In Vitro Cell Proliferation and Migration Properties of Oral Mucosal Fibroblasts: A Comparative Study on the Effects of Cord Blood- and Peripheral Blood-Platelet Lysate
Abstract
:1. Introduction
2. Results
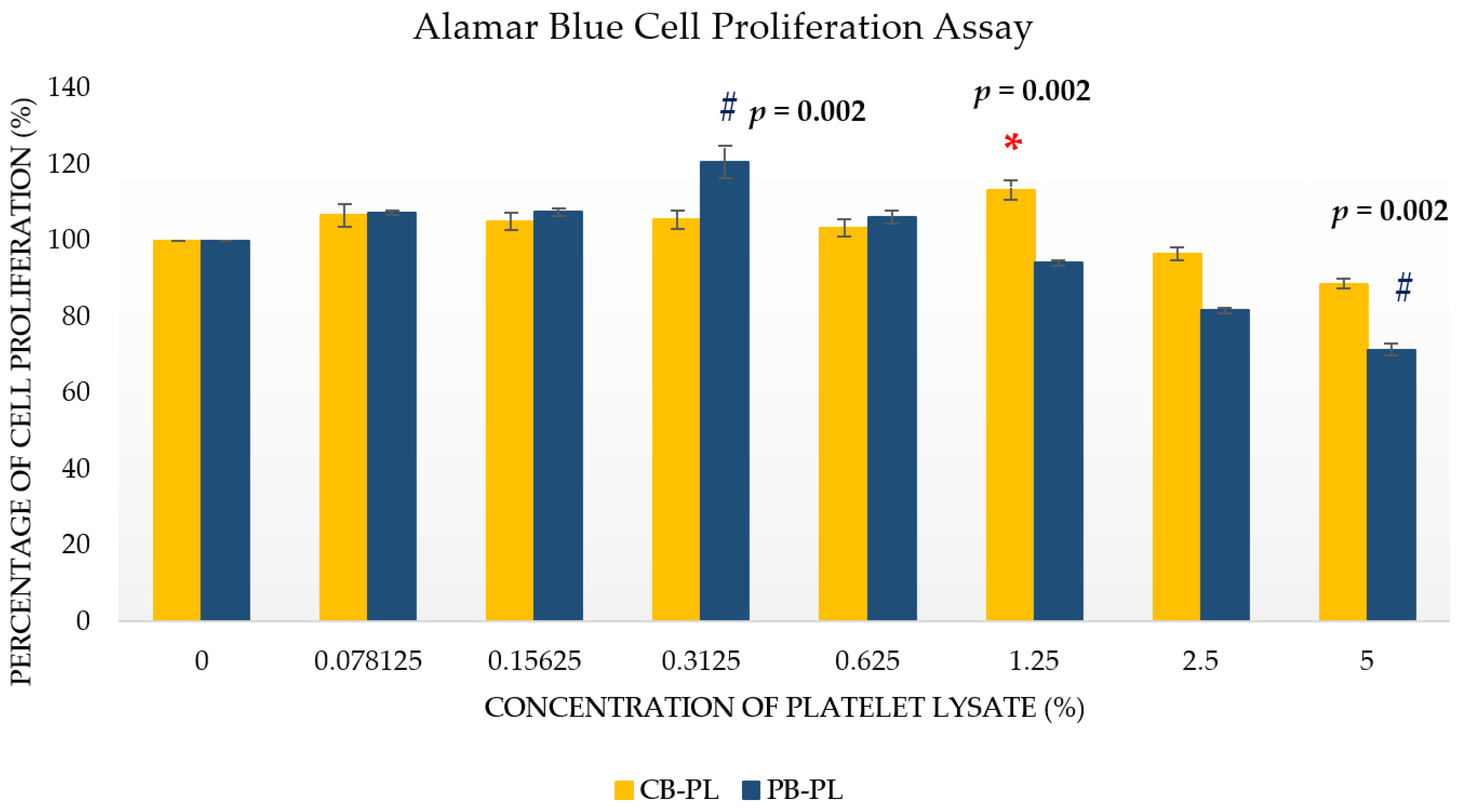
2.1. Optimal Concentrations of CB-PL and PB-PL in Cell Proliferation Assays
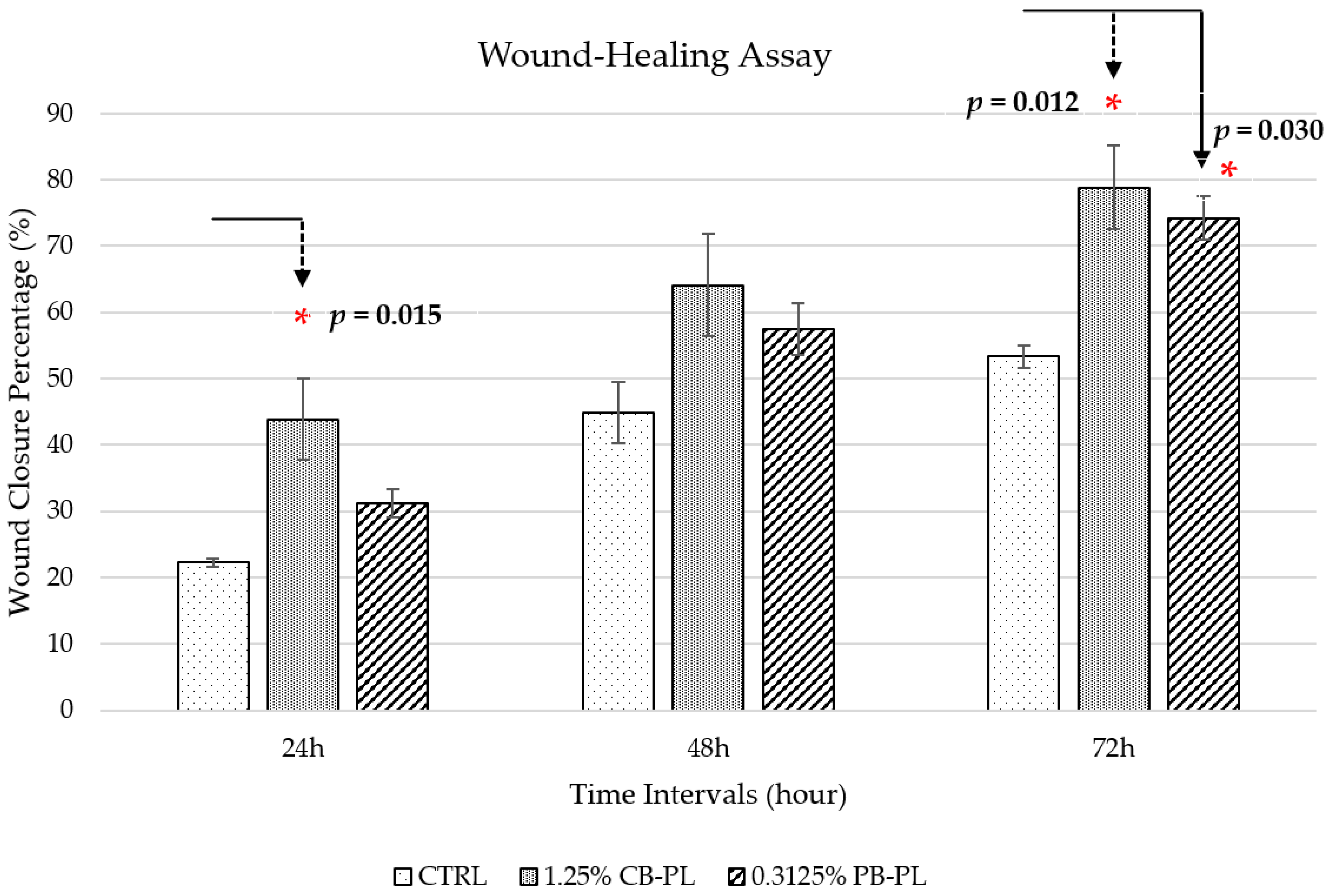
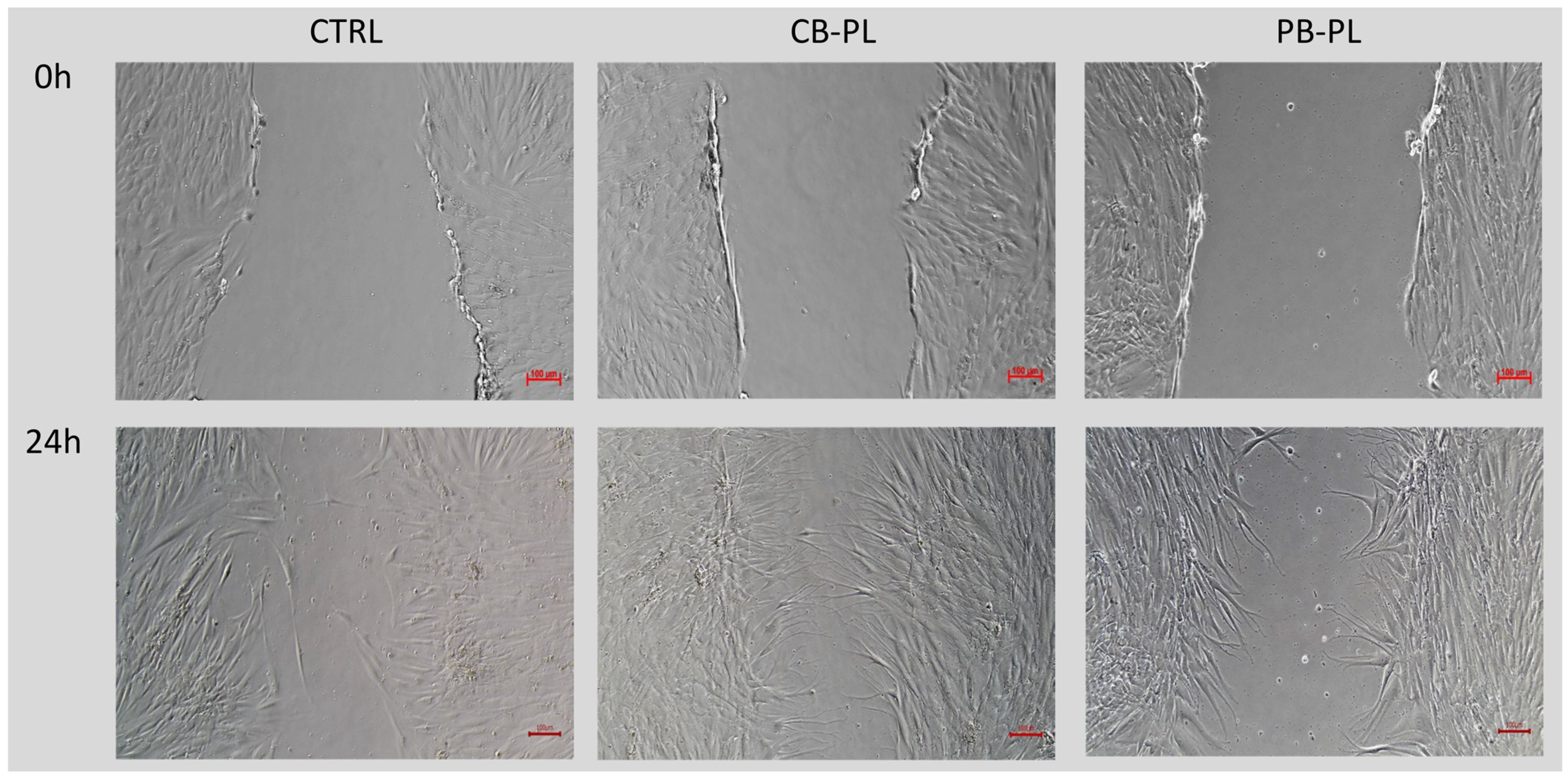
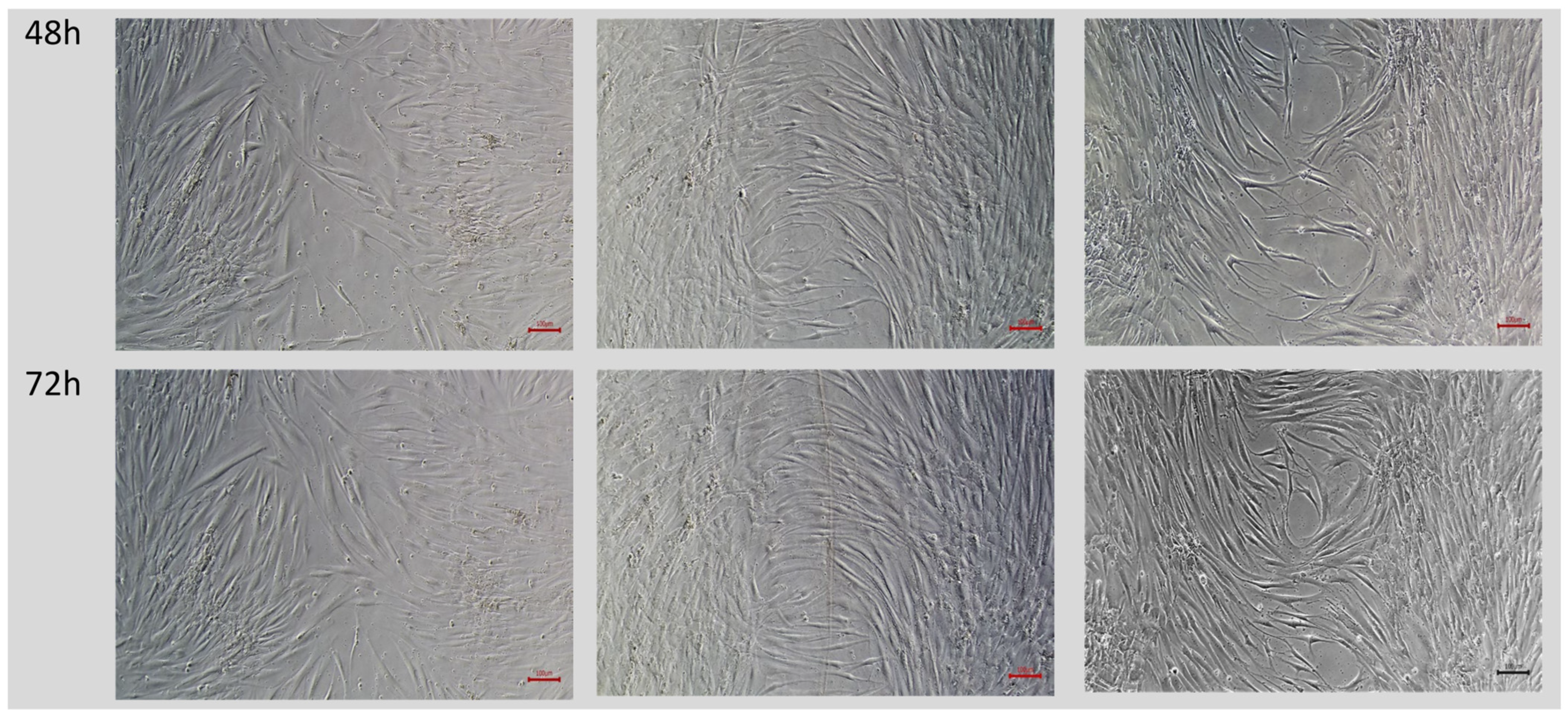
2.2. Wound Closure Percentage in Wound-Healing Assay
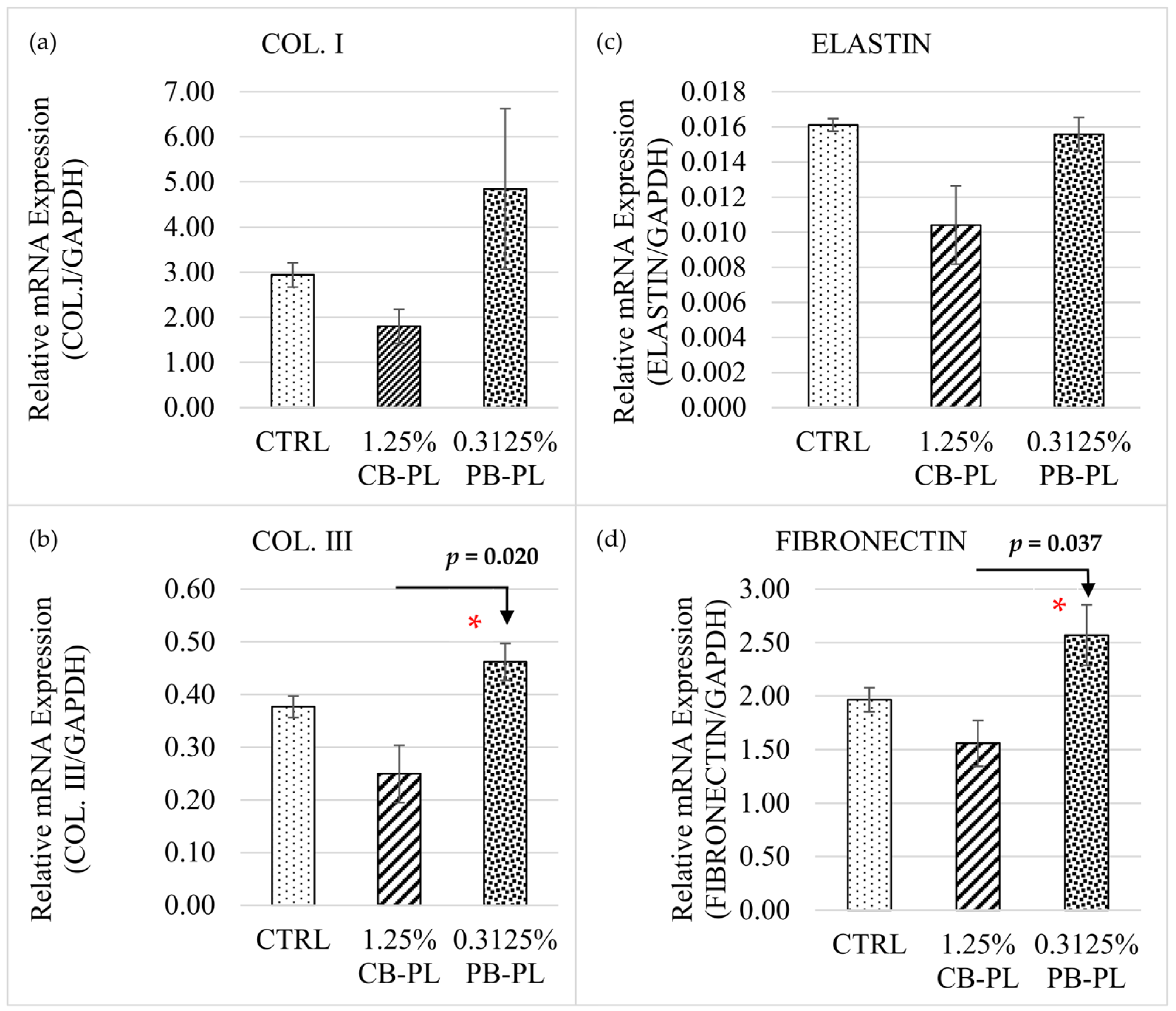
2.3. Relative mRNA Expressions of Cell Phenotypic Markers in HOMF
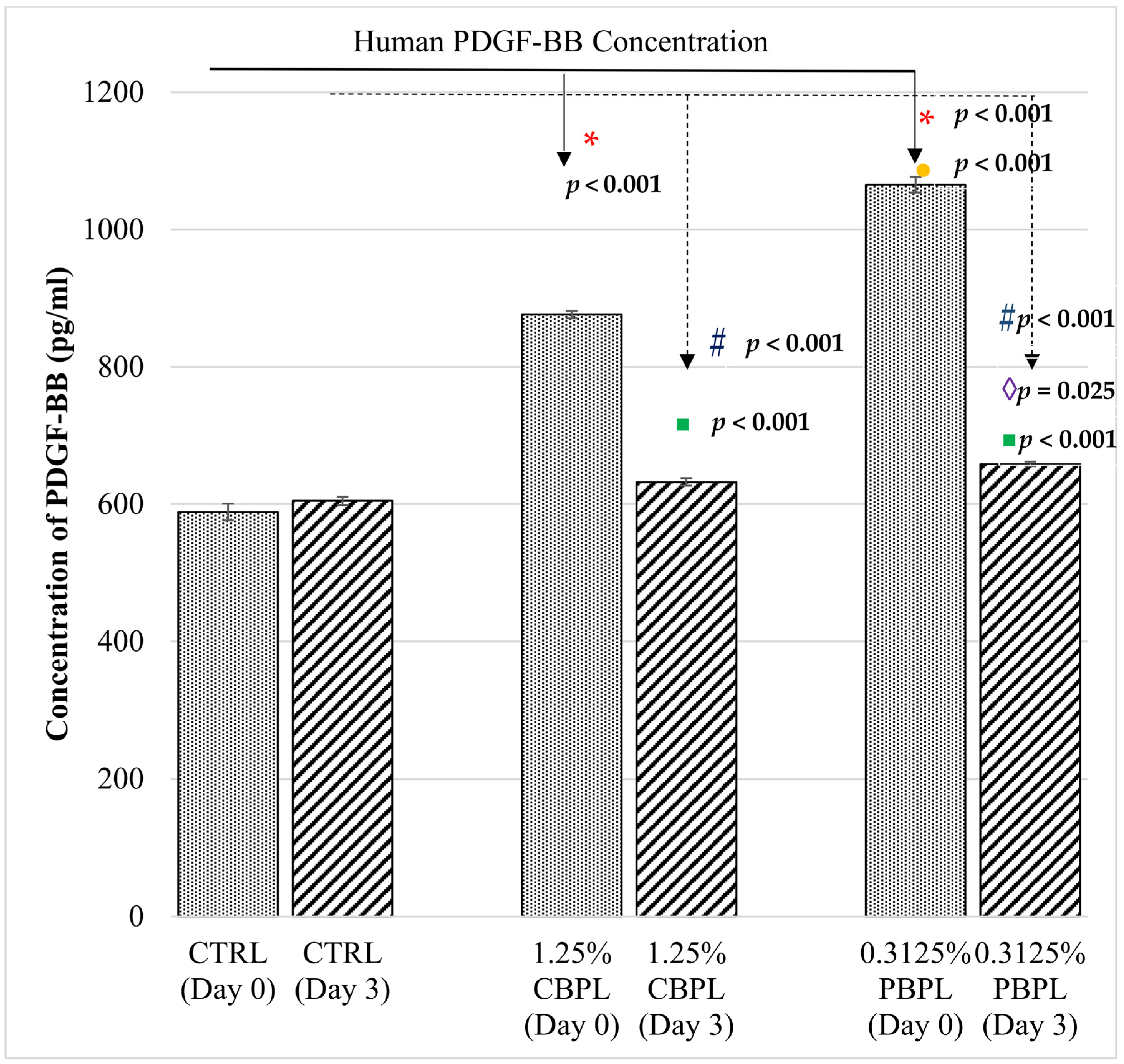
2.4. Human PDGF-BB Measurement
3. Discussion
3.1. Limitations and Advantages of PB-PL versus CB-PL
3.2. The Role of Platelet in Wound-Healing and the Biomarkers
3.3. Possible Reasons for the Low Platelet Count in Subjects and Risk Reduction Activities
3.4. Future Studies
4. Materials and Methods
4.1. Sample Collection and Preparation
4.1.1. Cord Blood Collection
4.1.2. Peripheral Blood Collection
4.1.3. Blood Processing
4.1.4. Primary Human Oral Mucosal Fibroblast Isolation and Culture
4.2. Alamar Blue Cell Proliferation Assay
4.3. Wound-Healing Assay
- At=0h = Area of wound measured immediately after scratching (t = 0 h);
- At=Δh = Area of wound measured at h hours after the scratch was performed.
- At=0h = Area of wound measured immediately after scratching (t = 0 h);
- At=Δh = Area of wound measured at h hours after the scratch was performed.
4.4. Two-Step Quantitative Polymerase Chain Reaction (qPCR)
4.5. Enzyme-Linked Immunosorbent Assay (ELISA) for PDGF-BB Measurement
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mortazavi, H.; Safi, Y.; Baharvand, M.; Rahmani, S. Diagnostic Features of Common Oral Ulcerative Lesions: An Updated Decision Tree. Int. J. Dent. 2016, 2016, 7278925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didona, D.; Caposiena Caro, R.D.; Sequeira Santos, A.M.; Solimani, F.; Hertl, M. Therapeutic strategies for oral lichen planus: State of the art and new insights. Front. Med. 2022, 9, 997190. [Google Scholar] [CrossRef] [PubMed]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Derakhshanfar, S.; Zhong, W. Biomaterials and tissue engineering for scar management in wound care. Burn. Trauma 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Rai, V.; Agrawal, D.K. Regulation of Collagen I and Collagen III in Tissue Injury and Regeneration. Cardiol. Cardiovasc. Med. 2023, 7, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, D.; Di Paolo, J.; Kumra, H.; Fois, G.; Candiani, G.; Reinhardt, D.P.; Mantovani, D. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials 2018, 180, 130–142. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Albanese, A.; Licata, M.E.; Polizzi, B.; Campisi, G. Platelet-rich plasma (PRP) in dental and oral surgery: From the wound healing to bone regeneration. Immun. Ageing 2013, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Huber, S.C.; de Lima Montalvão, S.A.; Sachetto, Z.; Santos Duarte Lana, J.F.; Annichino-Bizzacchi, J.M. Characterization of autologous platelet rich plasma (PRP) and its biological effects in patients with Behçet’s Disease. Regen 2021, 18, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell. Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Sanberg, P.R.; Garbuzova-Davis, S. Plasma derived from human umbilical cord blood: Potential cell-additive or cell-substitute therapeutic for neurodegenerative diseases. J. Cell. Mol. Med. 2018, 22, 6157–6166. [Google Scholar] [CrossRef] [PubMed]
- Mallis, P.; Gontika, I.; Dimou, Z.; Panagouli, E.; Zoidakis, J.; Makridakis, M.; Vlahou, A.; Georgiou, E.; Gkioka, V.; Stavropoulos-Giokas, C.; et al. Short Term Results of Fibrin Gel Obtained from Cord Blood Units: A Preliminary in Vitro Study. Bioengineering 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losi, P.; Barsotti, M.C.; Foffa, I.; Buscemi, M.; De Almeida, C.V.; Fabbri, M.; Gabbriellini, S.; Nocchi, F.; Ursino, S.; Urciuoli, P.; et al. In vitro human cord blood platelet lysate characterisation with potential application in wound healing. Int. Wound J. 2020, 17, 65–72. [Google Scholar] [CrossRef]
- Buzzi, M.; Versura, P.; Grigolo, B.; Cavallo, C.; Terzi, A.; Pellegrini, M.; Giannaccare, G.; Randi, V.; Campos, E.C. Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus. Apher. Sci. 2018, 57, 549–555. [Google Scholar] [CrossRef]
- Barsotti, M.C.; Losi, P.; Briganti, E.; Sanguinetti, E.; Magera, A.; Al Kayal, T.; Feriani, R.; Di Stefano, R.; Soldani, G. Effect of platelet lysate on human cells involved in different phases of wound healing. PLoS ONE 2013, 8, e84753. [Google Scholar] [CrossRef]
- Chen, L.W.; Huang, C.J.; Tu, W.H.; Lu, C.J.; Sun, Y.C.; Lin, S.Y.; Chen, W.L. The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates. PLoS ONE 2018, 13, e0194345. [Google Scholar] [CrossRef] [Green Version]
- Mallis, P.; Michalopoulos, E.; Sarri, E.F.; Papadopoulou, E.; Theodoropoulou, V.; Katsimpoulas, M.; Stavropoulos-Giokas, C. Evaluation of the Regenerative Potential of Platelet-Lysate and Platelet-Poor Plasma Derived from the Cord Blood Units in Corneal Wound Healing Applications: An In Vitro Comparative Study on Corneal Epithelial Cells. Curr. Issues Mol. Biol. 2022, 44, 4415–4438. [Google Scholar] [CrossRef]
- Beevi, S.; Bhale, A.; Panchal, N.; Siddiqui, S.; Anbarasu, K.; Verma, V. In vitro healing efficacy of stem cell secretome and cord blood platelet lysate on a chronic wound model. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2019, 27, 122–130. [Google Scholar] [CrossRef]
- Harto, P.H.B.; Mahmud, M.H.; Othman, A.H.; Ngadenin, N.H.; Azahar, N.S.M.; Hassan, M.N.F.; Yahaya, N.H.M.; Rani, R.A.; Leong, C.F.; Ng, M.H.; et al. Human platelet lysate promotes proliferation but fails to maintain chondrogenic markers of chondrocytes. Sains Malays. 2019, 48, 2169–2176. [Google Scholar] [CrossRef]
- Liau, L.L.; Hassan, M.N.F.B.; Tang, Y.L.; Ng, M.H.; Law, J.X. Feasibility of human platelet lysate as an alternative to foetal bovine serum for in vitro expansion of chondrocytes. Int. J. Mol. Sci. 2021, 22, 1269. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, N.S.; Shanskii, Y.D.; Sviridova, I.K.; Karalkin, P.A.; Kirsanova, V.A.; Akhmedova, S.A.; Kaprin, A.D. Analysis of Reparative Activity of Platelet Lysate: Effect on Cell Monolayer Recovery In Vitro and Skin Wound Healing In Vivo. Bull. Exp. Biol. Med. 2016, 162, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.C. Platelet-derived growth factor. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 343–347. [Google Scholar]
- Parazzi, V.; Lazzari, L.; Rebulla, P. Platelet gel from cord blood: A novel tool for tissue engineering. Platelets 2010, 21, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Merigo, E.; Oppici, A.; Parlatore, A.; Cella, L.; Clini, F.; Fontana, M.; Fornaini, C. Platelet-Rich Plasma (PRP) Rinses for the Treatment of Non-Responding Oral Lichen Planus: A Case Report. Biomedicines 2018, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- MH, E.L.-K.; Hassan, A.S.; Abdel Raheem, H.M.; Doss, S.S.; El-Kalioby, M.; Saleh, N.A.; Saleh, M.A. Platelet-rich plasma for resistant oral erosions of pemphigus vulgaris: A pilot study. Wound Repair Regen. 2015, 23, 953–955. [Google Scholar]
- Caramella, C.M.; Sandri, G.; Rossi, S.; Mori, M.; Cristina Bonferoni, M.; Ferrari, F.; Del Fante, C.; Perotti, C. New therapeutic platforms for the treatment of epithelial and cutaneous lesions. Curr. Drug Deliv. 2013, 10, 18–31. [Google Scholar] [CrossRef]
- Sindici, E.; Astesano, S.; Fazio, L.; Dragonetti, A.; Pugliese, M.; Scully, C.; Carossa, S.; Broccoletti, R.; Arduino, P.G. Treatment of Oral Lesions in Dystrophic Epidermolysis Bullosa: A Case Series of Cord Blood Platelet Gel and Low-level Laser Therapy. Acta Derm. Venereol. 2017, 97, 383–384. [Google Scholar] [CrossRef] [Green Version]
- Rebulla, P.; Pupella, S.; Santodirocco, M.; Greppi, N.; Villanova, I.; Buzzi, M.; De Fazio, N.; Grazzini, G.; Italian Cord Blood Platelet Gel Study Group. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016, 14, 73–79. [Google Scholar]
- Marx, R.E. Platelet-rich plasma (PRP): What is PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.N.F.; Yap, Z.Y.; Tang, Y.L.; Ng, M.H.; Law, J.X. Expired platelet concentrate as a source of human platelet lysate for Xenogeneic-free culture of human dermal fibroblasts. Sains Malays. 2021, 50, 2355–2365. [Google Scholar] [CrossRef]
- Meftahpour, V.; Malekghasemi, S.; Baghbanzadeh, A.; Aghebati-Maleki, A.; Pourakbari, R.; Fotouhi, A.; Aghebati-Maleki, L. Platelet lysate: A promising candidate in regenerative medicine. Regen. Med. 2021, 16, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.-S.; Mahmoodi, M.; Rafati, A.R.; Manafi, F.; Mehrabani, D. The role of human adult peripheral and umbilical cord blood platelet-rich plasma on proliferation and migration of human skin fibroblasts. World J. Plast. Surg. 2017, 6, 198–205. [Google Scholar] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, C.; Hao, Z.; Wen, S.; Leng, H. A quantitative study of the relationship between the distribution of different types of collagen and the mechanical behavior of rabbit medial collateral ligaments. PLoS ONE 2014, 9, e103363. [Google Scholar] [CrossRef] [PubMed]
- Sá, O.M.S.; Lopes, N.N.F.; Alves, M.T.S.; Caran, E.M.M. Effects of Glycine on Collagen, PDGF, and EGF Expression in Model of Oral Mucositis. Nutrients 2018, 10, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evrova, O.; Kellenberger, D.; Calcagni, M.; Vogel, V.; Buschmann, J. Supporting Cell-Based Tendon Therapy: Effect of PDGF-BB and Ascorbic Acid on Rabbit Achilles Tenocytes in Vitro. Int. J. Mol. Sci. 2020, 21, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.H.; Jeong, E.S.; Lee, S.; Jang, J.H. Bio-functionalization and in-vitro evaluation of titanium surface with recombinant fibronectin and elastin fragment in human mesenchymal stem cell. PLoS ONE 2021, 16, e0260760. [Google Scholar] [CrossRef]
- Sharma, S.; Rose, H. Incidentally detected thrombocytopenia in adults. Aust. Fam. Physician 2014, 43, 700–704. [Google Scholar]
- Jiang, D.; Portuguese, A.J.; Weatherford, A.; Garcia, D.; Gernsheimer, T. Platelet trends after COVID-19 vaccination in patients with chronic or persistent immune thrombocytopenia. Am. J. Hematol. 2021, 96, E472–E474. [Google Scholar] [CrossRef]
- Bianchetti, A.; Chinello, C.; Guindani, M.; Braga, S.; Neva, A.; Verardi, R.; Piovani, G.; Pagani, L.; Lisignoli, G.; Magni, F.; et al. A Blood Bank Standardized Production of Human Platelet Lysate for Mesenchymal Stromal Cell Expansion: Proteomic Characterization and Biological Effects. Front. Cell. Dev. Biol. 2021, 9, 650490. [Google Scholar] [CrossRef] [PubMed]
- Parazzi, V.; Lavazza, C.; Boldrin, V.; Montelatici, E.; Pallotti, F.; Marconi, M.; Lazzari, L. Extensive Characterization of Platelet Gel Releasate from Cord Blood in Regenerative Medicine. Cell Transpl. 2015, 24, 2573–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullard, J.D.; Lei, J.; Lim, J.J.; Massee, M.; Fallon, A.M.; Koob, T.J. Evaluation of dehydrated human umbilical cord biological properties for wound care and soft tissue healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Adtani, P.; Narasimhan, M.; Ranganathan, K.; Punnoose, A.; Prasad, P.; Natarajan, P.M. Characterization of oral fibroblasts: An in vitro model for oral fibrosis. J. Oral Maxillofac. Pathol. 2019, 23, 198–202. [Google Scholar] [CrossRef]
- Vanegas, D.; Triviño, L.; Galindo, C.; Franco, L.; Salguero, G.; Camacho, B.; Perdomo-Arciniegas, A.M. A new strategy for umbilical cord blood collection developed at the first Colombian public cord blood bank increases total nucleated cell content. Transfusion 2017, 57, 2225–2233. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.H.; Zarrabi, M.; Abroun, S.; Ahmadipanah, M.; Abbaspanah, B. Umbilical cord blood quality and quantity: Collection up to transplantation. Asian J. Transfus. Sci. 2019, 13, 79–89. [Google Scholar] [CrossRef]
- Xian, L.J.; Chowdhury, S.R.; Bin Saim, A.; Idrus, R.B. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy 2015, 17, 293–300. [Google Scholar] [CrossRef]
- Wong, P.-R.; Sahardi, N.F.N.M.; Tan, J.; Chua, K.-H.; Wan, W.Z.J.S.M. Characterization of Keratinocytes, Fibroblasts and Melanocytes Isolated from Human Skin using Gene Markers. Sains Malays. 2022, 51, 1425–1436. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J Investig. Derm. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]

| Gene | GenBank Accession Number | Primer Sequence (5′ to 3′) |
|---|---|---|
| GAPDH | NM_002046.5 | F: CAATGACCCCTTCATTGACC |
| R: TTGATTTTGGAGGGATCTCG | ||
| Col. I | NM_000088.3 | F: GTGCTAAAGGTGCCAATGGT |
| R: ACCAGGTTCACCGCTGTTAC | ||
| Col. III | NM_000090.3 | F: CCAGGAGCTAACGGTCTCAG |
| R: CAGGGTTTCCATCTCTTCCA | ||
| Elastin | NM_000501.4 | F: GGTGGCTTAGGAGTGTCTGC |
| R: CCAGCAAAAGCTCCACCTAC | ||
| Fibronectin | NM_212482.2 | F: AAAATGGCCAGATGATGAGC |
| R: TGGCACCGAGATATTCCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azmi, A.F.; Yahya, M.A.A.M.; Azhar, N.A.; Ibrahim, N.; Ghafar, N.A.; Ghani, N.A.A.; Nizar, M.A.M.; Yunus, S.S.M.; Singh, T.K.L.; Law, J.-X.; et al. In Vitro Cell Proliferation and Migration Properties of Oral Mucosal Fibroblasts: A Comparative Study on the Effects of Cord Blood- and Peripheral Blood-Platelet Lysate. Int. J. Mol. Sci. 2023, 24, 5775. https://doi.org/10.3390/ijms24065775
Azmi AF, Yahya MAAM, Azhar NA, Ibrahim N, Ghafar NA, Ghani NAA, Nizar MAM, Yunus SSM, Singh TKL, Law J-X, et al. In Vitro Cell Proliferation and Migration Properties of Oral Mucosal Fibroblasts: A Comparative Study on the Effects of Cord Blood- and Peripheral Blood-Platelet Lysate. International Journal of Molecular Sciences. 2023; 24(6):5775. https://doi.org/10.3390/ijms24065775
Chicago/Turabian StyleAzmi, Arief Faisal, Mohammad Amirul Asyraff Mohd Yahya, Nur Ain Azhar, Norliwati Ibrahim, Norzana Abd Ghafar, Nur Azurah Abdul Ghani, Muhammad Aiman Mohd Nizar, Siti Salmiah Mohd Yunus, Tashveender Kaur Lakhbir Singh, Jia-Xian Law, and et al. 2023. "In Vitro Cell Proliferation and Migration Properties of Oral Mucosal Fibroblasts: A Comparative Study on the Effects of Cord Blood- and Peripheral Blood-Platelet Lysate" International Journal of Molecular Sciences 24, no. 6: 5775. https://doi.org/10.3390/ijms24065775
APA StyleAzmi, A. F., Yahya, M. A. A. M., Azhar, N. A., Ibrahim, N., Ghafar, N. A., Ghani, N. A. A., Nizar, M. A. M., Yunus, S. S. M., Singh, T. K. L., Law, J.-X., & Ng, S.-L. (2023). In Vitro Cell Proliferation and Migration Properties of Oral Mucosal Fibroblasts: A Comparative Study on the Effects of Cord Blood- and Peripheral Blood-Platelet Lysate. International Journal of Molecular Sciences, 24(6), 5775. https://doi.org/10.3390/ijms24065775







