Elucidation of OSW-1-Induced Stress Responses in Neuro2a Cells
Abstract
1. Introduction
2. Results
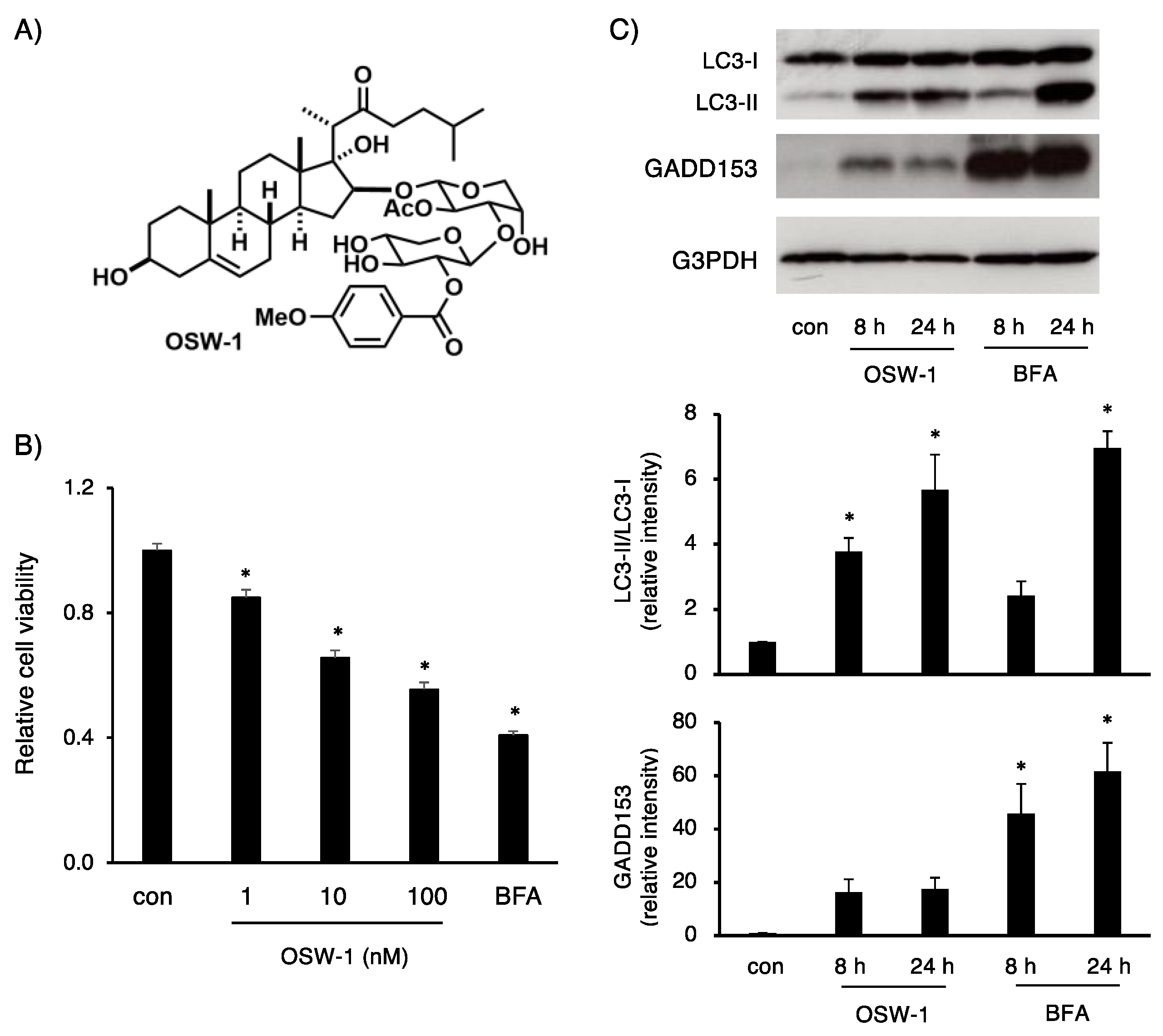
2.1. OSW-1 Stimulation in Neuro2a Induced Cell Death, Accompanied by Autophagy
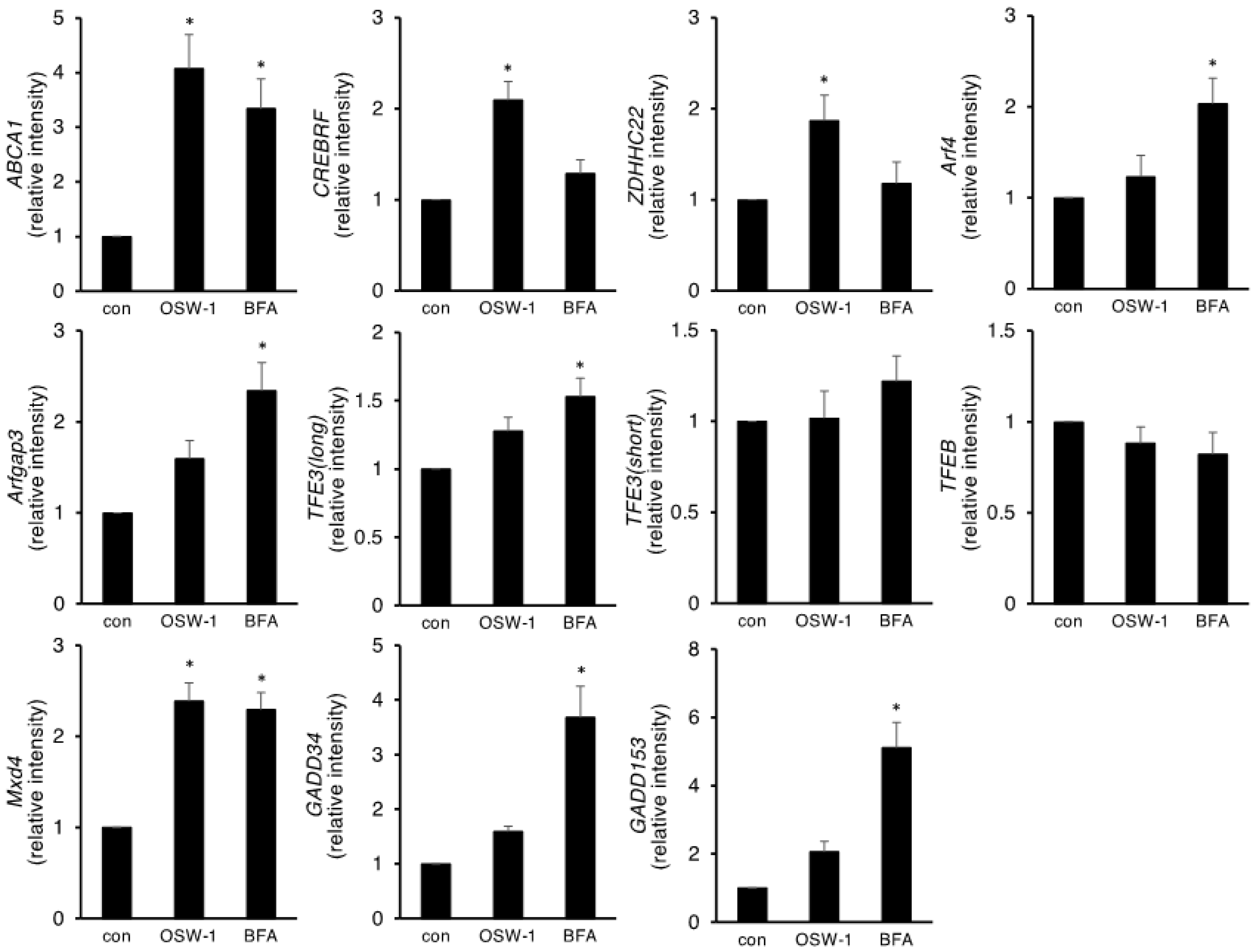
2.2. OSW-1 Affected Genes Involved in Lipid Metabolism, ER/Golgi Homeostasis, and Autophagy in Neuro2a Cells
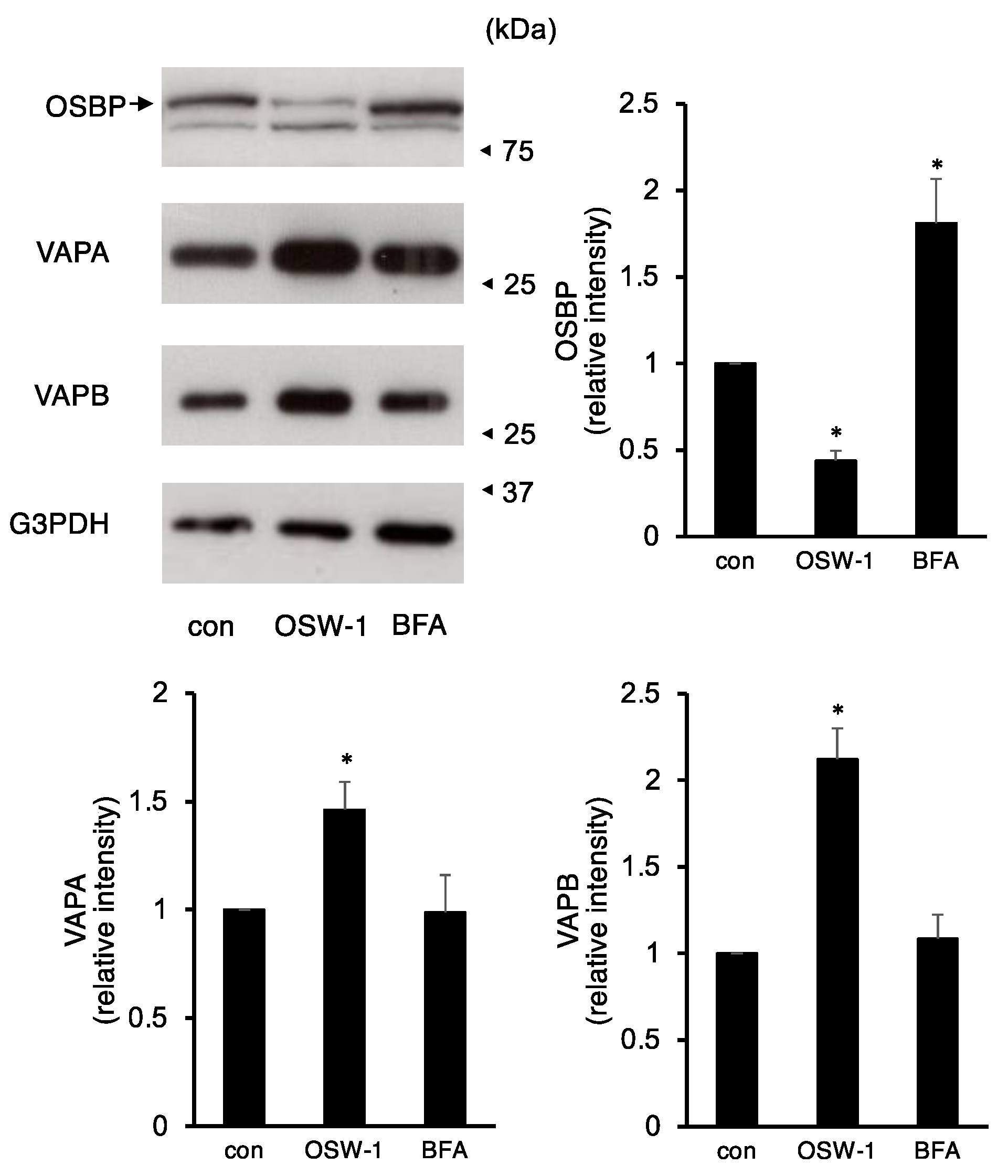
2.3. OSW-1 Downregulated ER–Golgi Transport in Neuro2a Cells
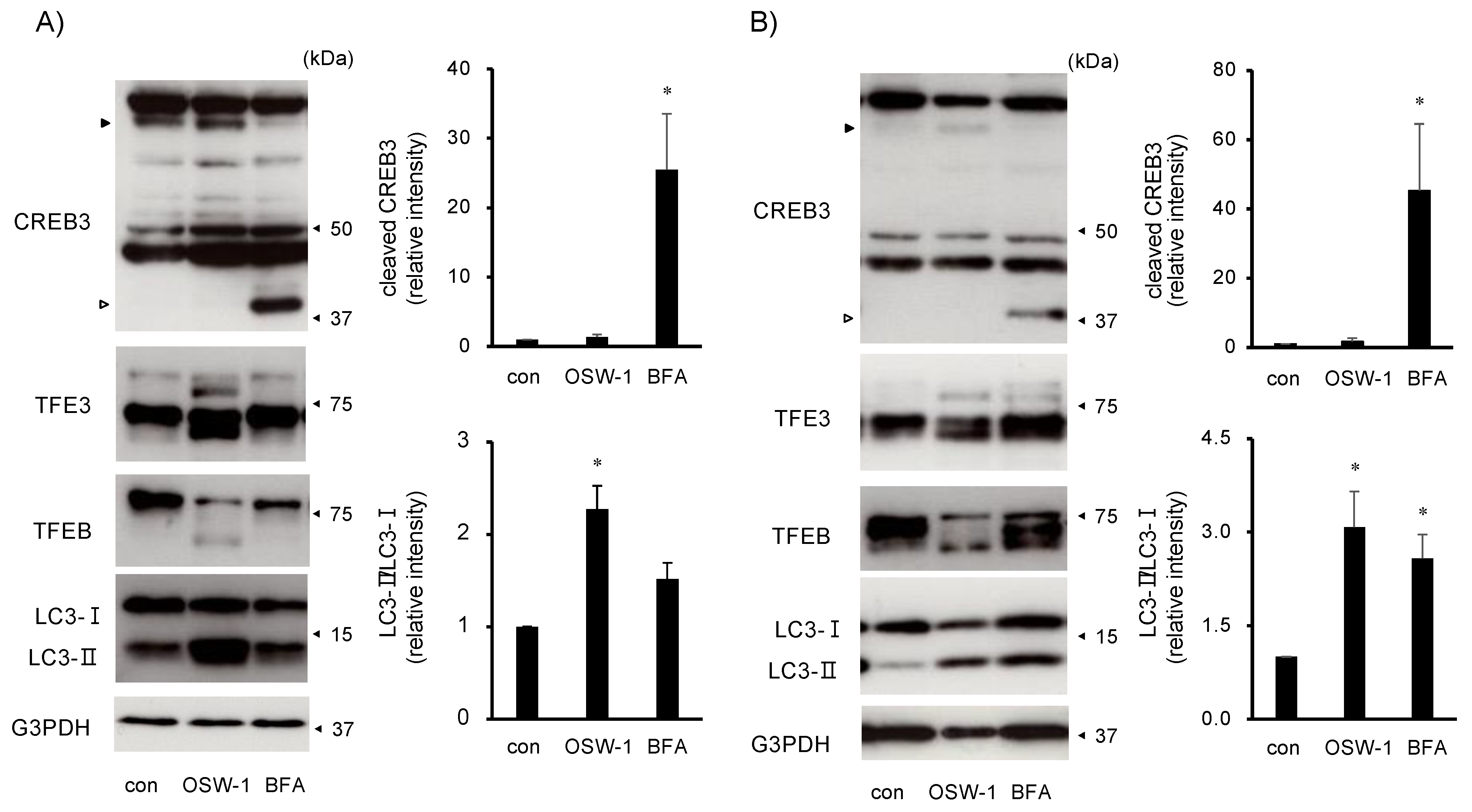
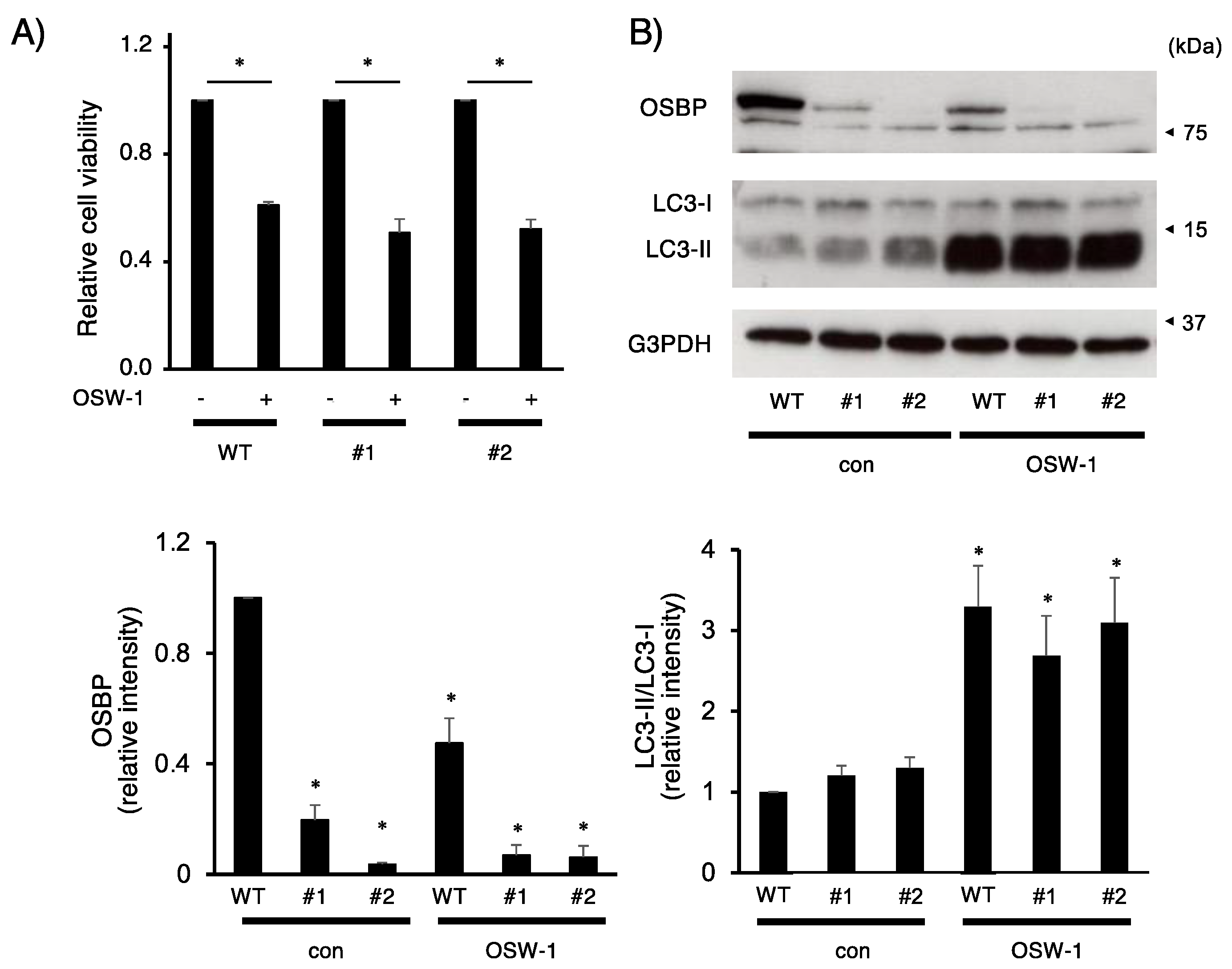
2.4. OSW-1 Induced Atypical Golgi Stress Responses, and Its Cytotoxicity Was OSBP-Independent in Neuro2a Cells
3. Discussion
4. Materials and Methods
4.1. Construction of Plasmids
4.2. Cell Culture and Treatment
4.3. Measurement of Cell Viability
4.4. Microarray Analysis
4.5. Reverse Transcription Polymerase Chain Reaction
4.6. Western Blotting Analysis
4.7. Measurement of Protein Secretion
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mimaki, Y.; Kuroda, M.; Kameyama, A.; Sashida, Y.; Hirano, T.; Oka, K.; Maekawa, R.; Wada, T.; Sugita, K.; Beutler, J.A. Cholestane glycosides with potent cytostatic activities on various tumor cells from Ornithogalum saundersiae bulbs. Bioorganic Med. Chem. Lett. 1997, 7, 633–636. [Google Scholar] [CrossRef]
- Burgett, A.W.G.; Poulsen, T.B.; Wangkanont, K.; Anderson, D.R.; Kikuchi, C.; Shimada, K.; Okubo, S.; Fortner, K.C.; Mimaki, Y.; Kuroda, M.; et al. Natural products reveal cancer cell dependence on oxysterol-binding proteins. Nat. Chem. Biol. 2011, 7, 639–647. [Google Scholar] [CrossRef]
- Zhou, Y.; Garcia-Prieto, C.; Carney, D.A.; Xu, R.-H.; Pelicano, H.; Kang, Y.; Yu, W.; Lou, C.; Kondo, S.; Liu, J.; et al. OSW-1: A Natural Compound with Potent Anticancer Activity and a Novel Mechanism of Action. J. Natl. Cancer Inst. 2005, 97, 1781–1785. [Google Scholar] [CrossRef]
- Nakatsu, F.; Kawasaki, A. Functions of Oxysterol-Binding Proteins at Membrane Contact Sites and Their Control by Phosphoinositide Metabolism. Front. Cell Dev. Biol. 2021, 9, 664758. [Google Scholar] [CrossRef] [PubMed]
- Mesmin, B.; Bigay, J.; Polidori, J.; Jamecna, D.; Lacas-Gervais, S.; Antonny, B. Sterol transfer, PI4P consumption, and control of membrane lipid order by endogenous OSBP. EMBO J. 2017, 36, 3156–3174. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Sasaki, K.; Fukutani, Y.; Yoshida, H.; Ohsawa, I.; Yohda, M.; Sakurai, K. Anticancer saponin OSW-1 is a novel class of selective Golgi stress inducer. Bioorganic Med. Chem. Lett. 2019, 29, 1732–1736. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; E Johansen, F.; Prywes, R. Interaction of ATF6 and serum response factor. Mol. Cell. Biol. 1997, 17, 4957–4966. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999, 397, 271–274. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004, 18, 3066–3077. [Google Scholar] [CrossRef]
- Galehdar, Z.; Swan, P.; Fuerth, B.; Callaghan, S.M.; Park, D.S.; Cregan, S.P. Neuronal Apoptosis Induced by Endoplasmic Reticulum Stress Is Regulated by ATF4–CHOP-Mediated Induction of the Bcl-2 Homology 3-Only Member PUMA. J. Neurosci. 2010, 30, 16938–16948. [Google Scholar] [CrossRef] [PubMed]
- Oh-Hashi, K.; Sugiura, N.; Amaya, F.; Isobe, K.-I.; Hirata, Y. Functional validation of ATF4 and GADD34 in Neuro2a cells by CRISPR/Cas9-mediated genome editing. Mol. Cell. Biochem. 2017, 440, 65–75. [Google Scholar] [CrossRef]
- Bi, M.; Naczki, C.; Koritzinsky, M.; Fels, D.; Blais, J.; Hu, N.; Harding, H.; Novoa, I.; Varia, M.; Raleigh, J.; et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005, 24, 3470–3481. [Google Scholar] [CrossRef]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Yoshida, H. TFE3, HSP47, and CREB3 Pathways of the Mammalian Golgi Stress Response. Cell Struct. Funct. 2017, 42, 27–36. [Google Scholar] [CrossRef]
- Reiling, J.H.; Olive, A.J.; Sanyal, S.; Carette, J.E.; Brummelkamp, T.R.; Ploegh, H.L.; Starnbach, M.N.; Sabatini, D.M. A CREB3–ARF4 signalling pathway mediates the response to Golgi stress and susceptibility to pathogens. Nat. Cell Biol. 2013, 15, 1473–1485. [Google Scholar] [CrossRef]
- Gao, J.; Gao, A.; Liu, W.; Chen, L. Golgi stress response: A regulatory mechanism of Golgi function. Biofactors 2021, 47, 964–974. [Google Scholar] [CrossRef]
- Sasaki, K.; Yoshida, H. Golgi stress response and organelle zones. FEBS Lett. 2019, 593, 2330–2340. [Google Scholar] [CrossRef]
- Oh-Hashi, K.; Soga, A.; Naruse, Y.; Takahashi, K.; Kiuchi, K.; Hirata, Y. Elucidating post-translational regulation of mouse CREB3 in Neuro2a cells. Mol. Cell. Biochem. 2018, 448, 287–297. [Google Scholar] [CrossRef]
- Oh-Hashi, K.; Kohno, H.; Kandeel, M.; Hirata, Y. Characterization of IRE1α in Neuro2a cells by pharmacological and CRISPR/Cas9 approaches. Mol. Cell. Biochem. 2019, 465, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Oh-Hashi, K.; Hasegawa, T.; Mizutani, Y.; Takahashi, K.; Hirata, Y. Elucidation of brefeldin A-induced ER and Golgi stress responses in Neuro2a cells. Mol. Cell. Biochem. 2021, 476, 3869–3877. [Google Scholar] [CrossRef]
- Helms, J.B.; Rothman, J.E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature 1992, 360, 352–354. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Q.; Liao, M.; Wu, X.; Xi, H.; Ma, H.; Li, S.; Zhang, Y.; Xia, Y. OSW-1 induces apoptosis and cyto-protective autophagy, and synergizes with chemotherapy on triple negative breast cancer metastasis. Cell. Oncol. 2022, 45, 1255–1275. [Google Scholar] [CrossRef]
- Lim, C.-Y.; Davis, O.B.; Shin, H.R.; Zhang, J.; Berdan, C.A.; Jiang, X.; Counihan, J.L.; Ory, D.S.; Nomura, D.K.; Zoncu, R. ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nat. Cell Biol. 2019, 21, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Nadanaka, S.; Tanakura, S.; Sawaguchi, S.; Midori, S.; Kawai, Y.; Yamaguchi, S.; Shimada, Y.; Nakamura, Y.; Matsumura, Y.; et al. TFE3 is a bHLH-ZIP-type transcription factor that regulates the mammalian Golgi stress response. Cell Struct. Funct. 2015, 40, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Raben, N.; Puertollano, R. TFEB and TFE3: Linking lysosomes to cellular adaptation to stress. Annu. Rev. Cell Dev. Biol. 2016, 32, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Martina, J.A.; Diab, H.I.; Lishu, L.; Jeong-A, L.; Patange, S.; Raben, N.; Puertollano, R. The Nutrient-Responsive Transcription Factor TFE3 Promotes Autophagy, Lysosomal Biogenesis, and Clearance of Cellular Debris. Sci. Signal. 2014, 7, ra9. [Google Scholar] [CrossRef] [PubMed]
- Plummer, A.M.; Culbertson, A.T.; Liao, M. The ABCs of Sterol Transport. Annu. Rev. Physiol. 2021, 83, 153–181. [Google Scholar] [CrossRef]
- Nishimura, A.L.; Mitne-Neto, M.; Silva, H.C.; Richieri-Costa, A.; Middleton, S.; Cascio, D.; Kok, F.; Oliveira, J.R.; Gillingwater, T.; Webb, J.; et al. A Mutation in the Vesicle-Trafficking Protein VAPB Causes Late-Onset Spinal Muscular Atrophy and Amyotrophic Lateral Sclerosis. Am. J. Hum. Genet. 2004, 75, 822–831. [Google Scholar] [CrossRef]
- Teuling, E.; Ahmed, S.; Haasdijk, E.; Demmers, J.; Steinmetz, M.O.; Akhmanova, A.; Jaarsma, D.; Hoogenraad, C.C. Motor Neuron Disease-Associated Mutant Vesicle-Associated Membrane Protein-Associated Protein (VAP) B Recruits Wild-Type VAPs into Endoplasmic Reticulum-Derived Tubular Aggregates. J. Neurosci. 2007, 27, 9801–9815. [Google Scholar] [CrossRef]
- Moumen, A.; Virard, I.; Raoul, C. Accumulation of Wildtype and ALS-Linked Mutated VAPB Impairs Activity of the Proteasome. PLoS ONE 2011, 6, e26066. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Yang, C.; Li, F.-P.; Yu, D.-H.; Pan, Z.-Y.; Wang, Z.-F.; Li, Z.-Q. Palmitoyl transferases act as potential regulators of tumor-infiltrating immune cells and glioma progression. Mol. Ther.-Nucleic Acids 2022, 28, 716–731. [Google Scholar] [CrossRef]
- Huang, J.; Li, J.; Tang, J.; Wu, Y.; Dai, F.; Yi, Z.; Wang, Y.; Li, Y.; Wu, Y.; Ren, G.; et al. ZDHHC22-mediated mTOR palmitoylation restrains breast cancer growth and endocrine therapy resistance. Int. J. Biol. Sci. 2022, 18, 2833–2850. [Google Scholar] [CrossRef]
- Audas, T.E.; Li, Y.; Liang, G.; Lu, R. A Novel Protein, Luman/CREB3 Reruitment Factor, Inhibits Luman Activation of the Unfolded Protein Response. Mol. Cell. Biol. 2008, 28, 3952–3966. [Google Scholar] [CrossRef]
- Oh-Hashi, K.; Hasegawa, T.; Naruse, Y.; Hirata, Y. Molecular characterization of mouse CREB3 regulatory factor in Neuro2a cells. Mol. Biol. Rep. 2021, 48, 5411–5420. [Google Scholar] [CrossRef]
- Minster, R.L.; Hawley, N.; Su, C.-T.; Sun, G.; E Kershaw, E.; Cheng, H.; Buhule, O.D.; Lin, J.; Reupena, M.S.; Viali, S.; et al. A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat. Genet. 2016, 48, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.J.F. CREBRF variant increases obesity risk and protects against diabetes in Samoans. Nat. Genet. 2016, 48, 976–978. [Google Scholar] [CrossRef]
- Prochownik, E.V.; Wang, H. Normal and Neoplastic Growth Suppression by the Extended Myc Network. Cells 2022, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Bensen, R.C.; Gunay, G.; Finneran, M.C.; Jhingan, I.; Acar, H.; Burgett, A.W.G. Small Molecule Targeting of Oxysterol-Binding Protein (OSBP)-Related Protein 4 and OSBP Inhibits Ovarian Cancer Cell Proliferation in Monolayer and Spheroid Cell Models. ACS Pharmacol. Transl. Sci. 2021, 4, 744–756. [Google Scholar] [CrossRef]
- Zhan, Z.; Liu, Z.; Zhang, C.; Gao, H.; Lai, J.; Chen, Y.; Huang, H. Anticancer effects of OSW-1 on glioma cells via regulation of the PI3K/AKT signal pathway: A network pharmacology approach and experimental validation in vitro and in vivo. Front. Pharmacol. 2022, 13, 967141. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Fan, K.; Zhang, Y.; Zhang, J.; Guo, H.; Yu, P.; Ma, J. Effective cytotoxic activity of OSW-1 on colon cancer by inducing apoptosis in vitro and in vivo. Oncol. Rep. 2017, 37, 3509–3519. [Google Scholar] [CrossRef]
- Iguchi, T.; Kuroda, M.; Naito, R.; Watanabe, T.; Matsuo, Y.; Yokosuka, A.; Mimaki, Y. Cholestane glycosides from Ornithogalum saundersiae bulbs and the induction of apoptosis in HL-60 cells by OSW-1 through a mitochondrial-independent signaling pathway. J. Nat. Med. 2018, 73, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Prieto, C.; Riaz Ahmed, K.B.; Chen, Z.; Zhou, Y.; Hammoudi, N.; Kang, Y.; Lou, C.; Mei, Y.; Jin, Z.; Huang, P. Effective killing of leukemia cells by the natural product OSW-1 through disruption of cellular calcium homeostasis. J. Biol. Chem. 2013, 288, 3240–3250. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, L.; Yu, B.; Wu, J. Apoptosis Induced by a New Member of Saponin Family Is Mediated through Caspase-8-Dependent Cleavage of Bcl-2. Mol. Pharmacol. 2005, 68, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, Y.; Li, J.; Yin, Y. OSW-1 inhibits tumor growth and metastasis by NFATc2 on triple-negative breast cancer. Cancer Med. 2020, 9, 5558–5569. [Google Scholar] [CrossRef] [PubMed]
- Esvelt, K.M.; Yang, L.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-Guided Human Genome Engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh-hashi, K.; Nakamura, H.; Ogawa, H.; Hirata, Y.; Sakurai, K. Elucidation of OSW-1-Induced Stress Responses in Neuro2a Cells. Int. J. Mol. Sci. 2023, 24, 5787. https://doi.org/10.3390/ijms24065787
Oh-hashi K, Nakamura H, Ogawa H, Hirata Y, Sakurai K. Elucidation of OSW-1-Induced Stress Responses in Neuro2a Cells. International Journal of Molecular Sciences. 2023; 24(6):5787. https://doi.org/10.3390/ijms24065787
Chicago/Turabian StyleOh-hashi, Kentaro, Hibiki Nakamura, Hirotaka Ogawa, Yoko Hirata, and Kaori Sakurai. 2023. "Elucidation of OSW-1-Induced Stress Responses in Neuro2a Cells" International Journal of Molecular Sciences 24, no. 6: 5787. https://doi.org/10.3390/ijms24065787
APA StyleOh-hashi, K., Nakamura, H., Ogawa, H., Hirata, Y., & Sakurai, K. (2023). Elucidation of OSW-1-Induced Stress Responses in Neuro2a Cells. International Journal of Molecular Sciences, 24(6), 5787. https://doi.org/10.3390/ijms24065787




