; et al. Glutamatergic Synapses from the Insular Cortex to the Basolateral Amygdala Encode Observational Pain. Neuron 2022, 110, 1993–2008.e6. [Google Scholar] [CrossRef]
- Zaniboni, C.R.; Pelarin, V.; Baptista-de-Souza, D.; Canto-de-Souza, A. Empathy for Pain: Insula Inactivation and Systemic Treatment with Midazolam Reverses the Hyperalgesia Induced by Cohabitation with a Pair in Chronic Pain Condition. Front. Behav. Neurosci. 2018, 12, 278. [Google Scholar] [CrossRef]
- Guldin, W.O.; Markowitsch, H.J. Cortical and Thalamic Afferent Connections of the Insular and Adjacent Cortex of the Rat. J. Comp. Neurol. 1983, 215, 135–153. [Google Scholar] [CrossRef]
- Dum, R.P.; Levinthal, D.J.; Strick, P.L. The Spinothalamic System Targets Motor and Sensory Areas in the Cerebral Cortex of Monkeys. J. Neurosci. 2009, 29, 14223–14235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apkarian, A.V.; Shi, T. Squirrel Monkey Lateral Thalamus. I. Somatic Nociresponsive Neurons and Their Relation to Spinothalamic Terminals. J. Neurosci. 1994, 14, 6779–6795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauriau, C.; Bernard, J.F. Posterior Triangular Thalamic Neurons Convey Nociceptive Messages to the Secondary Somatosensory and Insular Cortices in the Rat. J. Neurosci. 2004, 24, 752–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.J.; Cassell, M.D. Cascade Projections from Somatosensory Cortex to the Rat Basolateral Amygdala via the Parietal Insular Cortex. J. Comp. Neurol. 1998, 399, 469–491. [Google Scholar] [CrossRef]
- Jasmin, L.; Granato, A.; Ohara, P.T. Rostral Agranular Insular Cortex and Pain Areas of the Central Nervous System: A Tract-Tracing Study in the Rat. J. Comp. Neurol. 2004, 468, 425–440. [Google Scholar] [CrossRef]
- Bastuji, H.; Frot, M.; Perchet, C.; Hagiwara, K.; Garcia-Larrea, L. Convergence of Sensory and Limbic Noxious Input into the Anterior Insula and the Emergence of Pain from Nociception. Sci. Rep. 2018, 8, 13360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Z.; Li, H.; Naser, P.V.; Oswald, M.J.; Kuner, R. Suppression of Neuropathic Pain and Comorbidities by Recurrent Cycles of Repetitive Transcranial Direct Current Motor Cortex Stimulation in Mice. Sci. Rep. 2021, 11, 9735. [Google Scholar] [CrossRef]
- Shi, C.; Cassell, M.D. Cortical, Thalamic, and Amygdaloid Connections of the Anterior and Posterior Insular Cortices. J. Comp. Neurol. 1998, 399, 440–468. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, J.J.; Wang, L.; Kan, Y.P.; Liu, Y.M.; Wu, Y.J.; Gu, X.; Yi, X.; Lin, Z.J.; Wang, Q.; et al. Insular Cortical Circuits as an Executive Gateway to Decipher Threat or Extinction Memory via Distinct Subcortical Pathways. Nat. Commun. 2022, 13, 5540. [Google Scholar] [CrossRef]
- Reep, R.L.; Winans, S.S. Efferent Connections of Dorsal and Ventral Agranular Insular Cortex in the Hamster, Mesocricetus Auratus. Neuroscience 1982, 7, 2609–2635. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Xiao, X.; Yang, T.; Ritola, K.; Hantman, A.; Li, Y.; Huang, Z.J.; Li, B. A Genetically Defined Insula-Brainstem Circuit Selectively Controls Motivational Vigor. Cell 2021, 184, 6344–6360.e18. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Iwata, K.; Kobayashi, M. Insular Cortical Descending Projections Facilitate Neuronal Responses to Noxious but Not Innoxious Stimulation in Rat Trigeminal Spinal Subnucleus Caudalis. Brain Res. 2023, 1804, 148248. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Yamamoto, K.; Kobayashi, M. Descending Projections from the Insular Cortex to the Trigeminal Spinal Subnucleus Caudalis Facilitate Excitatory Outputs to the Parabrachial Nucleus in Rats. Pain 2022, 164, e157–e173. [Google Scholar] [CrossRef] [PubMed]
- Floyd, N.S.; Price, J.L.; Ferry, A.T.; Keay, K.A.; Bandler, R. Orbitomedial Prefrontal Cortical Projections to Distinct Longitudinal Columns of the Periaqueductal Gray in the Rat. J. Comp. Neurol. 2000, 422, 556–578. [Google Scholar] [CrossRef]
- Huang, J.; Gadotti, V.M.; Chen, L.; Souza, I.A.; Huang, S.; Wang, D.; Ramakrishnan, C.; Deisseroth, K.; Zhang, Z.; Zamponi, G.W. A Neuronal Circuit for Activating Descending Modulation of Neuropathic Pain. Nat. Neurosci. 2019, 22, 1659–1668. [Google Scholar] [CrossRef]
- Wager, T.D.; Rilling, J.K.; Smith, E.E.; Sokolik, A.; Casey, K.L.; Davidson, R.J.; Kosslyn, S.M.; Rose, R.M.; Cohen, J.D. Placebo-Induced Changes in FMRI in the Anticipation and Experience of Pain. Science 2004, 303, 1162–1167. [Google Scholar] [CrossRef]
- Huynh, V.; Lütolf, R.; Rosner, J.; Luechinger, R.; Curt, A.; Kollias, S.; Michels, L.; Hubli, M. Descending Pain Modulatory Efficiency in Healthy Subjects Is Related to Structure and Resting Connectivity of Brain Regions. Neuroimage 2022, 247, 118742. [Google Scholar] [CrossRef]
- Li, H.; Gan, Z.; Wang, L.; Oswald, M.J.; Kuner, R. Prolonged Suppression of Neuropathic Hypersensitivity upon Neurostimulation of the Posterior Insula in Mice. Cells 2022, 11, 3303. [Google Scholar] [CrossRef]
- Alonso-Matielo, H.; Gonçalves, E.S.; Campos, M.; Oliveira, V.R.S.; Toniolo, E.F.; Alves, A.S.; Lebrun, I.; de Andrade, D.C.; Teixeira, M.J.; Britto, L.R.G.; et al. Electrical Stimulation of the Posterior Insula Induces Mechanical Analgesia in a Rodent Model of Neuropathic Pain by Modulating GABAergic Signaling and Activity in the Pain Circuitry. Brain Res. 2021, 1754, 147237. [Google Scholar] [CrossRef]
- Zhuo, M. Contribution of Synaptic Plasticity in the Insular Cortex to Chronic Pain. Neuroscience 2016, 338, 220–229. [Google Scholar] [CrossRef]
- Ma, Q. A Functional Subdivision within the Somatosensory System and Its Implications for Pain Research. Neuron 2022, 110, 749–769. [Google Scholar] [CrossRef]
- Craig, A.D. A New View of Pain as a Homeostatic Emotion. Trends Neurosci. 2003, 26, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazeli, S.; Büchel, C. Pain-Related Expectation and Prediction Error Signals in the Anterior Insula Are Not Related to Aversiveness. J. Neurosci. 2018, 38, 6461–6474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, L.; Manders, T.; Wang, J. Neuroplasticity Underlying the Comorbidity of Pain and Depression. Neural Plast. 2015, 2015, 504691. [Google Scholar] [CrossRef] [Green Version]
- Kandilarova, S.; Stoyanov, D.; Kostianev, S.; Specht, K. Altered Resting State Effective Connectivity of Anterior Insula in Depression. Front. Psychiatry 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprengelmeyer, R.; Steele, J.D.; Mwangi, B.; Kumar, P.; Christmas, D.; Milders, M.; Matthews, K. The Insular Cortex and the Neuroanatomy of Major Depression. J. Affect. Disord. 2011, 133, 120–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labrakakis, C. The Role of the Insular Cortex in Pain. Int. J. Mol. Sci. 2023, 24, 5736. https://doi.org/10.3390/ijms24065736
Labrakakis C. The Role of the Insular Cortex in Pain. International Journal of Molecular Sciences. 2023; 24(6):5736. https://doi.org/10.3390/ijms24065736
Chicago/Turabian StyleLabrakakis, Charalampos. 2023. "The Role of the Insular Cortex in Pain" International Journal of Molecular Sciences 24, no. 6: 5736. https://doi.org/10.3390/ijms24065736
APA StyleLabrakakis, C. (2023). The Role of the Insular Cortex in Pain. International Journal of Molecular Sciences, 24(6), 5736. https://doi.org/10.3390/ijms24065736





